Abstract
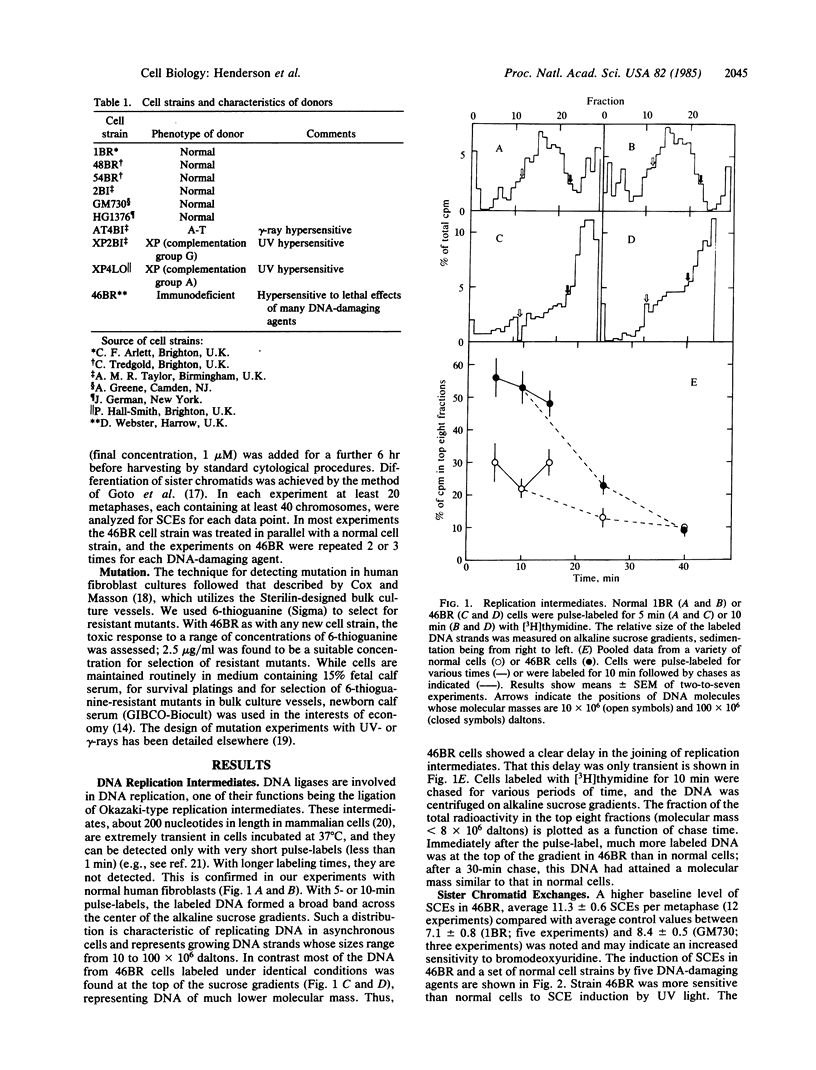
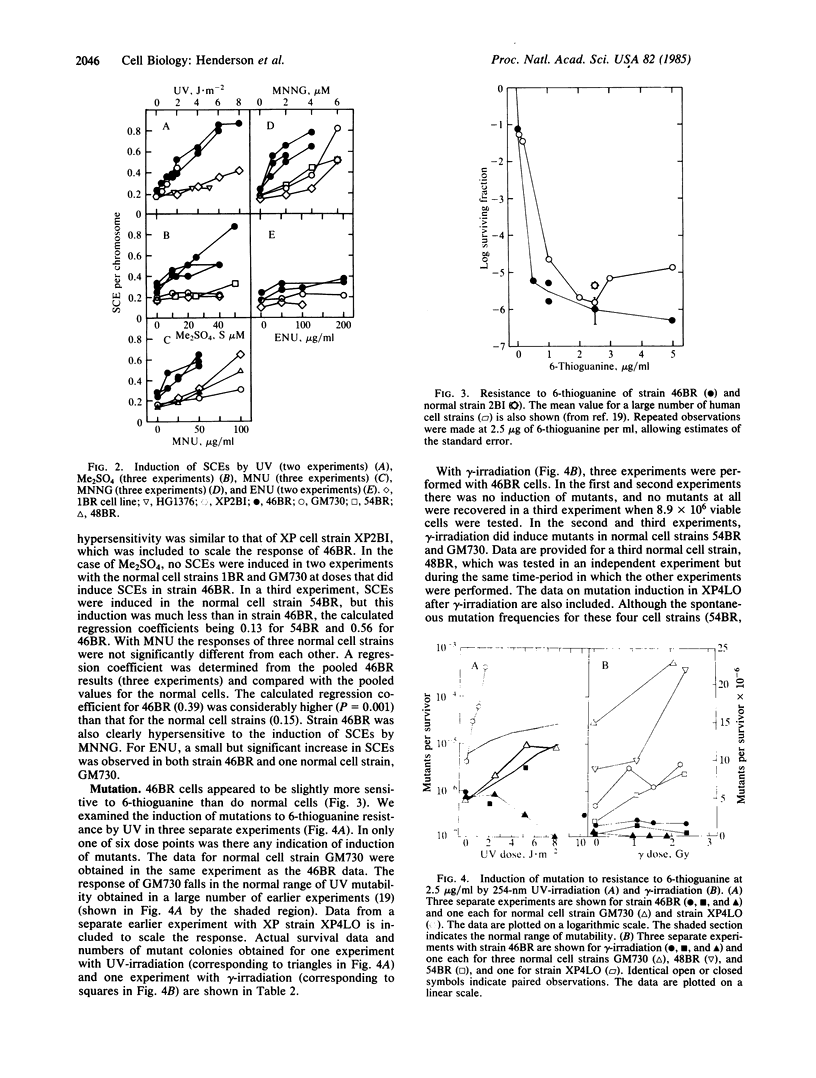
A fibroblast cell strain, 46BR, derived from an immunodeficient patient is hypersensitive to the lethal effects of a wide range of DNA-damaging agents. It is also defective in strand-break rejoining after treatment with dimethyl sulfate and UV light. The present study shows that the cells have a defect in joining Okazaki-type fragments during DNA replication, supporting the interpretation that the basic defect is in ligation of DNA strands. The baseline level of sister chromatid exchange is slightly higher than in normal cells but it does not approach that of Bloom's syndrome or dyskeratosis congenita cells. Sensitivity to the induction of sister chromatid exchange and the hypersensitivity to the lethal effects of a set of DNA-damaging agents are correlated, implying that the basic defect influences both end points in a similar manner. No 6-thioguanine-resistant mutants could be induced by either gamma- or UV-irradiation in these cells, suggesting that error-prone repair pathways for damage induced by these agents may contain a common ligation step in human cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgdorf W., Kurvink K., Cervenka J. Sister chromatid exchange in dyskeratosis congenita lymphocytes. J Med Genet. 1977 Aug;14(4):256–257. doi: 10.1136/jmg.14.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Masson W. K. X-ray-induced mutation to 6-thioguanine resistance in cultured human diploid fibroblasts. Mutat Res. 1976 Oct;37(1):125–136. doi: 10.1016/0027-5107(76)90060-9. [DOI] [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- De Weerd-Kastelein E. A., Keijzer W., Rainaldi G., Bootsma D. Induction of sister chromatid exchanges in xeroderma pigmentosum cells after exposure to ultraviolet light. Mutat Res. 1977 Nov;45(2):253–261. doi: 10.1016/0027-5107(77)90025-2. