Abstract
During the third and final larval instar stage, thousands of pluripotent cells within the Drosophila eye imaginal disc are transformed into a near perfect neurocrystalline lattice of 800 unit eyes called ommatidia. This transformation begins with the initiation of the morphogenetic furrow at the posterior margin of the eye field. The furrow, which marks the leading edge of a wave of differentiation, passes across the epithelium transforming unpatterned and undifferentiated cells into rows of periodically spaced clusters of photoreceptor neurons. As cells enter and exit the furrow they undergo dramatic alterations in cellular architecture and gene expression, many of which are required to propel the furrow forward and for proper cell fate specification. The Decapentaplegic (Dpp) and Hedgehog (Hh) signaling pathways are required for the initiation and progression of the furrow, respectively. Consistent with a role in furrow progression, the loss of Hh pathway activity results in a “furrow stop” phenotype. In contrast, reductions in levels of the helix-loop-helix transcription factor, Extramacrochaetae (Emc), lead to the polar opposite phenotype—the furrow accelerates. Recently, we demonstrated that the furrow stop and furrow acceleration phenotypes are molecularly connected. Emc appears to serve as a brake on the furrow by dampening the activity of the Hh pathway. Loss of Emc leads to an upsurge in Hh pathway activity and a faster moving furrow. The acceleration of the furrow appears to be due to an increase in levels of the full-length isoform of Cubitus Interruptus (Ci155) and Suppressor of Fused [Su(fu)]. Here we will briefly review the mechanisms by which Hh drives and Emc impedes the progression of the furrow across the developing retina.
Keywords: extramacrochaetae, Hedgehog, Drosophila, Suppressor of Fused, morphogenetic furrow
Introduction
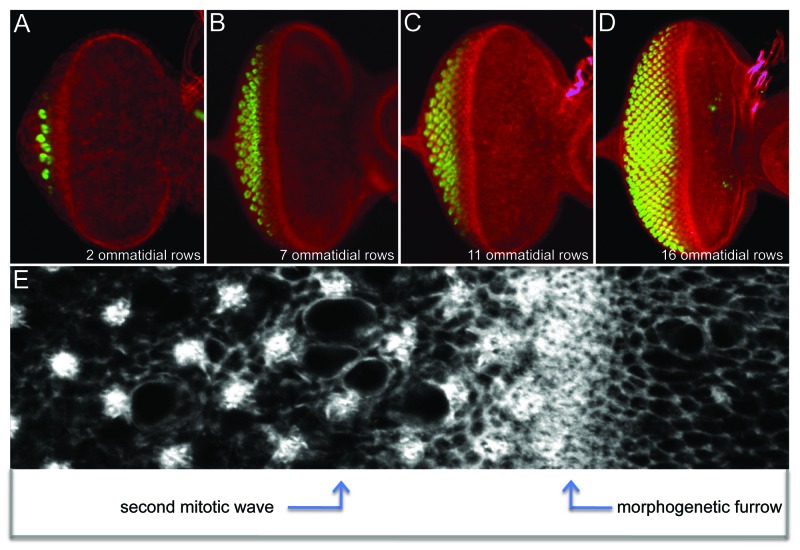
Eye development begins during mid-embryogenesis when 2 small groups of cells delaminate from the surface ectoderm to form a pair of eye-antennal imaginal discs. As development proceeds through the first and second larval instars, cells within each disc rapidly divide but remain unpatterned and undifferentiated. This period of prolonged quiescence comes to an abrupt end at the start of the third and final larval instar when the morphogenetic furrow initiates at the posterior margin and begins its long march across the eye primordium.1 As the furrow advances it transforms the unpatterned eye primordium into a highly ordered array consisting of several dozen columns of unit eyes that are also called ommatidia. This dramatic transformation can be seen in immunohistochemical preparations of developing eye discs (Fig. 1). The furrow is easily identified by an indentation in the epithelium that runs along the dorsal-ventral axis. Cells ahead of the advancing furrow are actively dividing, have yet to choose a final cellular fate, and are not arranged in any distinct pattern. Once cells enter the furrow they are quickly organized into a column of periodically spaced unit eyes. As the furrow moves forward this newly minted column is pushed out of the furrow and added to existing ommatidial columns that lie behind the advancing wave (Fig. 1). In this way, the eye grows by accretion and has been aptly referred to as a neurocrystalline lattice.1 Eventually, the furrow will reach the eye/antennal border shortly after the larva has transitioned to the pupal stage.
