Abstract
Here we report the first example of a hydrogelator made of the conjugate of nucleobase-saccharide-amino acids by incorporating L-3-(2-naphthyl)-alanine to the conjugate, which illustrates a facile and effective method for generating bioactive and functional hydrogelators from the basic biological building blocks.
Supramolecular hydrogels of small molecules, as an emerging, new type of biomaterials, have exhibited a wide range of potentials in biomedicine. For example, hydrogels formed by the self-assembly of oligopeptides1 and derivatives of small peptides2 have shown promises in tissue engineering,3 drug delivery,4 cancer therapy,5 protein separation,6 binding RNA7 or proteins,8 and wound healing.9 These advances and other developments of gelators made of small molecules10 have encouraged us to explore supramolecular hydrogelators constructed from the basic biological building blocks (i.e., nucleobase, saccharide, and amino acid). We recently have demonstrated that the covalent conjugates of nucleobase and amino acids11, 12 or the conjugates of nucleobase, amino acids, and saccharide13 not only are able to self-assemble in water to form hydrogels, but also are cell-compatible.11, 13, 14 Moreover, a conjugate of nucleobase, amino acids, and saccharide is able to assist single strand nucleic acids into cells.13 These successful results indicate that it is feasible to generate bioactive and functional hydrogelators from the pool of the basic building blocks used by nature.
Encouraged by the hydrogelation of the conjugates of nucleobase, amino acids, and saccharide (detonated as NAS type in this article), we mutate the sequence of the building blocks to make the conjugate of nucleobase, saccharide, and amino acids (detonated as NSA type) to expand the diversity of the hydrogelators. However, the covalent link of a nucleoside derivative to a dipeptide fails to produce a hydrogelator. Thus, we decide to introduce a naphthalene containing unnatural amino acid, 3-(2-naphthyl)-alanine (Nal), into the amino acid segment via solid phase peptide synthesis (SPPS) because Nal provides enhanced aromatic-aromatic interactions for molecular self-assembly in water.15 In addition, Nal already finds applications in the preparation of bioactive molecules. For example, Nal has served as a residue for making antagonists (e.g., SB-75) of luteinizing hormone-releasing hormone (LH-RH),16 inhibitors of protease,17 polyvalent inhibitors of influenza-mediated hemagglutination,18 and enantioselective catalysts.19 In fact, Nal is also a residue to enable lanreotide to form autogels.20 Despite these applications, it has yet to be used in the conjugates of nucleobase, saccharide, and amino acids.
We find that the introduction of L-NaI to a conjugate of NSA type enables the conjugate (1) to become a supramolecular hydrogelator (2) (Scheme 1). The nucleobase on the hydrogelator preserves its ability to interact with single strand nucleic acids (e.g., sDNA). The incorporation of D-Nal to a D-amino acid conjugate (3) yields a new conjugate, 4, which is unable to act as a hydrogelator. According to in vitro cell viability tests, these NSA conjugates are cell compatible. These results, as the first demonstration of converting a conjugate of nucleobase, saccharide, and amino acids into a hydrogelator for forming supramolecular nanofibers, illustrates a facile and effective method for generating diverse functional biomaterials from the basic biological building blocks.
Scheme 1.