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Galloway S. M. Ataxia telangiectasia: the effects of chemical mutagens and x-rays on sister chromatid exchanges in blood lymphocytes. Mutat Res. 1977 Dec;45(3):343–349. doi: 10.1016/0027-5107(77)90144-0. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Evans H. J. Sister chromatid exchange in human chromosomes from normal individuals and patients with ataxia telangiectasia. Cytogenet Cell Genet. 1975;15(1):17–29. doi: 10.1159/000130495. [DOI] [PubMed] [Google Scholar]
- Goto K., Maeda S., Kano Y., Sugiyama T. Factors involved in differential Giemsa-staining of sister chromatids. Chromosoma. 1978 May 16;66(4):351–359. doi: 10.1007/BF00328535. [DOI] [PubMed] [Google Scholar]
- Ishii Y., Bender M. A. Effects of inhibitors of DNA synthesis on spontaneous and ultraviolet light-induced sister-chromatid exchanges in Chinese hamster cells. Mutat Res. 1980 Sep;79(1):19–32. doi: 10.1016/0165-1218(80)90144-5. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Stetten G., Juergens L. A., Buchanan G. R., Gerald P. S. Induction by alkylating agents of sister chromatid exchanges and chromatid breaks in Fanconi's anemia. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4066–4070. doi: 10.1073/pnas.72.10.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. R., Arlett C. F., Harcourt S. A., Broughton B. A. Increased sensitivity of cell strains from Cockayne's syndrome to sister-chromatid-exchange induction and cell killing by UV light. Mutat Res. 1980 Jan;69(1):107–112. doi: 10.1016/0027-5107(80)90180-3. [DOI] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C., Slor H., Thomas G., Cleaver J. E. Defective thymine dimer excision by cell-free extracts of xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2757–2761. doi: 10.1073/pnas.73.8.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotná B., Goetz P., Surkova N. I. Effects of alkylating agents on lymphocytes from controls and from patients with Fanconi's anemia. Studies of sister chromatid exchanges, chromosome aberrations, and kinetics of cell division. Hum Genet. 1979 May 23;49(1):41–50. doi: 10.1007/BF00277685. [DOI] [PubMed] [Google Scholar]
