Abstract
Purpose
The present investigation was designed to study the modulation of abdomen and rib cage movements during vocalization over a period of development associated with rapid decreases in the compliance of the chest wall.
Method
Rib cage and abdominal kinematics were recorded during spontaneous vocalizations in 7- and 11-month old infants. Principal component analysis was used to represent each infant’s abdomen and rib cage traces as the weighted sum of a small number of principal component (PC) waveforms.
Results
The fundamental periods of infants’ PC waveforms in the 11-month group were significantly shorter than those in the 7-month group. In addition, the variance contributed by PCs describing unidirectional patterns of respiratory movement decreased in the 11-month group, whereas the variances contributed by PCs describing modulated patterns of movement increased. Lastly, the extent to which abdomen and rib cage movements predicted the duration of corresponding vocalizations also increased significantly in the 11-month group compared with the 7-month group.
Conclusions
The findings of the present study were consistent with the hypothesis that decreases in the compliance of the chest wall result in more rapid modulation of chest wall movements and greater control of those movements by the developing neuromuscular system.
Keywords: respiration, speech, infant, motor development, physiology
Movements of the abdomen and rib cage during early vocalizations are quite distinct from movements during rest breathing (Boliek, Hixon, Watson, & Morgan, 1996, 1997; Connaghan, Moore, & Higashakawa, 2004; Moore, Caulfield, & Green, 2001; Wilder & Baken, 1978). Abdomen and rib cage movements exhibit larger volume displacements and weaker coupling and have a higher incidence of paradoxing (oppositional movements of the rib cage and abdomen) during early vocal productions compared with rest breathing. A major challenge in understanding how these findings relate to the infant’s emerging respiratory motor control for speech production lies in accounting for other factors that may influence the child’s respiratory kinematics. In particular, the biomechanical characteristics of the infant chest wall are quite distinct from those of the adult and undergo dramatic changes in the first 2 years of life. Although biomechanical factors figure prominently in interpretations of respiratory kinematic data in adult speakers (Hixon, Goldman, & Mead, 1973), little is known of how these factors contribute to changes in respiratory kinematics during early speech behaviors or how biomechanical changes affect the development of respiratory control for speech.
Biomechanical Characteristics of the Developing Chest Wall
The defining characteristic of the developing chest wall is the minimal inspiratory forces generated by the infant rib cage. This property of the rib cage gives rise to the common observation that infants are obligate “belly-breathers”; they produce changes in lung volume by displacement of the diaphragm rather than the rib cage (Hershenson, Colin, Wohl, & Stark, 1990; Hershenson, 1992). Several characteristics of the developing chest wall contribute to the limited inspiratory forces generated by the rib cage. The lack of mineralization in the developing ribs reduces their mechanical inspiratory recoil and, as a result, the passive compliance of the developing chest wall is considerably higher than that of the adult (Gerhardt & Bancalari, 1980; Papastamelos, Panitch, England, & Allen, 1995; Sharp, Druz, Balagot, Bandelin, & Danon, 1970).
The inspiratory action of diaphragm and rib cage muscles on the rib cage is also greatly reduced in the infant. Rib orientation is relatively horizontal, and so contraction of external intercostals produces limited increases in rib cage cross-sectional area (Openshaw, Edwards, & Helms, 1984). In addition, the infant diaphragm is considerably flatter than that of the adult, and the angle at which the fibers of the diaphragm insert into the ribs is much larger. As a result, the portion of the diaphragm and abdomen that lie apposed to the inner surface of the rib cage, an area defined as the zone of apposition (Mead, 1979), is considerably smaller in the infant (Devlieger, Daniels, Marchal, Moerman, Casaer, & Eggermont, 1991).
This difference in the arrangement of chest wall structures in the infant affects the inspiratory action of the diaphragm on the rib cage (Devlieger et al., 1991). Because the fibers of the costal diaphragm insert into the lower rib cage in the adult, their contraction can expand the rib cage by elevating the lower ribs (Loring & Mead, 1982; Mead & Loring, 1982). In the infant, the oblique angle of insertion of these fibers into the lower ribs causes contraction of the costal diaphragm to draw the rib cage inward and, as a result, the action of the costal diaphragm on the lower rib cage is expiratory instead of inspiratory (Devlieger et al., 1991). The fibers of the crural diaphragm attach to the lumber vertebrae, and their contraction produces axial displacement of the diaphragmatic dome, which simultaneously lowers intra-thoracic pressure and increases abdominal pressure. Because abdominal contents are adjacent to the lower rib cage in the zone of apposition in the adult, the increased abdominal pressure can also expand the rib cage (Loring & Mead, 1982). The flatter shape of the infant diaphragm reduces the amount of axial displacement produced by diaphragm contraction and limits the inspiratory effects of increases in abdominal pressure on the rib cage (Devlieger et al., 1991).
Besides increasing the workload of the diaphragm, the high compliance of the chest wall alters the mechanical interaction between the rib cage and the lungs such that the volume at which the opposing forces of these structures are in equilibrium (i.e., the functional residual capacity) is approximately 10% of total lung capacity (Agostoni, 1959). Because passive expiration to this level would result in atelectasis, or alveolar collapse, infants actively maintain their end expiratory level (EEL) at a volume significantly higher than their passively determined functional residual capacity (Henderson-Smart & Read, 1979; Kosch & Stark, 1984; Thach et al., 1980). Several mechanisms for maintaining EEL above functional residual capacity have been identified, including activation of inspiratory muscles during expiration (Muller, Volgyesi, Becker, Bryan, & Bryan, 1979; Prechtl, Vaneykern, & O’Brien, 1977; Thach et al., 1980) and increases in glottal resistance (Kosch & Stark, 1984).
The infant’s compliant chest wall also predisposes the rib cage to paradoxical expiratory movement during inspiration (Hershenson, 1992; Muller & Bryan, 1979). During non-REM sleep and awake states, activation of the external intercostals stabilizes the rib cage to oppose inspiratory paradoxing (Pascucci, Hershenson, Sethna, Loring, & Stark, 1990; Thach et al., 1980). The depression in respiratory muscle activity occurring during REM sleep reduces the stabilizing effects of external intercostal activity and, as a result, increases the amount of rib cage paradoxing (Gaultier, Praud, Canet, Delaperche, & Dallest, 1987; Heldt & McIlroy, 1987; Henderson-Smart & Read, 1979; Prechtl et al., 1977; Thach et al., 1980).
Biomechanical Changes and Respiratory Development for Speech
The high compliance of the infant chest wall may limit the movement patterns exhibited by this structure during vocalization and early speech behaviors. In the adult, rapid modulation of respiratory movements is important for responding to the changing aeromechanical demands of speech production (Finnegan, Luschei, & Hoffman, 2000; Hixon, 1973; Huber & Stathopoulos, 2003). Rapid modulation of physiologic movements involves co-contraction of antagonist muscles to decrease the compliance of the moving structure and increase its response to small changes in the balance of muscle pressures (Cooke & Brown, 1990; Moore, Smith, & Ringel, 1988).
Modulation of respiratory movements in the infant may be more limited owing to the high compliance of the chest wall. In the infant, the passive forces tending to collapse the lungs are much greater than the passive forces tending to expand the rib cage (Gerhardt & Bancalari, 1980; Papastamelos et al., 1995; Sharp et al., 1970) and, as a result, the balance of inspiratory and expiratory muscle pressures during expiration has an important role in maintaining the integrity of the lungs (Muller et al., 1979; Prechtl et al., 1977; Thach et al., 1980). The importance of respiratory muscle pressures in preventing atelectasis may limit the involvement of these muscle systems in other functions such as vocalization. In addition, the smaller zone of apposition and horizontal orientation of the ribs in the infant reduce the inspiratory action of both diaphragm and rib cage muscle groups (Devlieger et al., 1991; Openshaw et al., 1984). As a result, changes in the activation of these muscles during vocalization will produce more limited changes in the movements of the chest wall.
The findings from investigations of speech respiratory function in infants and toddlers provide some support for biomechanical constraints on the modulation of chest wall movements. Connaghan and colleagues (Connaghan et al., 2004) observed 4 children longitudinally from the ages of 9–48 months. These researchers found that paradoxical movements of the abdomen and rib cage during speech increased over this time period and that this increase was due to increasing instances of inspiratory rib cage movements during speech expiration. This finding was consistent with a previous study by Moore and colleagues (Moore et al., 2001), who studied respiratory movements in 11 toddlers at 15 months of age. These investigators observed that the proportion of paradoxical movements during speech resulting from inspiratory movement of the rib cage was twice that resulting from inspiratory movement of the abdomen. In summary, these studies of respiratory behaviors during early speech behaviors suggest developmental increases in the occurrence of inspiratory rib cage movements during the first years of life. Investigations of changes in respiratory biomechanics have pointed toward the first 2 years of life as a period associated with large decreases in the compliance of the chest wall (Colin et al., 1989; Gaultier et al., 1987; Hershenson et al., 1990). These biomechanical data indicate that increases in inspiratory rib cage movements during early speech occur over a period of development associated with large increases in the inspiratory forces generated by the rib cage.