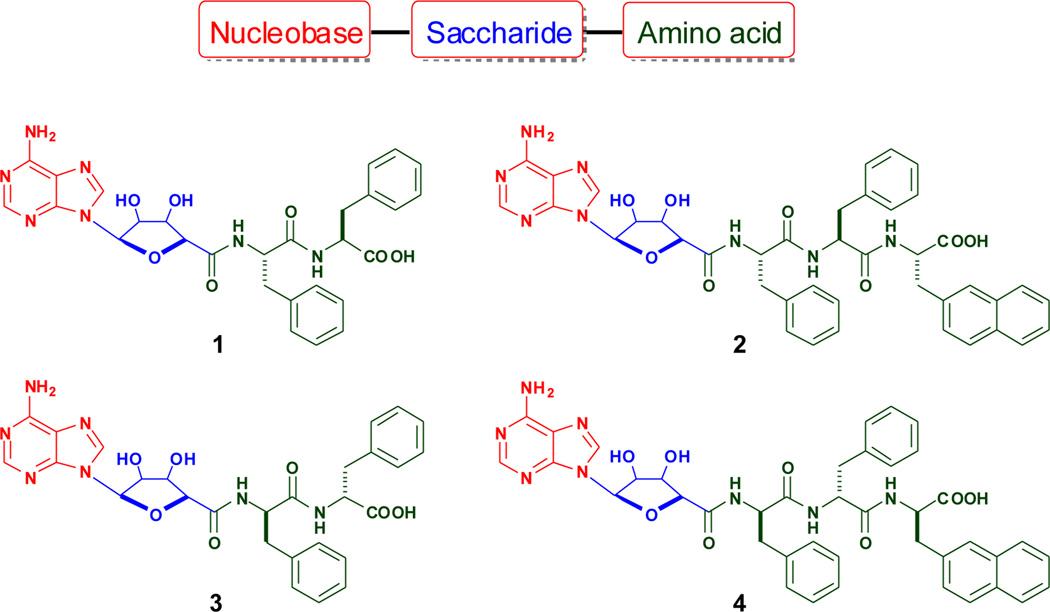
(A) The representation of the conjugate of nucleobase, saccharide, and amino acids, and the structures of the molecules explored in this work.
Scheme 1 shows four conjugates of NSA type investigated in this work. Both 1 and 3 consist of nucleobase (adenine), saccharide (ribofuranose) and amino acids (L-Phe-L-Phe for 1 and D-Phe-D-Phe for 2). The addition of an L-Nal at the C-terminal of 1 gives 2, and a D-Nal onto 3 affords 4. Since a nucleoside consists of both nucleobase and ribofuranose, the covalent linkage of a nucleoside to a small peptide provides a facile synthetic route for making these NSA type conjugates. We choose adenosine as an example to build the conjugates because an excellent procedure to convert adenosine the o-diol-protected adenosine acid derivative 5 (Scheme S1),21 which makes it compatible with SPPS.22 We also use D-amino acids for the construction of the conjugates (e.g., 3 and 4) for increasing their proteolytic resistance.23 The structural difference between 1 and 3 (or 2 and 4) also provides insights on the effect of stereochemistry on self-assembly of the conjugates. Scheme S1 shows the typical synthetic routes for making the conjugates. The synthesis of 1 or 2 starts with the link of N-Fmoc-protected amino acids to 2-chlorotrityl resin. After the construction of the peptide chains (e.g., L-Phe-L-Phe (for 1) and L-Phe-L-Phe-L-Nal (for 2) from the C-terminal towards the N-terminal through a step-by-step peptide chain elongation procedure, the direct coupling of the o-diol-protected adenosine acid derivative 5 to the peptide chains on the beads allows the formation of the protected conjugates. The use of TFA cleaves the conjugates from the resin and deprotects the o-diol-protected group from the ribofuranose. Using D-amino acids and following the same SPPS protocols, we obtain 3 and 4.