Figure 1. Progression of the morphogenetic furrow across the developing eye field of Drosophila. (A–D) Confocal images of third instar eye discs depicting the addition of ommatidial rows as the furrow moves across the eye field. Red = F-actin and gree = Elav. (E) An enlarged view of the area surrounding the morphogenetic furrow. Note that cells in the anterior are unpatterned while cells that lie behind the furrow are organized into periodically spaced photoreceptor clusters. White = F-actin. In all images anterior is to the right.
Hh Signaling Pushes the Furrow Across the Eye Field
In the decade following the discovery of the morphogenetic furrow, 2 competing models for how it moved across the eye field had been offered. The first model proposed that cells within and posterior to the furrow provided an inductive signal(s) that was required to “push” the furrow forward.1 The competing model, however, suggested that cells ahead of the furrow express factors that “pulled” the furrow across the disc.2 The solution to this puzzle came in the mid-1990s when a reexamination of the role that the Hedgehog (Hh) signaling pathway played in the eye took place. It had been known for some time that certain mutations within the hh gene resulted in viable flies whose only visible defect was a small rough eye.3,4 However, a connection between this phenotype and a possible defect in furrow movement was not made until the hh gene was cloned and the biochemical features of the encoded protein were determined.
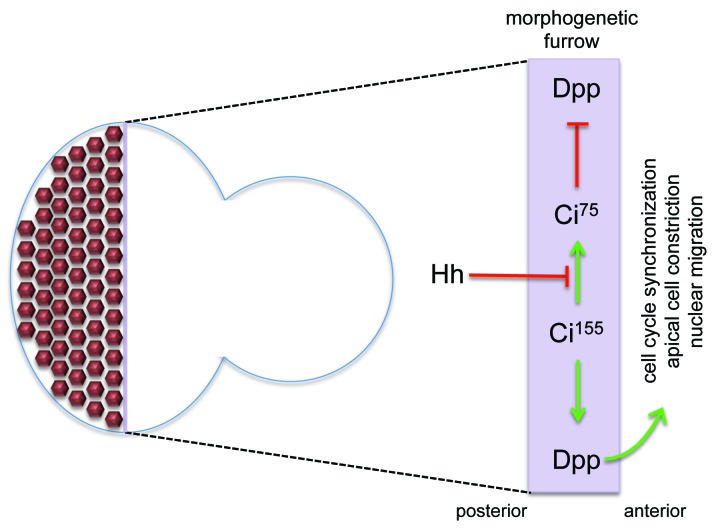
Three key findings relating to Hh signaling demonstrated a role for the pathway in furrow propagation and provided mechanistic support for the “furrow pushing” model. First, results from biochemical and genetic experiments indicated that Hh is a secreted morphogen that functions non-autonomously during development.4-6 Second, while hh is expressed in developing photoreceptors posterior to the furrow, it is non-autonomously required to regulate expression of several genes within the furrow.7-10 One key target is the decapentaplegic (dpp) gene, which itself encodes a long-range diffusible morphogen.11 And third, a temperature sensitive allele of hh was used to show that temporary removal of Hh signaling during eye development caused the furrow to stop.8 The Hh-Dpp relay system allows for cells well behind the furrow to induce cellular and gene expression changes within and ahead of the furrow. In doing so Hh signaling pushes morphogenesis forward (Fig. 2). These results began to provide an explanation for why several hh mutants had small eyes.
Figure 2. The Hh-Dpp relay system pushes the furrow forward across the eye field. Hh signaling emanating from developing photoreceptor neurons blocks the degradation of Ci155 activator into the smaller Ci75 repressor isoform within the furrow. As a consequence Ci155 can translocate to the nucleus and activate transcription of several target genes including dpp. Dpp is a long-range morphogen, which instructs cells in the anterior compartment to prepare for entrance into the morphogenetic furrow. Anterior is to the right.