Within-cycle respiratory behaviors were also investigated by Wilder and Baken (1978), who recorded abdomen and rib cage movements during cries in a semi-longitudinal study of 10 infants from the age of 2 days to 8 months. The researchers introduced a novel measure, the expiratory subcycle, which was described as a measurable expansion or contraction of the rib cage and/or abdomen during a single expiration. Wilder and Baken (1978) reported that the number of expiratory subcycles during cries increased over the first of year life and was significantly correlated (r = .99) with corresponding increases in expiratory duration over the same time period. The authors suggested that the correlation between expiratory duration and the number of expiratory subcycles per cry reflected the infants’ failure to effectively control the recoil forces of the lungs and chest wall for cries of longer duration. Another possibility is that changes to the compliance of the chest wall during this period gave rise to more rapid modulation of infants’ chest wall movements. If this was the case, then increases in the number of expiratory subcycles per cry should emerge independent of any increases in expiratory duration.
Experimental Approach
The findings of these studies suggest that the biomechanical characteristics of the developing chest wall might contribute to some of the trends observed in respiratory movements during early vocalizations and speech behaviors. The current study was designed to investigate whether biomechanical changes to the developing chest wall influence the modulation of chest wall movements during vocal productions. Direct measurement of the most important physiologic variables (e.g., passive compliance of the rib cage, rib cage orientation) is precluded by the invasiveness of available techniques. Instead, a developmental approach was adopted to allow for observation of biomechanical effects by examining infants during a narrow period of development associated with large decreases in the compliance of the chest wall. Infants were observed at 7 and 11 months of age, as several studies have indicated that the second half of the first year of life is associated with rapid decreases in the compliance of the infant chest wall. Colin and colleagues (1989) found that the transition from a dynamically maintained EEL to a passively determined EEL occurs during this period. In addition, Hershenson and colleagues (1990) reported that by 9 months of age, the contribution of rib cage to changes in lung volume during quiet sleep resembled that of adolescents during quiet sleep.
Although the current study was motivated in part by the findings of Wilder and Baken (1978), a problem with using expiratory subcyles to address these questions was the lack of detail provided by Wilder and Baken regarding the identification of these movement behaviors. An example of respiratory traces from a 7-month-old infant in the current investigation that seemed consistent with Wilder and Baken’s description of expiratory subcycles is displayed in the top panel of Figure 1. In this figure, the direction of rib cage and abdomen movements alternated between periods of expiration and periods of inspiration during vocalization. However, the identification of expiratory subcycles is not typically straightforward based on a visual inspection of respiratory traces. For example, the identification of expiratory subcycles in the respiratory movements displayed in the middle panel of Figure 1 is more difficult. The direction of abdomen and rib cage movements was generally expiratory throughout the vocalization and, therefore, would not seem to constitute a subcycle. However, the substantial velocity changes evident in the middle of the utterance give the appearance of a movement behavior that is similar to a subcycle but smaller in magnitude. Finally, many traces do not exhibit any type of modulation during expiration. An example of one such trace is shown in the bottom panel of Figure 1.
Figure 1.
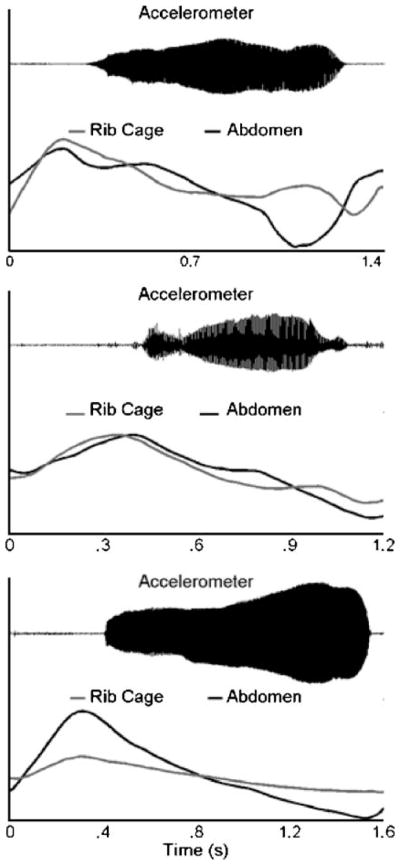
Abdomen, rib cage, and laryngeal accelerometer signals for three vocalizations produced by infants in the 7-month group. From top to bottom, the panels depict respiratory traces exhibiting decreasing amounts of modulation during vocalization.
The cyclic nature of respiratory movements described in the studies of Wilder and Baken (1978), Moore and colleagues (2001), and Connaghan and colleagues (2004) suggested that spectral analysis of respiratory traces would be an effective method to quantify respiratory movement patterns during early vocal productions. As detailed further in the Method section, principal component analysis (PCA) is used to decompose the set of abdomen and rib cage traces obtained from an infant into a few dominant movement patterns, or principal components (PCs). Spectral analysis is used to identify the fundamental period of the movement pattern described by the PC. The amplitude of the PC was expressed as the fraction of total variance that the PC movement pattern contributed to the infant’s abdomen and rib cage traces.
Expressing each infant’s abdomen and rib cage traces as the sum of a small number of PC waveforms of varying fundamental periods and amplitudes provides the basis for evaluating changes in the modulation of abdomen and rib cage movements during this period of development. It was predicted that, compared with the 7-month group, the PC waveforms of the 11-month group would be characterized by shorter fundamental periods and that the fractional variances of PCs describing movement patterns with shorter fundamental periods would increase relative to the fractional variances of PCs describing longer fundamental periods. These predictions were based on the expectation that decreases in the compliance of the chest wall would lead to more rapid modulation of respiratory movements that accounted for a greater amount of variance in infants’ respiratory kinematics.
Predictions regarding changes in the shapes of respiratory traces do not address how changes in the compliance of the chest wall might affect neuromuscular control of the chest wall for early vocalizations. An interaction between biomechanical and neuromuscular factors in this population was considered because the smaller zone of apposition and horizontal orientation of the ribs limit the effects of inspiratory muscle forces on the rib cage. At the same time, decreases in chest wall compliance resulting from increases in the action of muscle forces on the rib cage might lead to greater control of this structure by the developing neuromuscular system.
One indication of respiratory control for speech in adults is the reported co-variation between utterance length and respiratory measures such as termination lung volume (Hixon et al., 1973; Winkworth, Davis, Ellis, & Adams, 1994), inspired volume, and speech initiation lung volume (Winkworth et al., 1994). The finding that utterance length accounts for a significant amount of the variability in these respiratory measures suggests systematic planning and/or adjustments by the central nervous system for production of utterances of varying length. An analysis of how token-to-token changes in the amplitudes of PC waveforms co-varied with changes in vocal duration was performed to determine whether such behaviors were present in infants and whether these behaviors changed between 7 and 11 months.
Method
Participants
Forty infants were observed at 7 and 11 months of age (±3 days) for this investigation. The experimental protocol was approved by the Institutional Review Board/Human Subjects Review Committee at the University of Washington, and informed consent was obtained from a parent of each infant. All infants were born within 2 weeks of their due date; were at least 6 pounds at birth; and had negative medical histories for respiratory, cardiovascular, and neurological disease. Infants with a history of ear infections (more than two), an ear infection in the week prior to testing, or a family member with a hereditary hearing problem were also ineligible. Infants having an immediate family member with a speech or language disorder were also excluded from this study. All babies were from monolingual English-speaking homes in the Seattle metropolitan area. Participants in this study were part of a larger study investigating speech, language, and hearing development.
Measurements
All experiments were performed in a large, sound-treated booth. The child was seated comfortably in a high chair with his or her caregiver at the side. Respiratory kinematics (i.e., abdominal and rib cage cross-sectional areas) were transduced using respiratory inductance plethysmography (Inductotrace; Ambulatory Monitoring Inc., Ardsley, NY). The elastic bands of the Inducto-trace were placed around the infant’s chest just below the axilla for transduction of rib cage circumference and between the lowest vertebral rib and the iliac crest for transduction of abdominal circumference. The respi-bands were secured to the skin with micropore first aid tape (3M) to prevent their movement during the recording session. The child’s vocalizations were recorded with a Sony electret condenser lapel microphone (ECM-77B). For the 7-month group, this microphone was held in front of the baby by an experimenter. In the 11-month group, the microphone was adhered to the forehead of the infant with micropore tape. A miniature, high-sensitivity accelerometer (PCB Piezotronics Inc., Depew, NY; Model 352C67) was placed on the skin overlying the thyroid cartilage and was used to transduce the vibrations of the vocal folds. The laryngeal accelerometer signal facilitated identification of vocalization produced during periods of background chatter by the mother and/or experimenters.