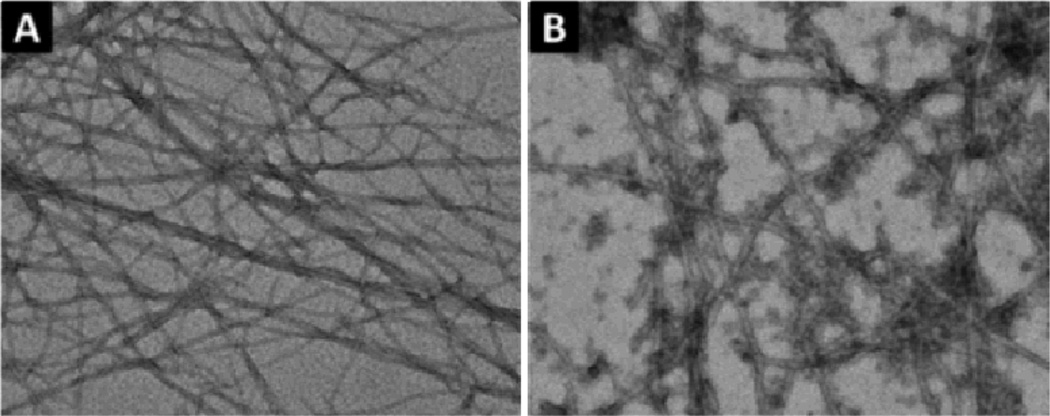
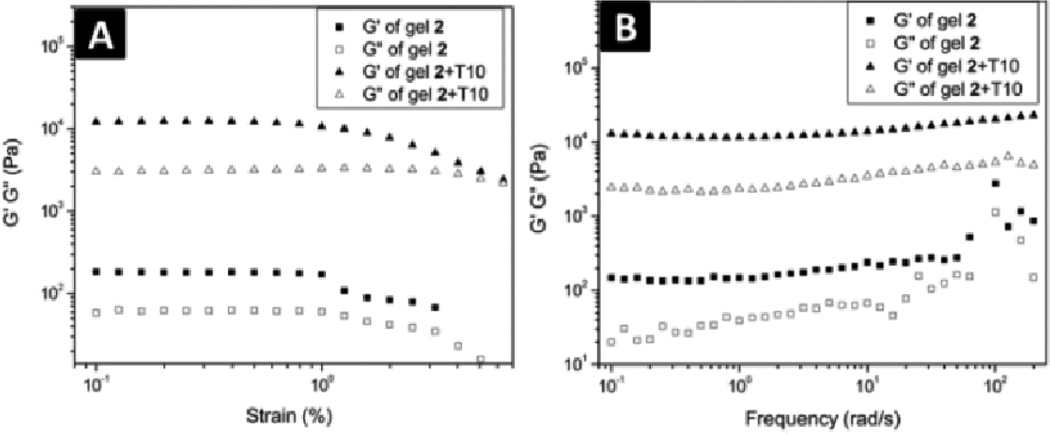
After the synthesis of the NSA conjugates, we tested their gelation properties. 1 affords the transparent solution (Fig. 1A) at pH 7.0 and concentration 1.0 wt %. The change of the pH value of the solution fails to lead a hydrogel, except the precipitation occurring at pH 4–5. The increase of the concentration of 1 up to 3.0 wt % is still unable to result in hydrogelation. The transmission electron microscopy (TEM)24 image of the solution of 1 (1.0 wt %) hardly shows any ordered nanostructures except few of aggregates (Fig. S1A, ESI). Unlike 1, 2 is able to form a transparent hydrogel at pH 7.0 and 1.0 wt %, (Fig. 1B). TEM reveals that the long and flexible nanofibers (7±2 nm in diameter, Fig. 2A) as the matrices of hydrogel of 2. Dynamic strain sweep of rheometry shows that the hydrogel of 2 has the critical strain value of 0.86%, indicating a relative weak network in the hydrogel. The hydrogel exhibits the maximum dynamic storage moduli (G’) to be 181 Pa, which is almost ten times higher than its dynamic loss moduli G”, suggesting that the material behaves as solid-like. (Fig. 3A) In addition, the G’ and G” values of the hydrogel of 2 are almost independent with the frequency from 0.1 to 50 rad/s (Fig. 3B). These results confirm that 2 forms a typical hydrogel (with mgc of 0.8 wt% at pH 7.0), which turns into a solution at 59 °C or at pH above 9.0. To evaluate