Regulation of hh Expression in the Eye
The viable hhbar3 allele (also known as hh1) was first identified by its small eye phenotype.3 In this mutant, hh expression in photoreceptor cells is completely eliminated and as a result the furrow lays down only 8–10 rows of ommatidia before coming to a halt.3,4,7,12 Molecular analysis of hhbar3 has provided a wealth of insight into the mechanisms by which hh expression is regulated in the developing eye. The complete loss of hh expression in photoreceptors is caused by a 1.9kb deletion within the first intron.5,13 A smaller ~200bp fragment is sufficient to drive transcription of a reporter within developing photoreceptors.12,14 Bioinformatic analysis of this fragment identified consensus sites for the Pointed (Pnt) and Sine Oculis (So) DNA binding proteins. The former is an Ets transcription factor and a terminal member of the Epidermal Growth Factor Receptor pathway while the latter is a homeobox DNA binding protein that functions within the retinal determination network. Both pnt and so are expressed in developing photoreceptor clusters. Mutations in either gene or deletion of the binding sites for the encoded proteins within the hh eye specific enhancer lead to complete abolition of hh expression.12,14
If hh expression is eliminated from the photoreceptor cells in the hhbar3 mutant then how does the furrow advance for 8–10 rows prior to halting? In addition to photoreceptor neurons, hh is expressed earlier at the posterior margin of the eye disc.9,15 This expression remains in the hhbar3 mutant12 and appears sufficient to support the initiation of the furrow and its progression across 25–33% of the eye field. The conclusion from these and many related studies is that Hh, which is produced and secreted from the photoreceptors, is required non-autonomously to “push” the furrow across the eye primordium.
Hh Signaling in the Eye
Within the embryo and imaginal discs, the Hh ligand is produced and secreted by cells that lie within the posterior compartment. The Hh receptor Patched (Ptc), while being expressed at low levels throughout the entire anterior compartment, is highly enriched within cells that lie at the border of the anterior and posterior compartment boundaries. These cells capture the vast majority of the diffusing Hh ligand and, as a consequence, the Hh pathway is robustly activated. At the bottom of the Hh pathway lies the zinc finger transcription factor Cubitus Interruptus (Ci). The full-length protein (Ci155) functions as a transcriptional activator and transduces the Hh signal. In the absence of Hh signaling it is cleaved into a smaller protein (Ci75) that functions as a transcriptional repressor. While Ci75 is present throughout all imaginal discs at low levels, Ci155 is highly enriched at the A/P axis. As a consequence, the transcription of downstream targets of Ci155 such as ptc and dpp are strikingly elevated along the A/P axis as well. Antibodies that detect Ci155 distinguish cells that have received and are actively transducing the Hh signaling from cells that are refractory to Hh. Once Hh signaling is activated, the long-range Dpp morphogen is transmitted to cells lying within the anterior compartment.
Hh signaling in the eye operates under similar constraints with one interesting exception. In contrast to non-ocular tissues, the eye is initially born without an anterior-posterior (A/P) axis—all cells are initially considered to be part of the anterior compartment and hh expression is absent. The initiation of the furrow at the posterior margin breaks this uniformity. During the late second/early third instar hh expression is activated at the firing point along the posterior margin.9 As the furrow proceeds, hh expression is then activated in developing photoreceptors.8 Based on the expression pattern of hh within the embryo and other imaginal discs, cells that lie behind the furrow (and express hh) are considered to be in the posterior compartment while cells that lie ahead of the furrow (and lack Hh protein) are treated as being anterior. Since the furrow moves across the eye field, the relative ratio of anterior to posterior tissue varies temporally. This contrasts with all other imaginal discs in that they have a stationary A/P boundary and the ratio of anterior to posterior tissue remains constant during development. Despite these differences, the configuration of the Hh-Dpp relay system is identical to other tissues. Hh is produced in the posterior compartment (behind the furrow) and captured by cells at the A/P border (within the furrow). These border cells have high levels of both Ci155 levels and Dpp. And lastly, cells anterior to the furrow receive and transduce the Dpp signal.