Various procedures were used to elicit vocal behaviors from the infants. These procedures involved the caretaker’s participation as well as environmental stimuli such as videos, pictures, and other toys. A typical session would yield between 30 and 45 min of continuous data. During these sessions, infants produced a number of different utterance forms consistent with this period of speech development (Oller, 1980; Stark, 1986). Utterances ranged from quasivowel sounds, cooing, and vocal play to more speech-like forms, such as marginal and canonical babbles. A few instances of variegated babbling were also observed. Respiratory traces associated with all of these utterances forms were included for analysis. Because the period of development between 7 and 11 months of age is associated with the emergence of canonical and, possibly, variegated babbles (Oller, 1980; Stark, 1986), it was quite possible that the data from the 11-month group contained a greater proportion of truly syllabic utterance forms than the data from the 7-month group. This possibility was not controlled for in the current study, but the influence of linguistic complexity on the respiratory movements in this data set is being investigated in a separate study.
Respiratory traces produced during reflexive vocalizations such as coughs and hiccups were excluded from the analysis, but those produced during cries were included. Stark (1986) has noted that cries may sometimes be associated with a forceful expulsion of air. In the present data set, these expiratory bursts were accompanied by dramatic changes in respiratory movements. These tokens were parsed so that only respiratory movements preceding the expiratory bursts were analyzed.
Each included vocalization had a minimum duration of 300 ms. All recording sessions were videotaped for identification and exclusion of tokens that exhibited movements of the upper and lower limbs, which would have compromised the validity of the respiratory kinematic signals. Tokens were judged acceptable if video inspection revealed that they were not accompanied by any movements of the limbs and that the child exhibited a stable trunk posture. In addition, vocalizations produced with the infant’s arms extended either upward or forward were excluded from analysis, as these postures generated artifactual signals even if the infant was not moving.
Because of these inclusion criteria, it was not possible to obtain data from all infants at both ages of observation. Several infants did not tolerate the respiratory sensors, which precluded acquisition of respiratory signals. Other infants produced too few vocalizations or, more often, produced vocalizations accompanied by excess movements of their torso and/or limbs. Data were obtained from 12 infants in the 7-month group and from 18 infants in the 11-month group. For 6 infants, data were obtained at both 7 and 11 months of age.
Data Acquisition
Abdomen and rib cage signals were analog low-pass filtered (flp = 30 Hz) and amplified using a 4-pole Butterworth filter (Krohn–Hite Model 3364). The accelerometer signal was also analog filtered (flp = 750 Hz) and amplified. The audio signal was amplified as necessary using a high-quality microphone preamplifier (Pro MPA, Applied Research and Technology, Rochester, NY). Analog outputs from all signals were then filtered for anti-aliasing (RC Electronics Inc., Santa Barbara, CA) and digitized direct-to-disk using a commercially available hardware/software system (WINDAQ; Dataq Inc., Akron, OH). For the 7-month group, the digital sampling rate for each channel was 35.7 kHz, and for the 11-month group, the sampling rate for each channel was 10.1 kHz. After digitization, abdomen and rib cage signals were digitally low-pass filtered (flp = 30 Hz) forward and reverse with a third order Butterworth filter to remove low-intensity computer noise introduced during the digitization process.
PCA
PCA was performed on infants’ abdomen and rib cage traces, as this technique has been shown to be an effective method for describing highly variable movement behaviors, including speech, in terms of a few characteristic movement patterns or principal components (Borghese, Bianchi, & Lacquaniti, 1996; Ramsay, Munhall, Gracco, & Ostry, 1996; Soechting & Flanders, 1997; Santello, Flanders, & Soechting, 1998). Variability in infants’ respiratory behaviors was an important consideration in the current study, as Boliek and colleagues (1996) characterized the variability of their infant respiratory measures as a hallmark of the data set. In the current investigation, each PC corresponded to a waveform depicting a spatio-temporal pattern of motion that contributed some percentage of variance (i.e., the fractional variance) to the total variance observed in the infant’s abdomen and rib cage traces.
Prior to decomposing an infant’s respiratory traces, the rib cage and abdomen traces were parsed 25 ms prior to vocal onset and 25 ms following vocal termination. After removing the mean from each signal (i.e., centering the signal on zero amplitude), the traces were time-and amplitude-normalized. Because respiratory traces were not calibrated with respect to lung volume, the amplitude of a respiratory trace did not reflect its contribution to changes in lung volume. Instead, an infant’s respiratory traces were amplitude-normalized so that the amplitude of an abdomen trace reflected the proportion of variance that it contributed to the set of abdomen traces for that infant, and the amplitude of a rib cage trace reflected the proportion of variance that it contributed to the set rib cage traces for that infant. First, the root-mean-square value of the entire set of abdomen traces for an infant was derived, and then each individual abdomen trace was multiplied by the inverse of this value so that the root mean square of all abdomen traces was equal to one. This procedure was repeated for the rib cage traces. The infant’s abdomen and rib cage traces were then time-normalized by setting the length of each trace to 1,000 points using a cubic spline interpolation.
For each infant, the normalized abdomen and rib cage traces were collapsed into a single n × 1,000 matrix, X. Since each token produced by an infant was associated with an abdomen and a rib cage trace, n was equal to twice the number of tokens obtained for the infant. The amplitude normalization procedure ensured that the variance of the abdomen traces in X and the variance of the rib cage traces in X were equal. Singular value decomposition of the matrix, X, was used to derive the n principal components for the infant. The singular value decomposition of the matrix, X, yielded the factorization X = U S VT. The matrix V contained the n PCs, each of which described a pattern of respiratory movement that accounted for a certain percentage of the variance in the infant’s respiratory traces. Because the PCs returned were orthogonal, these PCs accounted for nonoverlapping variances in the infant’s respiratory traces. The matrices U and S contained different scaling factors for fitting the PCs to the original data. The squared diagonal elements of S corresponded to the eigenvalues in decreasing order for each PC, and these values described the amount of variance each PC contributed to all of the traces obtained for that infant. The squared elements of the matrix U reflected the PC scores, which described the variance that each PC contributed to an individual respiratory trace in the matrix, X. These values were normalized so that each PC score reflected the percentage of the variance in the respiratory trace accounted for by the corresponding PC. Analyses involving the eigenvalues and the PC scores are described below.
Summing the scaled n PCs produced an exact fit to the original respiratory traces. In practice, however, the traces could be approximated to an acceptable level of accuracy with a smaller number of PCs, c. The difference between n and c reflected the dimensionality reduction achieved by the singular value decomposition. In the current investigation, c was set to the lowest number of PCs that could account for at least 95% of the total variance in all infants’ respiratory traces. This method ensured that almost all of the variability in each infant’s respiratory traces would be explained and also guaranteed that the same number of PCs would be available for analysis from each infant.
Preliminary analyses revealed that infants’ PCs were approximately sinusoidal; spectral analysis of the PCs was used to quantify the fundamental period of the movement pattern described by each PC. The autocorrelation function of each PC waveform was derived and the power spectrum was estimated from the resulting 1,999 point autocorrelation array using Welch’s averaged periodogram method (segment length = 512 points, FFT length = 2,048). This method of spectral estimation returned an effective frequency resolution of 0.49 cycles per vocalization. Because the respiratory traces were time normalized, the resulting units of the c PC peak frequencies were not cycles per second (i.e., Hz) but rather in cycles per vocalization. These peak frequencies were converted to Hz by dividing each of the c PC peak frequencies for an infant by that infant’s vocal duration values and deriving their c means. The mean frequencies described by each PC were then converted to fundamental periods by calculating their inverse.
The c eigenvalues for each infant’s data set were normalized to reflect the fraction of total variance that each of the c PCs contributed. The fractional variances for each PC were then separated into abdomen and rib cage fractional variances, which provided a means of testing whether the abdomen and rib cage exhibit contrasting movement patterns during early vocalizations. Since each PC represents a single movement pattern observed in an infant’s respiratory traces, the abdomen and rib cage fractional variances indicate the extent to which that pattern was present in the abdomen and rib cage traces. For example, if an infant’s PC1 had a fractional variance of 80%, the corresponding abdomen and rib cage fractional variances might be 45% and 35%, indicating that the movement pattern described by PC1 was slightly more prevalent in this infant’s abdomen traces than it was in the rib cage traces. Differences between the abdomen and fractional variances for PCs correspond to shape differences in the traces of the abdomen and rib cage, which might indicate discrepancies in the functional or biomechanical characteristics of these structures during early vocalizations. Differences between the abdomen and rib cage fractional variances for a PC do not correspond to differences in the contribution of these structures to changes in lung volume. Instead, the fractional variances denote the contribution of the movement pattern to the fit of the respiratory traces for an infant and only provide information about possible differences in the shapes of abdomen and rib cage traces.
Respiratory traces and vocal duration
Two final analyses were developed to test for modulation in abdomen and rib cage movements with changes in vocal duration. To address this issue, a new measure, the token variance, was developed to quantify the percentage of variance each PC waveform contributed to individual respiratory traces. Token variances were derived by calculating the product of the PC scores and their corresponding fractional variances. A respiratory trace whose first 3 three token variances were .50, .32, and .11 indicated that the first three PCs accounted for 50%, 32%, and 11% of the variance in that trace, respectively. Since the token variances reflected token-to-token changes in the contribution of a PC waveform to the shapes of infants’ respiratory traces, tests for linear associations between vocal durations and the token variances for each PC were used to determine how the shapes of abdomen and rib cage traces changed with changes in vocal duration. In a second analysis, vocal duration values were compared with token variances for all PCs to quantify how well respiratory movements predicted vocal duration values.