the ability of the nucleobase on 2 to interact with nucleic acids after the self-assembly of 2, we added a single-strand deoxyribonucleic acid (T10, Scheme S2) to the hydrogel of 2 and examined the morphological and rheological changes of the hydrogel. The addition of T10 appears to result in a mechanically stronger hydrogel (Fig. 1C), which is confirmed by the subsequent TEM and rheological measurements. TEM image of the hydrogel of 2+T10 shows that the width of the nanofibers increases to 17±2 nm (Fig. 2B), confirming that the addition of T10 is responsible for the morphology change of the matrices of the hydrogel. Another difference between the TEM images of 2 and 2+T10 is that there are non-fibrillar aggregates in the TEM images of 2+T10, which might be T10 since most of the aggregates attach or twine on the nanofibers. TEM reveals that T10 alone is unable to form any fibers (Fig. S1D). The rheological test (Fig. 3A, B) shows that the addition of T10 increases the maximum dynamic storage moduli of the hydrogel for about two orders of magnitudes (i.e., from 181 Pa of the hydrogel of 2 to 12317 Pa of the hydrogel 2+T10). The frequency sweep of the hydrogel of 2+T10 (Fig. 3B) also shows frequency-independent G’ values, confirming the solid-like behaviour of the hydrogel of 2+T10. Interestingly, TEM reveals that the addition of 2 (not the hydrogel of 2) to the solution of T10 results in nanofibers with the diameter of 8±2 nm (Fig. S2).