- Painter R. B. A replication model for sister-chromatid exchange. Mutat Res. 1980 May;70(3):337–341. doi: 10.1016/0027-5107(80)90023-8. [DOI] [PubMed] [Google Scholar]
- Squires S., Johnson R. T. U.v. induces long-lived DNA breaks in Cockayne's syndrome and cells from an immunodeficient individual (46BR): defects and disturbance in post incision steps of excision repair. Carcinogenesis. 1983;4(5):565–572. doi: 10.1093/carcin/4.5.565. [DOI] [PubMed] [Google Scholar]
- Teo I. A., Arlett C. F., Harcourt S. A., Priestley A., Broughton B. C. Multiple hypersensitivity to mutagens in a cell strain (46BR) derived from a patient with immuno-deficiencies. Mutat Res. 1983 Feb;107(2):371–386. doi: 10.1016/0027-5107(83)90177-x. [DOI] [PubMed] [Google Scholar]
- Teo I. A., Broughton B. C., Day R. S., James M. R., Karran P., Mayne L. V., Lehmann A. R. A biochemical defect in the repair of alkylated DNA in cells from an immunodeficient patient (46BR). Carcinogenesis. 1983;4(5):559–564. doi: 10.1093/carcin/4.5.559. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Brookman K. W., Dillehay L. E., Carrano A. V., Mazrimas J. A., Mooney C. L., Minkler J. L. A CHO-cell strain having hypersensitivity to mutagens, a defect in DNA strand-break repair, and an extraordinary baseline frequency of sister-chromatid exchange. Mutat Res. 1982 Aug;95(2-3):427–440. doi: 10.1016/0027-5107(82)90276-7. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. DNA synthesis in human lymphocyts: intermediates in DNA synthesis, in vitro and in vivo. J Mol Biol. 1975 Dec 5;99(2):317–337. doi: 10.1016/s0022-2836(75)80149-5. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. Sister chromatid exchange. Annu Rev Genet. 1977;11:183–201. doi: 10.1146/annurev.ge.11.120177.001151. [DOI] [PubMed] [Google Scholar]
- Yarosh D. B. UV-induced mutation in bacteriophage T4. J Virol. 1978 May;26(2):265–271. doi: 10.1128/jvi.26.2.265-271.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]