Statistical Analyses
Although the current study was designed to evaluate longitudinal trends in infants’ respiratory measures, the high dropout rate among infants yielded a mixed cross-sectional longitudinal data set. To address the modified design of the study, linear mixed-effects models were used for all statistical testing in this study because of the flexibility with which these models are capable of handling both repeated-measures data with missing values and unbalanced experimental designs (Pinheiro & Bates, 2000). In the current study, random effects modeled individual infant effects; fixed effects modeled factors of interest such as age or PC number. These analyses were supplemented by mixed effects models that considered only the longitudinal trends of the 6 infants who were observed at both 7 and 11 months of age.
Results
In the 7-month group, 462 tokens were obtained from 12 infants, and in the 11-month group, 663 tokens were obtained from 18 infants. The number of tokens obtained from each infant in each age group, as well as the group means and standard deviations, are shown in the left side of Table 1. As is evident in this table, the mean number of tokens obtained from infants in each age group was comparable for the two groups (38.5 versus 38.6 for the 7- and 11-month groups, respectively). However, there was greater variability in the number of accepted tokens from infants in the 7-month group, as indicated by the higher SD for this group. This difference was primarily due to the large number of tokens (115) obtained from Infant 21 at 7 months. For 6 infants, data were obtained at both 7 and 11 months of age (see Table 1).
Table 1.
The number of tokens, PC fractional variances, and mean vocal durations for infants in each age group.
| Participant | No. of tokens
|
Fractional variance (%)
|
Mean vocal duration (s)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 mos
|
11 mos
|
|||||||||
| 7 mos | 11 mos | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | 7 mos M (SD) | 11 mos M (SD) | |
| 02 | — | 85 | 83.5 | 13.3 | 1.7 | 1.1 (0.6) | ||||
| 04 | 17 | — | 88.3 | 8.8 | 1.8 | 0.7 (0.2) | ||||
| 05 | — | 24 | 77.6 | 14.5 | 3.8 | 1.9 (0.9) | ||||
| 06 | 21 | — | 93.1 | 4.5 | 1.7 | 1.0 (0.5) | ||||
| 08 | 47 | — | 87.0 | 9.3 | 1.9 | 1.3 (0.7) | ||||
| 09 | — | 31 | 73.2 | 19.0 | 4.3 | 1.0 (1.1) | ||||
| 10* | 32 | 18 | 83.1 | 10.7 | 2.8 | 77.8 | 12.6 | 4.8 | 1.8 (0.8) | 1.4 (1.1) |
| 13* | 33 | 62 | 93.0 | 4.4 | 1.5 | 79.7 | 12.6 | 4.6 | 1.1 (0.4) | 0.8 (0.6) |
| 14* | 48 | 39 | 80.1 | 13.3 | 3.1 | 75.3 | 17.8 | 4.3 | 1.4 (0.9) | 1.5 (1.4) |
| 16 | 17 | — | 94.6 | 2.5 | 1.6 | 1.0 (0.4) | ||||
| 17* | 17 | 24 | 94.5 | 4.3 | 0.7 | 82.1 | 15.4 | 1.2 | 0.8 (0.4) | 0.9 (0.5) |
| 21* | 115 | 47 | 88.4 | 8.5 | 1.8 | 86.2 | 9.6 | 2.2 | 1.5 (0.6) | 0.8 (0.3) |
| 24 | — | 23 | 68.3 | 25.2 | 2.7 | 1.6 (0.9) | ||||
| 25 | — | 52 | 61.6 | 34.4 | 2.5 | 0.7 (0.4) | ||||
| 27 | — | 35 | 81.1 | 14.6 | 2.9 | 0.7 (0.4) | ||||
| 28 | — | 37 | 83.1 | 12.3 | 2.9 | 1.2 (0.7) | ||||
| 29 | 39 | — | 86.0 | 10.6 | 2.1 | 0.9 (0.4) | ||||
| 31* | 44 | 45 | 93.5 | 5.0 | 0.7 | 77.0 | 19.1 | 1.7 | 0.7 (0.2) | 1.4 (0.7) |
| 32 | — | 32 | 90.5 | 6.8 | 2.0 | 0.5 (0.1) | ||||
| 33 | — | 20 | 83.7 | 13.0 | 2.0 | 0.9 (0.9) | ||||
| 36 | — | 29 | 76.3 | 15.2 | 5.4 | 1.1 (0.8) | ||||
| 38 | — | 14 | 91.2 | 6.5 | 1.2 | 0.5 (0.2) | ||||
| 40 | 32 | — | 84.7 | 10.3 | 3.5 | 0.9 (0.3) | ||||
| 41 | — | 56 | 77.3 | 16.8 | 2.7 | 1.1 (0.8) | ||||
|
| ||||||||||
| TOTAL | 462 | 663 | ||||||||
| M | 38.5 | 36.8 | 88.8 | 7.7 | 1.9 | 79.2 | 15.5 | 2.9 | 1.1 | 1.1 |
| SD | 26.7 | 18.5 | 4.9 | 3.4 | 0.9 | 7.2 | 6.5 | 1.3 | 0.3 | 0.4 |
Note. mos = months; PC = principal component. Asterisks indicate infants who were recorded at both 7 and 11 months of age.
Singular value decomposition was performed on the set of abdomen and rib cage traces obtained from each infant to derive that infant’s PCs, their abdomen and rib cage fractional variances, and their abdomen and rib cage token variances. Three PCs were required to account for at least 95% of the total variance in each infant’s respiratory traces. Including the fourth PC from each infant accounted for a minimum of 97% of the respiratory variance in each infant’s data. The finding that the fourth PC accounted for only an additional 2% of the variance suggests that the first three PCs were sufficient to characterize the major components of variance in these data. The fractional variances of the first three PCs from each infant in each age group are displayed in the middle columns of Table 1. For infants in both age groups, the first PC accounted for a majority of the variance in the respiratory traces (between 61% and 95%) compared with the fractional variances of PC2 and PC3.
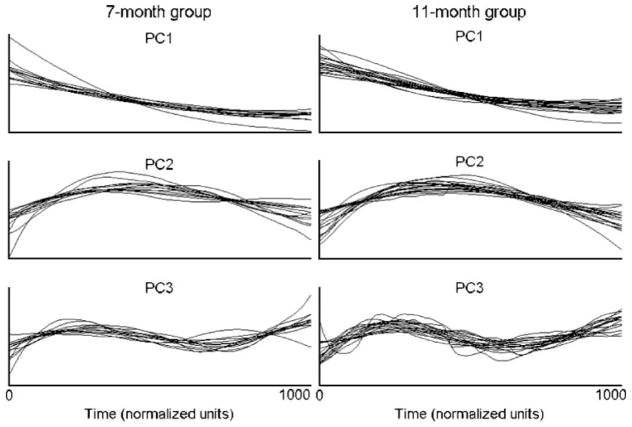
The first three PCs for each infant in the 7-month group are shown in the left panel of Figure 2, and those for the 11-month group are shown in the right panel. Because PCAwas performed separately on each infant’s respiratory data, the resulting PCs reflected respiratory movement patterns that were specific to that infant’s respiratory traces. Despite this fact, an examination of the PCs in Figure 2 suggests that the pattern of respiratory modulation described by each PC waveform was quite similar across infants. To quantify this similarity, correlation coefficients were computed between infants’ PC waveforms for each PC. In the 7-month group, the average correlations for PCs 1–3 were .98 (SD = .02), .90 (SD = .14), and .74 (SD = .31), respectively. It was possible that the large differences in number of tokens obtained from different infants produced systematic differences in the shapes of the PCs in this group. However, the correlation coefficients between the infant with 115 tokens and the three infants with 17 tokens were comparable to the averages for each PC. The correlations were .96, .99, and .99 for PC1; .95 .70, and .99 for PC2; and .95, .67, and .97 for PC3.
Figure 2.
PCs 1 through 3 are displayed in descending order for the 7-month (left) and 11-month (right) groups. For each PC, the shapes of the PC waveforms exhibited a high degree of similarity both within and across age groups.
For the 11-month group, the average correlations were .97 (SD = .04) for PC1, .93 (SD = .06) for PC2, and .78 (SD = .17) for PC3. A comparison of the correlation coefficients between the infant with 85 tokens and the infants with 14, 18, and 19 tokens did not indicate that the number of tokens obtained from an infant affected the shape of the PC waveform. The correlations were .97, .99, and .99 for PC1; .96, .89, and .99 for PC2; and .81, .75, and .97 for PC3. In general, the correlational analysis of infants’ PC waveforms indicated that infants’ PCs were quite similar for each PC although the similarity did decrease for PC3. In addition, the number of tokens obtained did not appear to have any systematic effects on the shapes of PC waveforms.