Fig. 1.

Optical images of solution of (A) 1, hydrogels of (B) 2 and (C) 2 + T10 (2 : T10 = 10:1), and solutions of (D) 3 and (E) 4. All at pH 7.0 and 1.0 wt %.
Fig. 2.
Transmission electron micrographs (TEM) of the hydrogels of (A) 2 (pH 7.0, 1.0 wt %), (B) 2+T10 (pH 7.0, 1.0 wt %). Scale bar is 100 nm
Fig. 3.
(A) Strain dependence of the storage moduli (G’) and the loss moduli (G”) of the hydrogels of 2 and 2 + T10; (B) Frequency dependence of the dynamic storage moduli (G’) and the loss moduli (G”) of the hydrogels of 2 and 2 + T10.
Like 1, 3 dissolves in water to form a transparent solution (Fig. 1D) at the same condition (pH 7.0 and 1.0 wt %). TEM image of the solution of 3 shows little of aggregates (Fig. S1B, ESI). As a diastereomer of 2, 4 is unable to form a hydrogel. At 1.0 wt % and pH 7, 4 affords a solution (Fig. 1E), though the TEM image of the solution of 4 reveals non-fibrillar aggregates in the solution (Fig. S1C, ESI). The increase of the concentration of 4 or change the pH value of the solution of 4 yields no hydrogel. Since 2 and 4 exhibit similar solubility in water, the difference between 2 and 4 in gelation likely originates their different stereochemistry. In addition, the addition of T10 in the solution of 1, 3, or 4 also fails to induce hydrogelation.
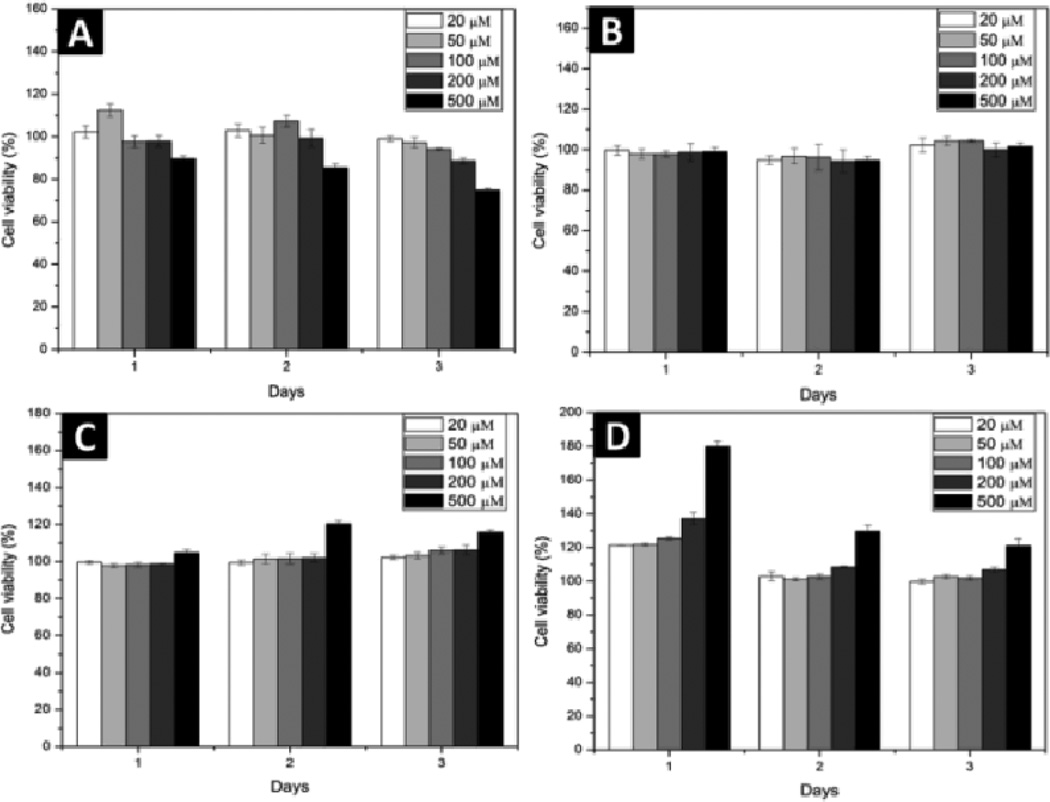
We also examined the cell compatibility of these four conjugates towards mammalian cells. After the conjugates being incubated with HeLa cells for three days at 37 °C, cell proliferation assay indicates all the conjugates are cell compatible (Fig. 4). For example, there are almost 80% cells alive after incubated with 500 µM of 1 for three days. Similarly, the cell viability remains at 100% after incubated with 2 at the concentrations up to 500 µM, confirming excellent cell compatibility of 2. Both 3 and 4 exhibit cell compatibility. Although the MTT assay shows increased absorption when cells are incubated with 4 at 500 µM, the cell counting excludes enhanced proliferation of HeLa cells. These results suggest that the replacement of L-amino acid by D-amino acid residues in 1 or 2 affects little on the cell compatibility of the NSA type conjugates.
Fig. 4.
Cell viability of HeLa cells incubated with (A) 1 (B) 2 (C) 3 (D) 4 for 72 hours.
In conclusion, we have demonstrated a new type of hydrogelator from the conjugate of nucleobase, saccharide, and amino acids. The use of 3-(2-Naphthyl)-alanine and derivatives of nucleoside for SPPS illustrates a facile route to introduce nucleobase to peptides for making biofunctional soft materials. These results greatly expand the pool of building blocks for generating functional hydrogelators and exploring supramolecular chemistry25 in cellular environment or other settings.26
Supplementary Material
Acknowledgments
This work was partially supported by NIH (R01CA142746).