Cell Cycle Profile and Cellular Morphology of the Furrow
As cells exit the furrow a subset of cells leave the cell cycle, self-organize into ommatidial clusters and initiate neuronal differentiation. The remaining cells will undergo one final round of mitosis, known as the second mitotic wave (Fig. 1E), before differentiating and joining the ommatidial cluster.1,16-18 While these decisions are occurring on an individual cell-by-cell basis, the timing of their exit or re-entry into the cell cycle is coordinated along the entire length of the posterior face of the furrow. In order for these post-furrow events to be synchronized all cells within the furrow are arrested in the G1 phase of the cell cycle.1 G1 arrest is induced by the downregulation of several cyclin genes (cycA, cycB, and cycE) and concomitant elevation of string, the fly homolog of cdc25.19-22 Hh and Dpp prevent cells within the furrow from progressing to S phase thus maintaining the state of G1 arrest.23-26
Cells entering and residing within the furrow also undergo several morphological changes including constriction of apical profiles and a displacement of their nuclei.1 Apical cell constriction, particularly when coordinated among large populations of cells, creates the appropriate force for tissue invagination.27 It appears that Hh, and to a lesser extent Dpp, signaling is required for apical cell constriction within the furrow.28 Loss of signaling leads to a decrease in F-actin accumulation at the apical surface as well as reductions in the amount of β-catenin (encoded by armadillo), myosin regulatory light chain (encoded by spaghetti squash), and at least one cadherin (encoded by Cad86C).28,29 The Hh induced apical constriction (and accompanying increase in F-actin accumulation) forms part of a negative feedback loop by restricting the distance that the Hh ligand can travel. Cells in the furrow that are mutant for act-up (acu), fail to constrict their apical profiles. As a result, Hh protein is located more anteriorly than in wild type and cells undergo precocious neuronal differentiation.30
Extramacrochaetae Regulates the Furrow
The reception of the Hh signal is tightly regulated such that only the narrow band of cells within the furrow receives and implements the signal. This is essential for preventing precocious neuronal development and for maintaining synchrony over cell cycle exit and re-entry decisions. Over the years, 2 sets of observations have guided our thinking on how Hh signaling is regulated. We have already seen that Hh signaling induces morphological changes within cells of the furrow restricting the distance over which the Hh ligand can travel.28,30 It is thought that this constriction increases the concentration of apical membrane, thus increasing the likelihood that cells within the furrow will capture the vast majority of Hh molecules.30 A second method of restricting Hh signaling appears to involve the helix-loop-helix (HLH) transcription factor, Extramacrochaetae (Emc). Removal of emc (either alone or in combination with a hairy null allele) in the eye disc results in an acceleration of the furrow.31,32 This phenotype is the diametric opposite of the furrow-stop phenotype that characterizes Hh signaling mutants. However, despite the obvious connection between the 2 phenotypes the possibility that Emc directly regulates the Hh pathway had not been directly considered.
To address this issue, we examined Hh signaling in emc null mutant clones that crossed the furrow.33 We first observed that the loss of emc leads to an increase in both the spatial distribution and levels of Ci155. Since the furrow accelerates through emc null clones, we also witnessed predictable changes in the expression of genes that are targets of the Hh pathway (increase in dpp-lacZ, arm, and Cad86C) as well as alterations in cellular architecture (apical constriction, tissue ingression, and nuclear migration). Together, these results suggested that the loss of Emc increases Hh signaling and thus puts cells into a furrow-like state. Is an independent increase in Ci155 sufficient to induce all of the changes that we see in emc null mutants? We used the flp-out method to induce clones that overexpressed Ci155 and indeed see an acceleration of the furrow. Based on these results, we concluded that Emc regulates the pace by which the furrow progresses across the eye disc by achieving an optimal balance between the levels of Ci155 (activator) and Ci75 (repressor). This mechanism does not seem to be under Hh control as emc expression is unaltered in clones that are insensitive to Hh signaling.33
A role for Suppressor of Fused [Su(fu)] in Furrow Progression?
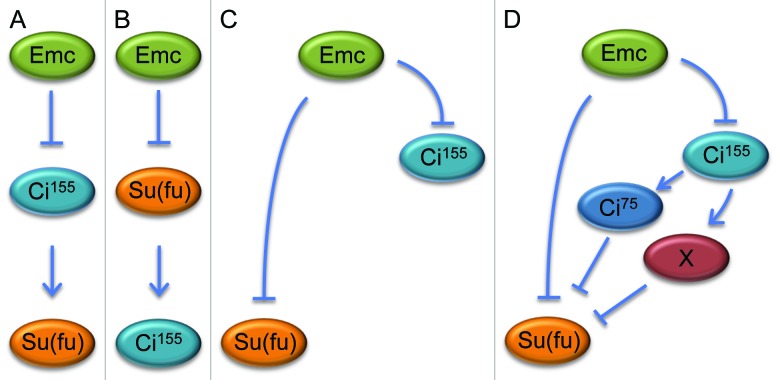
There are 3 broad mechanisms by which Ci155 levels could be elevated in emc mutants. First, the loss of emc could result in a dramatic increase in the transcription of ci. In this scenario the levels of Ci155 would be beyond the capacity of the degradation machinery to convert it to Ci75. We were able to rule out this possibility by demonstrating that a ci-lacZ transcriptional reporter was unaffected in emc mutant tissue. Second, the production of the Hh ligand in developing photoreceptors and/or induction of the pathway (via decreased levels of Ptc or increased levels of Smo) might be significantly elevated. We saw no evidence for this, as levels of Ptc, Smo and hh-lacZ were unchanged in emc clones. Finally, levels of 1 or more members of the Hh signaling complex could be altered such that the conversion of Ci155 into Ci75 is greatly reduced or completely blocked. We examined the expression levels of most Hh signaling components and saw that only Suppressor of Fused [Su(fu)] was altered by the absence of Emc. Consistent with Su(fu) being necessary to prevent degradation of Ci155, we observed that the loss of Su(fu) leads to the near elimination of Ci155. Taken together, our data indicates that there is a connection between Emc, Ci155 and Su(fu) but the exact genetic relationship between the 3 remains unclear. Emc could independently regulate the expression of Ci155 and Su(fu) or the increased level of either factor in emc mutant clones could be dependent upon the expression of the other (Fig. 3A-C).