An examination of the PC waveforms indicated that their shapes described interpretable aspects of the respiratory movements from which they were derived. PC1 described a unidirectional movement pattern that was most prominent in respiratory traces like the ones shown in the bottom panel of Figure 1. The display of these PCs in Figure 2 suggested that this unidirectional movement pattern reflected a net expiratory movement. But negative coefficients in the first column of U would indicate that PC1 described inspiratory movements for those respiratory traces. The sign of the coefficients for PC1 was negative for only 8% of the traces in the 7-month group and 16% of the traces in the 11-month group. This finding revealed that PC1 typically reflected a net expiratory movement.
PC2 described a movement pattern consisting of a single directional change, and PC3 described a movement pattern consisting of two or more directional changes. When the token variances for PC2 and PC3 were large, the corresponding respiratory traces tended to exhibit inspiratory–expiratory directional changes, such as the traces displayed in the top panel of Figure 1. When the contribution of these PCs was not as large, they tended to describe a modulation in the speed of the corresponding respiratory movements, such as is exhibited by the traces shown in the middle panel of Figure 1.
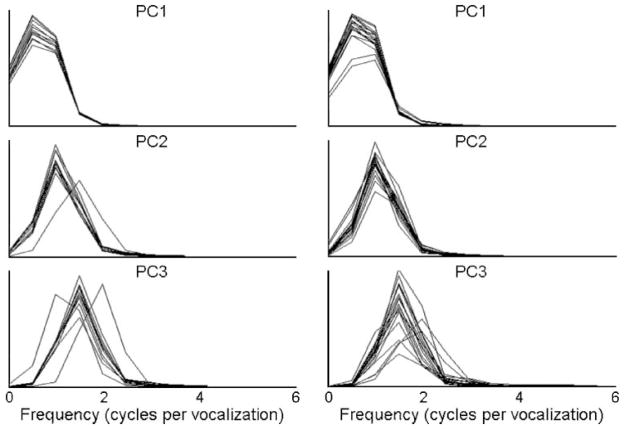
To quantify the patterns of modulation described by the PC waveforms, spectral analysis was performed on the first three PC waveforms derived for each infant. Figure 3 displays the resulting power spectra for each infant in the 7-month group (left) and each infant in the 11-month group (right). This figure reveals that the power spectra derived from infants’ PC were generally characterized by a single peak, which was consistent with the observation that these waveforms were sinusoidal.
Figure 3.
Power spectra of PC waveforms for infants in the 7-month and 11-month groups. These spectra were generally characterized by a single peak whose relative frequency was similar across PCs.
Fundamental Periods of PC Waveforms
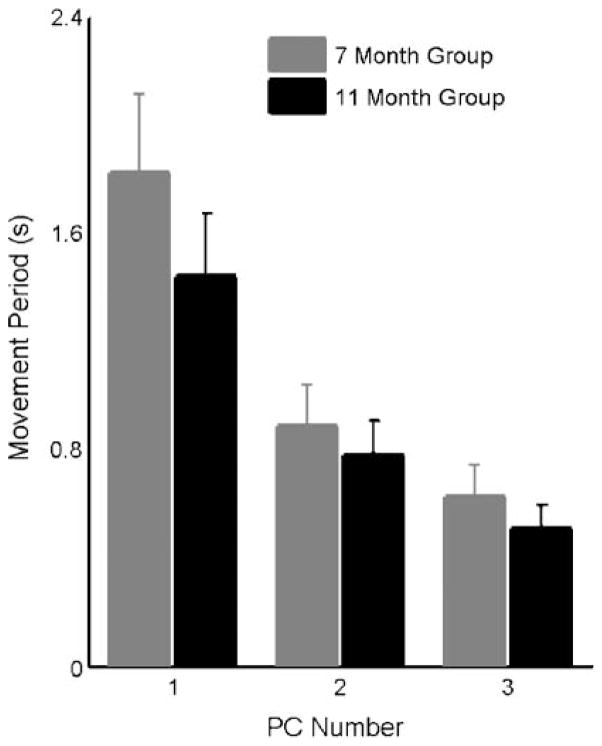
Figure 4 displays the means and 95% confidence intervals of the average fundamental periods of PCs 1–3 for the 7-month (gray bars) and 11-month (black bars) groups. This figure suggests that the fundamental period of the movement pattern described by each PC decreased with each increase in PC number for infants in both age groups. This figure also reveals that the fundamental periods of the PCs in the 11-month group tended to be shorter than those of the PCs in 7-month group. A linear mixed-effects model was used to test for the effects of PC number and age on the fundamental period of infants’ PCs. A significant main effect of PC number on fundamental period was observed, F(2, 84) = 85.06, p < .0001, and multiple comparisons across PCs were performed using a Bonferroni correction. These tests confirmed that the fundamental period of the movement pattern described by each PC tended to decrease with increases in PC number. The fundamental period for PC1 was significantly longer than PC2 (p < .001) and PC3 (p < .001) by averages of 800 ms and 1.07 s, respectively. In addition, the fundamental period of PC2 was longer than that of PC3 (p < .01) by an average of 267 ms. This statistical correlation provided the basis for linking age-related changes in the PC fractional variances to changes in the modulation characteristics of infants’ respiratory traces.
Figure 4.
Barplots showing the average fundamental periods obtained for each infant’s PC waveforms for the 7-month (gray) and 11-month (black) groups. For both age groups, the fundamental period of PC waveforms decreased with each increase in PC number. In addition, the fundamental periods of the 11-month group were significantly shorter than those of the 7-month group. Error bars represent 95% confidence intervals.
A significant main effect of age on fundamental period was also observed, F(1, 84) = 8.40, p < .01, with the fundamental period of PCs in the older group exhibiting an average decrease of 200 ms compared with the younger group. This test failed to identify a significant interaction effect between age and PC number, F(2, 84) = 1.70, p = .19, indicating that the effect of age on fundamental period was similar across PCs.
These tests were also performed on the 6 infants with data in both age groups. A main effect of PC number was observed for fundamental period, F(2, 30) = 39.61, p < .001. Pairwise comparisons using a Bonferroni correction revealed significant decreases in fundamental period with increases in PC number. PC1 exhibited average fundamental periods that were 800 ms longer than those of PC2 (p < .001) and 1.10 s longer than those of PC3 (p < .001). No significant difference was observed betweenthe fundamentalperiodsforPC2andPC3(p=.07). A significant main effect for age was also observed in these 6 infants, F(1, 30) = 4.64, p < .05. The average fundamental periods of the 11-month group were 227 ms shorter than those of the 7-month group. No interaction was observed between the main effects of age and PC number on fundamental period, F(2, 30) = 1.27, p = .30.
Fractional Variances of PCs
A linear mixed-effects model was also used to test for the main effects of PC number, age, and chest wall structure (abdomen or rib cage) on infants’ fractional variances. Because it was hypothesized that the effects of age on fractional variances would be different for different PCs, the two-way interaction between age and PC number was included in this model. Similarly, the two-way interaction between chest wall structure and PC number was evaluated to determine whether the abdomen and rib cage fractional variances were different for different PCs. Finally, the three-way interaction of age, PC number, and chest wall structure was included to test for differences in the effects of age on PC fractional variances between abdomen and rib cage fractional variances.
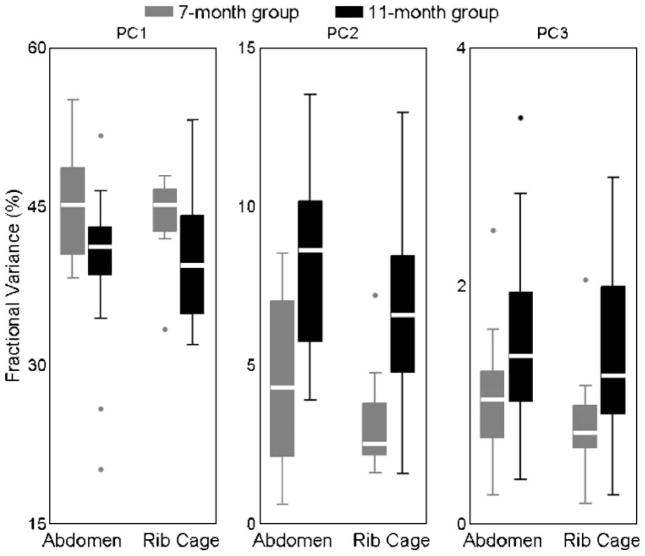
Boxplots showing the abdomen and rib cage fractional variances by PC for each age group are displayed in Figure 5. A significant main effect was observed for PC number, F(2, 168) = 1,894.8, p < .0001, and multiple comparisons were made using a Bonferroni correction. These comparisons revealed that the fraction of variance contributed by PC1 was significantly greater than that contributed by PC2 (p < .001, mean difference = 36.2%) and PC3 (p < .001, mean difference = 40.8%). Similarly, the fraction of variance contributed by PC2 was significantly greater than that of PC3 (p < .001, mean difference = 4.6%). No main effects were observed for age, F(1, 168) = 0.05, p = .82, or chest wall structure, F(1, 168) = 1.54, p = .22.
Figure 5.