Footnotes
Electronic Supplementary Information (ESI) available: [Synthesis and characterizations, supplementary figures and schemes, 1H NMR of all the conjugates]. See DOI: 10.1039/c000000x/
Notes and references
- 1.Matson JB, Stupp SI. Chem. Commun. 2012;48:26. doi: 10.1039/c1cc15551b. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rajagopal K, Schneider JP. Curr. Opin. Struct. Biol. 2004;14:480. doi: 10.1016/j.sbi.2004.06.006. [DOI] [PubMed] [Google Scholar]; Cui HG, Pashuck ET, Velichko YS, Weigand SJ, Cheetham AG, Newcomb CJ, Stupp SI. Science. 2010;327:555. doi: 10.1126/science.1182340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ulijn RV, Smith AM. Chem. Soc. Rev. 2008;37:664. doi: 10.1039/b609047h. [DOI] [PubMed] [Google Scholar]; Raeburn J, Cardoso AZ, Adams DJ. Chem. Soc. Rev. 2013;42:5143. doi: 10.1039/c3cs60030k. [DOI] [PubMed] [Google Scholar]; Yang Z, Liang G, Xu B. Acc. Chem. Res. 2008;41:315. doi: 10.1021/ar7001914. [DOI] [PubMed] [Google Scholar]
- 3.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]; Zhou M, Smith AM, Das AK, Hodson NW, Collins RF, Ulijn RV, Gough JE. Biomaterials. 2009;30:2523. doi: 10.1016/j.biomaterials.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Li JY, Kuang Y, Gao Y, Du XW, Shi JF, Xu B. J. Am. Chem. Soc. 2013;135:542. doi: 10.1021/ja310019x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Li JY, Li XM, Kuang Y, Gao Y, Du XW, Shi JF, Xu B. Adv. Healthcare Mater. 2013 [Google Scholar]
- 5.Li JY, Gao Y, Kuang Y, Shi JF, Du XW, Zhou J, Wang HM, Yang ZM, Xu B. J. Am. Chem. Soc. 2013;135:9907. doi: 10.1021/ja404215g. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gao Y, Kuang Y, Guo ZF, Guo ZH, Krauss IJ, Xu B. J. Am. Chem. Soc. 2009;131:13576. doi: 10.1021/ja904411z. [DOI] [PubMed] [Google Scholar]; Lin R, Cheetham AG, Zhang PC, Lin YA, Cui HG. Chem. Commun. 2013;49:4968. doi: 10.1039/c3cc41896k. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cheetham AG, Zhang PC, Lin YA, Lock LL, Cui HG. J. Am. Chem. Soc. 2013;135:2907. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamichi S, Jinno Y, Haraya N, Oyoshi T, Tomitori H, Kashiwagi K, Yamanaka M. Chem. Commun. 2011;47:10344. doi: 10.1039/c1cc13826j. [DOI] [PubMed] [Google Scholar]
- 7.Yang ZM, Kuang Y, Li XM, Zhou N, Zhang Y, Xu B. Chem. Commun. 2012;48:9257. doi: 10.1039/c2cc34935c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuang Y, Yuan D, Zhang Y, Kao A, Du XW, Xu B. RSC Advances. 2013;3:7704. doi: 10.1039/C3RA41523F. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gao Y, C. Long MJ, Shi JF, Hedstrom L, Xu B. Chem. Commun. 2012;48:8404. doi: 10.1039/c2cc33631f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang ZM, Liang GL, Ma ML, Abbah AS, Lu WW, Xu B. Chem. Commun. 2007:843. doi: 10.1039/b616563j. [DOI] [PubMed] [Google Scholar]; Gao J, Zheng WT, Zhang JM, Guan D, Yang ZM, Kong DL, Zhao Q. Chem. Commun. 2013;49:9173. doi: 10.1039/c3cc45666h. [DOI] [PubMed] [Google Scholar]