Figure 3. Models of potential genetic relationships between Emc, Ci155 and Su(fu). In emcAP6 null clones the levels of Su(fu) and Ci155 are elevated (Spratford and Kumar, 2013). In that report and this manuscript we tested several models (panel A–C) to determine the genetic relationship between the 3 factors. Our results indicate that Emc independently represses Ci155 and Su(fu) and based on the data in Figure 4 it is possible that Ci155 contributes to the inhibition of Su(fu) via an unknown intermediate or through its partial degradation into the Ci75 isoform (panel D).
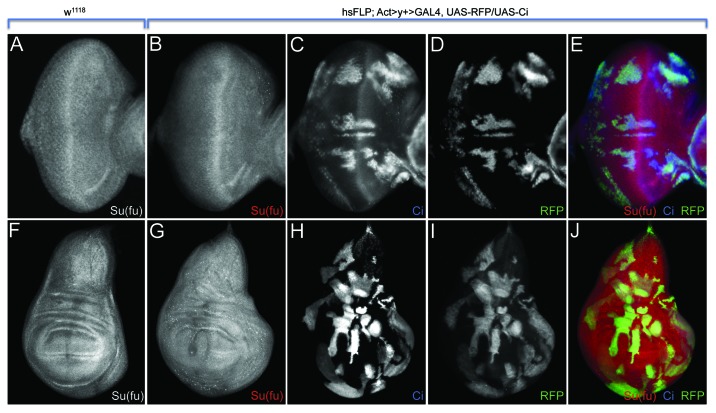
We began to tease apart this regulatory circuit by analyzing the expression levels of Ci155 when Su(fu) was overexpressed. Overexpression of Su(fu) had no effect on Ci155 levels; therefore, it is highly unlikely that the increase in Su(fu) levels in emc mutant clones is sufficient to stabilize Ci155. Consequently, although Su(fu) is necessary for Ci155 stabilization, it is not sufficient. Consistent with this result is our observation that the furrow did not accelerate through clones overexpressing Su(fu). Here we report the results of the converse experiment. We used the flp-out method to generate clones in the eye and wing that overexpress Ci155 and asked whether Su(fu) levels are elevated. Unexpectedly, we observe that Su(fu) levels are actually reduced in response to high Ci155 levels (Fig. 4). We conclude from this and our previous experiment that Emc independently represses Su(fu) and Ci155 levels ahead of the morphogenetic furrow (Fig. 3C and D). What requirement is there for Emc to repress Su(fu) in such a manner? Since many Hh signaling components are transcribed broadly in the developing eye disc, the restriction of Hh pathway activity to the furrow requires inhibition Ci155 stabilization throughout the rest of the eye field. In addition to binding to and stabilizing Ci155, Su(fu) translocate with Ci155 to the nucleus as a complex (Ci155 – Su(fu)).34 The repression of Su(fu) by Emc may be required to ensure that Ci155 is converted to the smaller Ci75 repressor form.
Figure 4. Expression of Ci155 leads to a downregulation of Su(fu) in the developing eye and wing. (A and F) Wild type expression patterns of Su(fu) in third instar eye and wing discs. (B–E) Overexpression of full-length Ci in flp-out clones (hsFLP; Act > y+ > GAL4, UAS-RFP/UAS-Ci: RFP positive cells in D and E) leads to the downregulation of Su(fu) in the developing eye. (G–J) Flp-out clones overexpressing Ci in the wing (same genotypes as in B-E: RFP positive cells in I and J) also show a reduction in Su(fu) expression.