Boxplots of the abdomen and rib cage fractional variances of PCs 1 through 3 (left to right) for the 7-month (gray) and 11-month (black) groups. Compared to the 7-month group, the 11-month group exhibited significant decreases in the fractional variances for PC1, and significant increases in the fractional variances for PC2 and PC3.
A significant interaction was observed between age and PC number, F(2, 168) = 18.36, p < .001, indicating that the effects of age on fractional variances were not the same across PCs. Pairwise comparisons revealed that the fractional variance for PC1 was significantly smaller in the 11-month group than in the 7-month group (p < .05, mean difference = 4.8%). The opposite was observed for the fractional variances of PC2 (p < .05) and PC3 (p < .05), which were significantly greater in the 11-month group by averages of 3.9% and 0.51%, respectively. No interaction effect between chest wall structure and PC number was observed on fractional variances, F(2, 168) = 0.73, p = .48, suggesting that abdomen and rib cage fractional variances were similar for each PC. Lastly, no significant interaction between age, PC number, and chest wall structure was observed, F(2, 168) = 0.16, p = .85, indicating that age-related changes in the fractional variances of different PCs were not significantly different for the abdomen and rib cage.
An analysis of the fractional variances in the 6 longitudinal infants revealed similar findings. A significant main effect on fractional variances was observed for PC number, F(2, 60) = 1,425.24, p < .001, but not for age, F(1, 60) = 0.04, p = .84, or chest wall structure, F(1, 61) = 3.61, p = .06. Pairwise comparisons using a Bonferroni correction revealed that the fractional variances for PC1 were significantly greater than those of PC2 (p < .001) and PC3 (p < .001) by averages of 37% and 41%, respectively. In addition, the average fractional variances of PC2 were 4% greater than those of PC3 (p < .001).
A significant interaction between age and PC number was also observed, F(2, 60) = 11.51, p < .001, in the 6 longitudinal infants. Multiple comparisons revealed that the fractional variances of PC1 in the 11-month group were significantly less than those of the 7-month group (p < .05) by an average of 4.5%. In addition, the fractional variances for PC2 in the 11-month group were significantly greater than those of the 7-month group (p < .05) by an average of 3%. No interaction effects were observed between PC number and chest wall structure, F(2, 60) = 0.95, p = .39, or between PC number, chest wall structure, and age, F(2, 60) = 0.17, p = .84.
Token Variances and Vocal Duration
The overall shape of each respiratory trace was described by three parameters, the token variances, which represented the percentage of variance that each of the first three PCs contributed to that trace. Token variances for abdomen and rib cage traces were analyzed to examine whether, and to what extent, infants’ respiratory movements were related to the duration of the corresponding vocalization. Mixed-effects models were used to test for linear associations between infants’ vocal duration values and the corresponding abdomen and rib cage token variances in each age group. The number of token variances (either one, two, or three) exhibiting a linear association with vocal duration provided a qualitative index of the extent to which the shapes of abdomen and rib cage traces changed with changes in vocal duration. Because each analysis involved three separate tests (one for each PC), a Bonferroni correction was applied to each test, and the adjusted p value for determining statistical significance was .016. A second analysis was designed to quantify the extent to which vocal durations could be predicted from shapes of either the abdomen or rib cage traces. For this analysis, a mixed-effects model using all three token variances was fit to the vocal duration values to determine the amount of variance in vocal duration that could be accounted for by movements of either the abdomen or the rib cage.
An analysis of the histogram of infants’ vocal durations revealed that it was skewed away from the minimum duration criterion for accepting tokens (i.e., 300 ms). To address this issue, the duration data were log-transformed using a base 10 prior to statistical testing. The skew of the original duration values was 2.20, and the skew of the log-transformed values was 0.26.
In the 7-month group, a significant linear association was identified between vocal durations and the abdomen token variances for PC1, F(1, 449) = 7.98, p < .005; PC2, F(1, 449) = 10.20, p < .005; and PC3, F(1, 449) = 12.30, p < .001. For all PCs, the fixed effect coefficient was positive, indicating that the contribution of each PC to the variance in abdomen traces increased with increases in vocal duration. The goodness-of-fit of the statistical model comparing all three abdomen token variances to vocal durations was derived to quantify the extent to which abdomen movements predicted vocal duration. The average correlation between abdomen token variances and vocal duration across infants in the 7-month group was .26, and the average squared correlation was .08, indicating that the abdomen token variances for all three PCs accounted for only 8% of the variance in infants’ vocal duration values.
Rib cage token variances exhibiting a significant linear association with vocal duration were identified for PC2, F(1, 449) = 13.15, p < .001, but not for PC1, F(1, 449) = 3.10, p = .08, or PC3, F(1, 449) = 5.92, p = .015. As with the abdomen token variances, the fixed-effect coefficient of the rib cage token variances for PC2 was positive, indicating that the contribution of this PC to the variance in rib cage traces increased with increases in vocal duration. A linear mixed-effect model using the rib cage token variances of the first three PCs was fit to the vocal duration values to evaluate the extent to which rib cage movements predicted the duration of corresponding vocal durations. This model revealed that the average correlation between infants’ rib cage token variances and their vocal durations was .26, and the average squared correlation was .08, indicating that the rib cage token variances accounted for 8% of the variance in infants’ vocal durations.
In the 11-month group, abdomen token variances exhibited a significant linear association with vocal duration for PC1, F(1, 644) = 20.91, p < .0001; PC2, F(1, 644) = 38.24, p < .0001; and PC3, F(1, 644) = 65.32, p < .0001. A similar relation with vocal duration was identified for the rib cage, as rib cage token variances also exhibited a significant linear relationship with vocal duration for PC1, F(1, 644) = 37.41, p < .0001; PC2, F(1, 644) = 68.74, p < .001; and PC3, F(1, 644) = 87.58, p < .0001. As with the previous results, the fixed-effects coefficients for these tests were all positive, indicating that the amplitude of the PC movement patterns for both the abdomen and rib cage increased with increases in vocal duration. A linear mixed-effects model with the abdomen token variances for all three PCs revealed that abdomen token variances accounted for an average of 22% of the variance in infants’ vocal durations. The same model revealed that rib cage token variances accounted for 26% of the variance in vocal durations.
A comparison of the correlations between token variances and vocal durations in each age group was performed to determine whether the increased correlations observed in the 11-month group were significant. The average correlations of the fits of the abdomen and rib cage token variances for each infant were Fisher-z transformed, and a linear mixed-effects analysis was performed to test for the effects of age and chest wall structure (i.e., abdomen token variances and rib cage token variances) on infants’ correlations. This analysis identified a significant effect for age, F(1, 56) = 19.17, p < .001, but not for chest wall structure, F(1, 56) = 0.26, p < .61, or for the interaction between age and chest wall structure, F(1, 56) = 0.15, p < .70. The correlations in the 11-month group were significantly greater than those of the 7-month group by an average of .27.
The results of the same analysis on the six longitudinal infants were identical to those observed in the full set of infants. A significant main effect of age was observed, F(1, 20) = 18.63, p < .001, and revealed that the correlations in the 11-month group were significantly greater than those of the 7-month group by an average of .31. This analysis failed to identify a significant main effect for chest wall structure, F(1, 20) = 0.03, p < .86, or for the interaction between age and chest wall structure, F(1, 20) = 1.25, p < .28.
To evaluate whether changes in the durations of infants’ vocal productions might have contributed to the differences observed between the 7- and 11-month groups, the mean of each infant’s vocal durations (right side of Table 1) and the mean of the log-transformed durations were calculated, and group differences in these variables were evaluated using a linear mixed-effects model. This test failed to identify any significant differences in either the raw vocal durations of the two groups, F(1, 28) = 0.04, p = .84, or in the log-transformed durations, F(1, 28) = 1.02, p = .32. An analysis of the duration data from the 6 longitudinal infants also failed to identify a significant group difference in both the vocal durations, F(1, 10) = 0.05, p = .82, and the log-transformed durations, F(1, 10) = 0.26, p = .62. These findings suggested that age-related changes in the relationship between vocal durations and the token variances for the abdomen and rib cage could not be attributed to changes in the durations of vocalizations.
It should be noted that the failure to identify a difference in vocal durations between the two age groups may have been due to the criteria for inclusion of vocalizations. A large number of tokens were rejected because they were associated with excess movement artifact, which confounded the interpretability of the corresponding respiratory traces. It is also possible that restrictions on trunk and limb movements imposed in the current study acted as a bias against accepting longer-duration vocalizations, as there was probably greater chance of observing a confounding limb or trunk movement during a longer utterance. For these reasons, the failure to identify significant differences between the two age groups studied in this investigation should not be taken as conclusive evidence against such differences.
Discussion
PCA of abdomen and rib cage traces revealed distinct changes in the shapes of infants’ respiratory traces between the 7- and 11-month groups. These developmental changes were consistent with the hypothesized effects of decreases in the compliance of the chest wall on respiratory movements. In addition, significant increases in the correlation between respiratory movement patterns and vocal durations were observed, suggesting increased modulation of chest wall movements for productions of utterances of varying durations. Finally, differences between the 7- and 11-month groups could not be attributed to increases in the durations of infants’ vocalizations, as no difference was identified in this measure between the two age groups.