- 10.Sangeetha NM, Maitra U. Chem. Soc. Rev. 2005;34:821. doi: 10.1039/b417081b. [DOI] [PubMed] [Google Scholar]; Terech P, Weiss RG. Chem. Rev. 1997;97:3133. doi: 10.1021/cr9700282. [DOI] [PubMed] [Google Scholar]; Kumar DK, Jose DA, Das A, Dastidar P. Chem. Commun. 2005:4059. doi: 10.1039/b506941f. [DOI] [PubMed] [Google Scholar]; Becker T, Goh CY, Jones F, McIldowie MJ, Mocerino M, Ogden MI. Chem. Commun. 2008:3900. doi: 10.1039/b807248e. [DOI] [PubMed] [Google Scholar]; Pal A, Shrivastava S, Dey J. Chem. Commun. 2009:6997. doi: 10.1039/b914665b. [DOI] [PubMed] [Google Scholar]; Qiu ZJ, Yu HT, Li JB, Wang Y, Zhang Y. Chem. Commun. 2009:3342. doi: 10.1039/b822840j. [DOI] [PubMed] [Google Scholar]; Li XM, Gao Y, Kuang Y, Xu B. Chem. Commun. 2010;46:5364. doi: 10.1039/c0cc00163e. [DOI] [PubMed] [Google Scholar]; Kuang Y, Gao Y, Shi JF, Lin HC, Xu B. Chem. Commun. 2011;47:8772. doi: 10.1039/c1cc13115j. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mecca TL, Messina GM, Marletta G, Cunsolo F. Chem. Commun. 2013;49:2530. doi: 10.1039/c3cc40374b. [DOI] [PubMed] [Google Scholar]; Pan SF, Luo S, Li S, Lai YS, Geng YY, He B, Gu ZW. Chem. Commun. 2013;49:8045. doi: 10.1039/c3cc44767g. [DOI] [PubMed] [Google Scholar]
- 11.Li XM, Kuang Y, Lin HC, Gao Y, Shi JF, Xu B. Angew. Chem.-Int. Edit. 2011;50:9365. doi: 10.1002/anie.201103641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Llansola F, Escuder B, Miravet JF, Hermida-Merino D, Hamley IW, Cardin CJ, Hayes W. Chem. Commun. 2010;46:7960. doi: 10.1039/c0cc02338h. [DOI] [PubMed] [Google Scholar]
- 13.Li XM, Kuang Y, Shi JF, Gao Y, Lin HC, Xu B. J. Am. Chem. Soc. 2011;133:17513. doi: 10.1021/ja208456k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XM, Du XW, Gao Y, Shi JF, Kuang Y, Xu B. Soft Matter, 2012;8:7402. doi: 10.1039/C2SM25725D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma M, Kuang Y, Gao Y, Zhang Y, Gao P, Xu B. J. Am. Chem. Soc. 2010;132:2719. doi: 10.1021/ja9088764. [DOI] [PubMed] [Google Scholar]; Mahler A, Reches M, Rechter M, Cohen S, Gazit E. Adv. Mater. 2006;18:1365. [Google Scholar]; Banerjee A, Palui G, Banerjee A. Soft Matter, 2008;4:1430. doi: 10.1039/b802205b. [DOI] [PubMed] [Google Scholar]
- 16.Korkut E, Bokser L, Comaruschally AM, Groot K, Schally AV. Proc. Natl. Acad. Sci. U. S. A. 1991;88:844. doi: 10.1073/pnas.88.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowe PD, Walter BN, Mohler KM, Ottenevans C, Black RA, Ware CF. J. Exp. Med. 1995;181:1205. doi: 10.1084/jem.181.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SK, Mammen M, Whitesides GM. J. Am. Chem. Soc. 1997;119:4103. [Google Scholar]
- 19.Sakakura A, Hori M, Fushimi M, Ishihara K. J. Am. Chem. Soc. 2010;132:15550. doi: 10.1021/ja1081603. [DOI] [PubMed] [Google Scholar]
- 20.Caron P, Beckers A, Cullen DR, Goth MI, Gutt B, Laurberg P, Pico AM, Valimaki M, Zgliczynski W. J. Clin. Endocrinol. Metab. 2002;87:99. doi: 10.1210/jcem.87.1.8153. [DOI] [PubMed] [Google Scholar]
- 21.Debnath J, Dasgupta S, Pathak T. Bioorg. Med. Chem. 2010;18:8257. doi: 10.1016/j.bmc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 22.White WCCaPD. Fmoc Solid Phase Peptide Synthesis: A Practical Approach. New York: Oxford University Press Inc.; 2000. [Google Scholar]
- 23.Li X, Du X, Li J, Gao Y, Pan Y, Shi J, Zhou N, Xu B. Langmuir, 2012;28:13512. doi: 10.1021/la302583a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frado LL, Craig R. J. Mol. Biol. 1992;223:391. doi: 10.1016/0022-2836(92)90659-8. [DOI] [PubMed] [Google Scholar]
- 25.Lehn J-M. Angew. Chem.-Int. Edit. 2013;52:2836. doi: 10.1002/anie.201208397. [DOI] [PubMed] [Google Scholar]
- 26.Steed Jonathan W. Chem. Commun. 2011;47:1379. doi: 10.1039/c0cc03293j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.