How does Emc regulate Su(fu) transcription and the balance between Ci155 and Ci75 levels? Emc is a member of the bHLH superfamily but it lacks the basic domain.35,36 Thus, unlike other bHLH proteins, Emc is unable to bind DNA.37 It can, however, form heterodimers with other bHLH proteins such as Daughterless (Da) and proteins of the Achaete-Scute Complex (AS-C).35-39 These Emc-bHLH heterodimers also cannot bind DNA;37 therefore, Emc influences transcription by sequestering bHLH proteins away from target DNA sequences. Mutations of the AS-C do not affect the compound eye40,41 and therefore can be ruled out as potential regulators of either Ci155 or Su(fu). Da, on the other hand, may be a good candidate since furrow progression slows as the furrow passes through mutant da clones.42 Consistent with this idea is the initiation of precocious neuronal development in clones overexpressing da.43 It is yet to be determined if the loss or overexpression of da is sufficient to modulate either Ci155 or Su(fu) levels. In addition, other bHLH proteins may participate in the regulation of the Hh signaling and may be sequestered by Emc.
Concluding Remarks
The work described here and in33 indicates that 2 competing forces regulate the pace at which the morphogenetic furrow moves across the eye field. Hh signaling, acting as an irresistible force, pushes the furrow across the eye field. Countering this forward momentum is an immovable object, in the form of Emc, which regulates Hh signaling to maintain the appropriate balance between Ci155 and Ci75 levels ahead of the morphogenetic furrow. Maintaining this balance, in turn, not only allows the furrow to progress across the eye disc but also regulates the velocity that at which the furrow migrates. This appears to involve Su(fu) as well as at least 1 unidentified Hh signaling component. Future studies will be aimed at finding these additional players. Furthermore, as Emc neither binds to DNA by itself or as part of a protein complex, the molecular link between Emc and Hh pathway members remains elusive. There are hints that Da may be involved in furrow progression.43 It will be important to determine if the apparent slowing and acceleration of the furrow through loss and overexpression da clones is due to changes in Ci155 levels and Su(fu). And, finally, for a complete understanding of how Emc regulates Hh signaling, it will be important to identify the entire suite of bHLH proteins that are bound and sequestered by Emc within the developing eye.
Materials and Methods
Fly stocks
(1) w1118; (2) hsFLP22; (3) Act5C > y+ > Gal4, UAS-RFP/TM3; (4) UAS-Ci
Antibodies and microscopy
Primary antibodies used: (1) mouse anti-Su(fu) (1:200, DSHB); (2) rat anti-CiACT (1:50, gift from Robert Holmgren). Fluorophore-conjugated secondary antibodies were obtained from Jackson Laboratories and Molecular Probes. Imaginal discs were prepared as described in Anderson et al., 2012.
Temperature shift regimes
Heat shock clones were induced by a 20 min heat shock at 37þ 48hrs after egg lay.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Robert Holmgren, the Bloomington Drosophila Stock Center and the Developmental Studies Hybridoma Bank for gifts of fly strains and antibodies. C.M.S. is supported by the Frank W. Putnam Research Fellowship, the Robert Briggs Research Fellowship and a stipend from the National Institutes of Health (NIH) GCMS Training Grant (T32 GM007757). J.P.K. is supported by a grant from the National Eye Institute (R01 EY014863).