Only 3 PCs were required to account for greater than 95% of the total variance observed in infants’ abdomen and rib cage traces. The movement patterns described by each PC waveform varied systematically with PC number such that the fraction of variance and the fundamental period described the PC waveforms decreased with increases in PC number. The first PC represented a unidirectional movement pattern that was most often expiratory and accounted for most of the variance in respiratory traces such as those depicted in the bottom panel of Figure 1. The other two PCs represented modulation of either the speed or direction of the PC1 movement pattern. The variance accounted for by these PCs was larger in respiratory traces such as those depicted in the middle and top panels of Figure 1. The finding that the PC waveforms described fundamentally similar movement patterns across infants provided a quantitative model for testing hypotheses regarding age-related changes in the modulation of infants’ chest wall movements.
The Effects of Age on PC Fundamental Periods and Fractional Variances
Spectral analysis of infants’ PC waveforms revealed that their average fundamental period decreased by 200 ms between 7 and 11 months of age. A difference of 200 ms in fundamental period was regarded as large in this study, as the average vocal duration in each group was only 1,100 ms, and the age difference between the two groups was only 4 months. In addition, the 11-month group exhibited smaller fractional variances for PC1, which exhibited the longest fundamental period, and larger fractional variances for PC2 and PC3, which possessed shorter fundamental periods. Together, these findings indicated that modulation of chest wall movements was more prevalent and more rapid in the 11-month group compared with the 7-month group.
In addition, age-related changes in the subset of infants with longitudinal data were generally consistent with age-related differences observed in all infants. The only differences between these analyses involved pairwise comparisons between PC2 and PC3, which were not significant in the longitudinal infants. Both PC2 and PC3 described modulatory patterns of movement; the failure to observe differences between these two PCs would not weaken the general findings from the full set of infants. Rather, the comparisons of PC2 and PC3 with PC1 in the 6 longitudinal infants supported the findings observed in all infants: Respiratory modulation increased between 7 and 11 months of age.
These changes in the shapes of infants’ respiratory traces were consistent with the predicted effects of decreases in the compliance of the chest wall on infants’ respiratory movements. Early in life, the passive forces tending to collapse the lungs are approximately three times greater than the passive forces tending to expand the ribs (Papastamelos et al., 1995), and recruitment of inspiratory muscles during expiration assists in preventing atelectasis (Muller et al., 1979; Prechtl et al., 1977; Thach et al., 1980). Progressive mineralization of the ribs associated with crawling and sitting increases the passive inspiratory recoil of the ribs. Colin and colleagues (1989) observed that during the second half of the first year of life, infants transitioned from a dynamically maintained EEL to a passively determined EEL. This finding indicates large increases in the passive forces driving expansion of the rib cage that, in turn, reduce the involvement of inspiratory muscles during expiration. As their role in maintaining the integrity of lungs decreases, inspiratory muscle groups expand their role in vocalization and speech, entailing a greater range of movement patterns.
Active mechanisms relating to the effectiveness of the respiratory muscles to expand the rib cage may also have contributed to the present findings. With changes in the orientation of the ribs and increases in the zone of apposition, the movements of the rib cage become increasingly influenced by the muscular activity of the diaphragm and the external intercostals. These changes in the efficiency of muscular action on the rib cage can increase the rate and the amplitude at which this structure’s movements are modulated by the neuromuscular system.
The present findings are relevant to some developmental trends identified in previous studies of speech respiration. Moore and colleagues (2001) and Connaghan and colleagues (2004) observed increases in inspiratory movements of the rib cage (i.e., rib cage paradoxing) during speech expiration during the first years of life. Although it is not clear why rib cage paradoxing is so prevalent during speech development, the current findings suggested that biomechanical changes increasing the inspiratory forces of the rib cage may account for some of the observed increases in rib cage paradoxing during development. The age-related changes in PC fundamental periods and fractional variances observed in this study were also consistent with the increased number of expiratory subcycles observed by Wilder and Baken (1978) during cries over the first year of life. Unlike the findings of Wilder and Baken, the current findings cannot be attributed to changes in vocal duration, as such changes were not observed, but instead reflected more rapid modulation of chest wall movements in the 11-month group. As a result, the current findings suggested that differences in infants’ respiratory movement patterns reflected biomechanical changes to the chest wall that allowed for greater and more rapid modulation of abdomen and rib cage movements.
Because changes in the biomechanical properties of the chest wall during the second half of the first year largely involve factors influencing movements of the rib cage, it was possible that changes in the patterning of chest wall movements would be more evident in infants’ rib cage traces than in their abdomen traces. However, a comparison of age-related changes in abdomen versus rib cage movements failed to detect any differences. The failure to observe differential effects between the abdomen and the rib cage might have been expected if increases in the zone of apposition contributed to changes in chest wall compliance during this period. A larger zone of apposition increases the inspiratory action of both the crural and costal diaphragm on the rib cage (Loring & Mead, 1982; Mead & Loring, 1982). As a result, movements of the diaphragm–abdomen will have greater effects on rib cage movements and vice versa.
Respiratory Kinematics and Vocal Duration
Token variances for the abdomen and rib cage were analyzed to determine how infants’ respiratory movement patterns were related to vocal duration. In the 7-month group, abdomen and rib cage token variances each accounted for only 8% variance observed in infants’ vocal duration values. This finding suggested that the difference between chest wall movements associated with shorter utterances and those associated with longer utterances was quite small. Abdomen token variances for all three PCs co-varied with vocal duration, indicating that changes in abdomen movements with vocal duration, although not large, were quite stereotyped: The variance contributed by each PC was smaller for production of shorter utterances and larger for production of longer utterances. This was not the case for the rib cage, as the rib cage token variances for PC2 that exhibited a significant linear association with vocal duration were limited to PC2. This analysis produced the only differential findings between movements of the abdomen and rib cage in the current study. The failure to identify more stereotypic changes in rib cage movements with vocal duration raises the possibility that neuromuscular control of the rib cage may lag that of the abdomen for this aspect of vocal behaviors. Differences in neuromuscular control at this point in development may be due to the horizontal orientation of infant ribs, which limits the effects of rib cage muscles on rib cage movements (Openshaw et al., 1984). Alternatively, such a difference could result from the smaller zone of apposition in infants, which reduces the inspiratory action of the diaphragm on the rib cage (Devlieger et al., 1991).
The extent to which abdomen and rib cage movements predicted vocal duration increased significantly in the 11-month group. Abdomen and rib cage token variances accounted for 22% and 26% of the variance in infants’ vocal durations, respectively. In addition, rib cage token variances for all three PCs exhibited a linear association with vocal duration values, indicating that changes in the movements of the rib cage for utterances of different durations became more stereotyped between 7 and 11 months of age. As a result, infants in the 11-month group exhibited increases in the relative displacements of both abdomen and the rib cage with increases in vocal duration.
In general, these findings were consistent with the prediction that decreases in the compliance of the chest wall during this period of development would lead to greater control of respiratory movements by the developing neuromuscular system. The increase in the number of PCs with rib token variances co-varying with vocal duration may have resulted from changes in the orientation of the infant rib that increased the action of rib cage muscles on rib cage movements and/or increases in the zone of apposition that increased the mechanical coupling between diaphragm–abdomen movements and rib cage movements. Linguistic complexity may also have contributed to this finding. The period between 7 and 11 months of age is associated with the emergence of canonical babbling and, possibly, variegated babbling. As a result, it is quite likely that the utterances of the 11-month group contained a greater proportion of truly syllabic utterance forms. The emergence of these linguistic forms might have influenced the relationship between respiratory movement patterns and vocal duration in ways that were directly related to biomechanical factors.
The findings of the present study were consistent with the hypothesized effects on respiratory movements of developmental decreases in chest wall compliance. The current study was designed to investigate respiratory movements during a period of development associated with rapid decreases in the compliance of the chest wall. The time between observations of the two groups was just 4 months, which reduced the potential for large changes in other developing speech processes to also influence respiratory behaviors. However, the complex and interacting factors associated with speech development preclude conclusive statements regarding the contribution of other factors to the current findings. In particular, changes in speech complexity during this period may have entailed respiratory behaviors in ways that are unrelated to the biomechanical properties of the chest wall (Boliek et al., 1996, 1997). Analyses of phonetic and linguistic aspects of infants’ utterance can determine whether these changes in respiratory movement patterns were accompanied by changes in linguistic complexity and may also provide insight into the interaction of respiratory, articulatory, and linguistic factors during speech development. Future efforts will assess these combined effects of linguistic, articulatory, and respiratory development.
Acknowledgments
This work was supported by Grants T32 DC00033, R01 DC00822, and F31 DC00295 from the National Institute on Deafness and Other Communication Disorders; the Talaris Research Institute; and the Institute for Learning and Brain Sciences (formerly called The Center for Mind, Brain, and Learning) at the University of Washington. We are also grateful for the support that we received from the University of Washington, Boston University, and Northeastern University.