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/27691
References
- 1.Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–40. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- 2.Lebovitz RM, Ready DF. Ommatidial development in Drosophila eye disc fragments. Dev Biol. 1986;117:663–71. doi: 10.1016/0012-1606(86)90335-0. [DOI] [PubMed] [Google Scholar]
- 3.Ives P. New mutant report: bar-3. Drosoph Inf Serv. 1950;24:58. [Google Scholar]
- 4.Mohler J. Requirements for hedgehog, a segmental polarity gene, in patterning larval and adult cuticle of Drosophila. Genetics. 1988;120:1061–72. doi: 10.1093/genetics/120.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JJ, von Kessler DP, Parks S, Beachy PA. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-D. [DOI] [PubMed] [Google Scholar]
- 6.Tabata T, Kornberg TB. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 7.Heberlein U, Wolff T, Rubin GM. The TGF beta homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–26. doi: 10.1016/0092-8674(93)90535-X. [DOI] [PubMed] [Google Scholar]
- 8.Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–38. doi: 10.1016/0092-8674(93)90536-Y. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez M, Hafen E. Hedgehog directly controls initiation and propagation of retinal differentiation in the Drosophila eye. Genes Dev. 1997;11:3254–64. doi: 10.1101/gad.11.23.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenwood S, Struhl G. Progression of the morphogenetic furrow in the Drosophila eye: the roles of Hedgehog, Decapentaplegic and the Raf pathway. Development. 1999;126:5795–808. doi: 10.1242/dev.126.24.5795. [DOI] [PubMed] [Google Scholar]
- 11.Padgett RW, St Johnston RD, Gelbart WM. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987;325:81–4. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- 12.Rogers EM, Brennan CA, Mortimer NT, Cook S, Morris AR, Moses K. Pointed regulates an eye-specific transcriptional enhancer in the Drosophila hedgehog gene, which is required for the movement of the morphogenetic furrow. Development. 2005;132:4833–43. doi: 10.1242/dev.02061. [DOI] [PubMed] [Google Scholar]
- 13.Ma C, Liu H, Zhou Y, Moses K. Identification and characterization of autosomal genes that interact with glass in the developing Drosophila eye. Genetics. 1996;142:1199–213. doi: 10.1093/genetics/142.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauli T, Seimiya M, Blanco J, Gehring WJ. Identification of functional sine oculis motifs in the autoregulatory element of its own gene, in the eyeless enhancer and in the signalling gene hedgehog. Development. 2005;132:2771–82. doi: 10.1242/dev.01841. [DOI] [PubMed] [Google Scholar]
- 15.Borod ER, Heberlein U. Mutual regulation of decapentaplegic and hedgehog during the initiation of differentiation in the Drosophila retina. Dev Biol. 1998;197:187–97. doi: 10.1006/dbio.1998.8888. [DOI] [PubMed] [Google Scholar]
- 16.Tomlinson A, Ready DF. Neuronal differentiation in Drosophila ommatidium. Dev Biol. 1987;120:366–76. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson A, Ready DF. Cell fate in the Drosophila ommatidium. Dev Biol. 1987;123:264–75. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- 18.Wolff T, Ready DF. The beginning of pattern formation in the Drosophila compound eye: the morphogenetic furrow and the second mitotic wave. Development. 1991;113:841–50. doi: 10.1242/dev.113.3.841. [DOI] [PubMed] [Google Scholar]
- 19.Thomas BJ, Gunning DA, Cho J, Zipursky L. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994;77:1003–14. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- 20.Thomas BJ, Zavitz KH, Dong X, Lane ME, Weigmann K, Finley RL, Jr., Brent R, Lehner CF, Zipursky SL. roughex down-regulates G2 cyclins in G1. Genes Dev. 1997;11:1289–98. doi: 10.1101/gad.11.10.1289. [DOI] [PubMed] [Google Scholar]
- 21.Richardson H, O’Keefe LV, Marty T, Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995;121:3371–9. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- 22.Dong X, Zavitz KH, Thomas BJ, Lin M, Campbell S, Zipursky SL. Control of G1 in the developing Drosophila eye: rca1 regulates Cyclin A. Genes Dev. 1997;11:94–105. doi: 10.1101/gad.11.1.94. [DOI] [PubMed] [Google Scholar]
- 23.Firth LC, Baker NE. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell. 2005;8:541–51. doi: 10.1016/j.devcel.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Firth LC, Bhattacharya A, Baker NE. Cell cycle arrest by a gradient of Dpp signaling during Drosophila eye development. BMC Dev Biol. 2010;10:28. doi: 10.1186/1471-213X-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horsfield J, Penton A, Secombe J, Hoffman FM, Richardson H. decapentaplegic is required for arrest in G1 phase during Drosophila eye development. Development. 1998;125:5069–78. doi: 10.1242/dev.125.24.5069. [DOI] [PubMed] [Google Scholar]
- 26.Penton A, Selleck SB, Hoffmann FM. Regulation of cell cycle synchronization by decapentaplegic during Drosophila eye development. Science. 1997;275:203–6. doi: 10.1126/science.275.5297.203. [DOI] [PubMed] [Google Scholar]
- 27.Kimberly EL, Hardin J. Bottle cells are required for the initiation of primary invagination in the sea urchin embryo. Dev Biol. 1998;204:235–50. doi: 10.1006/dbio.1998.9075. [DOI] [PubMed] [Google Scholar]
- 28.Corrigall D, Walther RF, Rodriguez L, Fichelson P, Pichaud F. Hedgehog signaling is a principal inducer of Myosin-II-driven cell ingression in Drosophila epithelia. Dev Cell. 2007;13:730–42. doi: 10.1016/j.devcel.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Schlichting K, Dahmann C. Hedgehog and Dpp signaling induce cadherin Cad86C expression in the morphogenetic furrow during Drosophila eye development. Mech Dev. 2008;125:712–28. doi: 10.1016/j.mod.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Benlali A, Draskovic I, Hazelett DJ, Treisman JE. act up controls actin polymerization to alter cell shape and restrict Hedgehog signaling in the Drosophila eye disc. Cell. 2000;101:271–81. doi: 10.1016/S0092-8674(00)80837-5. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya A, Baker NE. The HLH protein Extramacrochaetae is required for R7 cell and cone cell fates in the Drosophila eye. Dev Biol. 2009;327:288–300. doi: 10.1016/j.ydbio.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown NL, Sattler CA, Paddock SW, Carroll SB. Hairy and emc negatively regulate morphogenetic furrow progression in the Drosophila eye. Cell. 1995;80:879–87. doi: 10.1016/0092-8674(95)90291-0. [DOI] [PubMed] [Google Scholar]
- 33.Spratford CM, Kumar JP. Extramacrochaetae imposes order on the Drosophila eye by refining the activity of the Hedgehog signaling gradient. Development. 2013;140:1994–2004. doi: 10.1242/dev.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sisson BE, Ziegenhorn SL, Holmgren RA. Regulation of Ci and Su(fu) nuclear import in Drosophila. Dev Biol. 2006;294:258–70. doi: 10.1016/j.ydbio.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 35.Ellis HM, Spann DR, Posakony JW. extramacrochaetae, a negative regulator of sensory organ development in Drosophila, defines a new class of helix-loop-helix proteins. Cell. 1990;61:27–38. doi: 10.1016/0092-8674(90)90212-W. [DOI] [PubMed] [Google Scholar]
- 36.Garrell J, Modolell J. The Drosophila extramacrochaetae locus, an antagonist of proneural genes that, like these genes, encodes a helix-loop-helix protein. Cell. 1990;61:39–48. doi: 10.1016/0092-8674(90)90213-X. [DOI] [PubMed] [Google Scholar]
- 37.Van Doren M, Ellis HM, Posakony JW. The Drosophila extramacrochaetae protein antagonizes sequence-specific DNA binding by daughterless/achaete-scute protein complexes. Development. 1991;113:245–55. doi: 10.1242/dev.113.1.245. [DOI] [PubMed] [Google Scholar]
- 38.Alifragis P, Poortinga G, Parkhurst SM, Delidakis C. A network of interacting transcriptional regulators involved in Drosophila neural fate specification revealed by the yeast two-hybrid system. Proc Natl Acad Sci U S A. 1997;94:13099–104. doi: 10.1073/pnas.94.24.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Doren M, Powell PA, Pasternak D, Singson A, Posakony JW. Spatial regulation of proneural gene activity: auto- and cross-activation of achaete is antagonized by extramacrochaetae. Genes Dev. 1992;6(12B):2592–605. doi: 10.1101/gad.6.12b.2592. [DOI] [PubMed] [Google Scholar]
- 40.Jiménez F, Campos-Ortega JA. Genes in subdivision 1B of the Drosophila melanogaster X-chromosome and their influence on neural development. J Neurogenet. 1987;4:179–200. [PubMed] [Google Scholar]
- 41.Dambly-Chaudiere C, Ghysen A. Independent subpatterns of sense organs require independent genes of the achaete-scute complex in the Drosophila larva. Genes Dev. 1987;1:1297–306. doi: 10.1101/gad.1.3.297. [DOI] [Google Scholar]
- 42.Brown NL, Paddock SW, Sattler CA, Cronmiller C, Thomas BJ, Carroll SB. daughterless is required for Drosophila photoreceptor cell determination, eye morphogenesis, and cell cycle progression. Dev Biol. 1996;179:65–78. doi: 10.1006/dbio.1996.0241. [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharya A, Baker NE. A network of broadly expressed HLH genes regulates tissue-specific cell fates. Cell. 2011;147:881–92. doi: 10.1016/j.cell.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]