Contributor Information
Kevin J. Reilly, Northeastern University, Boston, MA
Christopher A. Moore, National Institute on Deafness and Other Communication Disorders, Bethesda, MD
References
- Agostini E. Volume-pressure relationships of the thorax and lung in the newborn. Journal of Applied Physiology. 1959;14:909–913. doi: 10.1152/jappl.1959.14.6.909. [DOI] [PubMed] [Google Scholar]
- Boliek CA, Hixon TJ, Watson PJ, Morgan WJ. Vocalization and breathing during the first year of life. Journal Voice. 1996;10:1–22. doi: 10.1016/s0892-1997(96)80015-4. [DOI] [PubMed] [Google Scholar]
- Boliek CA, Hixon TJ, Watson PJ, Morgan WJ. Vocalization and breathing during the second and third years of life. Journal of Voice. 1997;11:373–390. doi: 10.1016/s0892-1997(97)80033-1. [DOI] [PubMed] [Google Scholar]
- Borghese NA, Bianchi L, Lacquaniti F. Kinematic determinants of human locomotion. Journal of Physiology–London. 1996;494:863–879. doi: 10.1113/jphysiol.1996.sp021539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin AA, Wohl MEB, Mead J, Ratjen FA, Glass G, Stark AR. Transition from dynamically maintained to relaxed end-expiratory volume in human infants. Journal of Applied Physiology. 1989;67:2107–2111. doi: 10.1152/jappl.1989.67.5.2107. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown SH. Movement-related phasic muscle activation: II. Generation and functional role of the triphasic pattern. Journal of Neurophysiology. 1990;63:465–472. doi: 10.1152/jn.1990.63.3.465. [DOI] [PubMed] [Google Scholar]
- Connaghan KP, Moore CA, Higashakawa M. Respiratory kinematics during vocalization and nonspeech respiration in children from 9 to 48 months. Journal of Speech, Language, and Hearing Research. 2004;47:70–84. doi: 10.1044/1092-4388(2004/007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlieger H, Daniels H, Marchal G, Moerman P, Casaer P, Eggermont E. The diaphragm of the newborn infant—Anatomical and ultrasonographic studies. Journal of Developmental Physiology. 1991;16:321–329. [PubMed] [Google Scholar]
- Finnegan EM, Luschei ES, Hoffman HT. Modulations in respiratory and laryngeal activity associated with changes in vocal intensity during speech. Journal of Speech, Language, and Hearing Research. 2000;43:934–950. doi: 10.1044/jslhr.4304.934. [DOI] [PubMed] [Google Scholar]
- Gaultier C, Praud JP, Canet E, Delaperche MF, Dallest AM. Paradoxical inward rib cage motion during rapid eye movement sleep in infants and young children. Journal of Developmental Physiology. 1987;9:391–397. [PubMed] [Google Scholar]
- Gerhardt T, Bancalari E. Chest wall compliance in full term and premature infants. Acta Paediatrica Scandinavica. 1980;69:359–364. doi: 10.1111/j.1651-2227.1980.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Heldt GP, McIlroy MB. Distortion of chest wall and work of diaphragm in preterm infants. Journal of Applied Physiology. 1987;62:164–169. doi: 10.1152/jappl.1987.62.1.164. [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Read DJC. Reduced lung volume during behavioral active sleep in the newborn. Journal of Applied Physiology. 1979;46:1081–1085. doi: 10.1152/jappl.1979.46.6.1081. [DOI] [PubMed] [Google Scholar]
- Hershenson MB. The respiratory muscles and chest wall. In: Beckerman RC, Brouillette RT, Hunt CE, editors. Respiratory control disorders in infants and children. Baltimore: Williams & Wilkins; 1992. pp. 28–46. [Google Scholar]
- Hershenson MB, Colin AA, Wohl MEB, Stark AR. Changes in the contribution of the rib cage to tidal breathing during infancy. American Review of Respiratory Disease. 1990;141:922–925. doi: 10.1164/ajrccm/141.4_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- Hixon TJ. Respiratory function in speech. In: Minifie F, Hixon TJ, Williams F, editors. Normal aspects of speech, hearing, and language. Englewood Cliffs, NJ: Prentice-Hall; 1973. pp. 73–125. [Google Scholar]
- Hixon TJ, Goldman MD, Mead J. Kinematics of the chest wall during speech production: Volume displacement of the rib cage, abdomen, and lung. Journal of Speech and Hearing Research. 1973;16:78–115. doi: 10.1044/jshr.1601.78. [DOI] [PubMed] [Google Scholar]
- Huber JE, Stathopoulos ET. Respiratory and laryngeal responses to an oral air pressure bleed during speech. Journal of Speech, Language, and Hearing Research. 2003;46:1207–1220. doi: 10.1044/1092-4388(2003/094). [DOI] [PubMed] [Google Scholar]
- Kosch PC, Stark AR. Dynamic maintenance of end expiratory lung volume in full term infants. Journal of Applied Physiology. 1984;57:1126–1133. doi: 10.1152/jappl.1984.57.4.1126. [DOI] [PubMed] [Google Scholar]
- Loring SH, Mead J. Action of the diaphragm on the rib cage inferred from a force-balance analysis. Journal of Applied Physiology. 1982;53:756–760. doi: 10.1152/jappl.1982.53.3.756. [DOI] [PubMed] [Google Scholar]
- Mead J. Functional significance of the area of apposition of diaphragm to rib cage. American Review of Respiratory Disease. 1979;119:31–32. doi: 10.1164/arrd.1979.119.2P2.31. [DOI] [PubMed] [Google Scholar]
- Mead J, Loring SH. Analysis of volume displacement and length changes of the diaphragm during breathing. Journal of Applied Physiology. 1982;53:750–755. doi: 10.1152/jappl.1982.53.3.750. [DOI] [PubMed] [Google Scholar]
- Moore CA, Caulfield TJ, Green JR. Relative kinematics of the rib cage and abdomen during speech and nonspeech behaviors of 15-month-old children. Journal of Speech, Language, and Hearing Research. 2001;44:80–94. doi: 10.1044/1092-4388(2001/008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel RL. Task-specific organization of activity in human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Muller NL, Bryan AC. Chest wall mechanics and respiratory muscles in infants. Pediatric Clinics of North America. 1979;26:503–516. doi: 10.1016/s0031-3955(16)33745-2. [DOI] [PubMed] [Google Scholar]
- Muller N, Volgyesi G, Becker L, Bryan MH, Bryan AC. Diaphragmatic muscle tone. Journal of Applied Physiology. 1979;47:279–284. doi: 10.1152/jappl.1979.47.2.279. [DOI] [PubMed] [Google Scholar]
- Oller DK. The emergence of the sounds of speech in infancy. In: Yeni-Komshian GH, Ferguson CA, Kavanaugh J, editors. Child phonology: Production. New York: Academic Press; 1980. pp. 93–112. [Google Scholar]
- Openshaw P, Edwards S, Helms P. Changes in rib cage geometry during childhood. Thorax. 1984;39:624–627. doi: 10.1136/thx.39.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. Journal of Applied Physiology. 1995;78:179–184. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- Pascucci RC, Hershenson MB, Sethna NF, Loring SH, Stark AR. Chest-wall motion of infants during spinal-anesthesia. Journal of Applied Physiology. 1990;68:2087–2091. doi: 10.1152/jappl.1990.68.5.2087. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-Plus. New York: Springer-Verlag; 2000. [Google Scholar]
- Prechtl HFR, Vaneykern LA, O’Brien MJ. Respiratory muscle EMG in newborns: A non-intrusive method. Early Human Development. 1977;1:265–283. doi: 10.1016/0378-3782(77)90040-8. [DOI] [PubMed] [Google Scholar]
- Ramsay JO, Munhall KG, Gracco VL, Ostry DJ. Functional data analyses of lip motion. The Journal of the Acoustical Society of America. 1996;99:3718–3727. doi: 10.1121/1.414986. [DOI] [PubMed] [Google Scholar]
- Santello M, Flanders M, Soechting JF. Postural hand synergies for tool use. Journal of Neuroscience. 1998;18:10105–10115. doi: 10.1523/JNEUROSCI.18-23-10105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp JT, Druz WS, Balagot RC, Bandelin VR, Danon J. Total respiratory compliance in infants and children. Journal of Applied Physiology. 1970;29:775–779. doi: 10.1152/jappl.1970.29.6.775. [DOI] [PubMed] [Google Scholar]
- Soechting JF, Flanders M. Flexibility and repeatability of finger movements during typing: Analysis of multiple degrees of freedom. Journal of Computational Neuroscience. 1997;4:29–46. doi: 10.1023/a:1008812426305. [DOI] [PubMed] [Google Scholar]
- Stark R. Prespeech segmental feature development. In: Fletcher P, Garman M, editors. Language acquisition. New York: Cambridge University Press; 1986. pp. 149–173. [Google Scholar]
- Thach BT, Abroms IF, Frantz ID, Sotrel A, Bruce EN, Goldman MD. Intercostal muscle reflexes and sleep breathing patterns in the human infant. Journal of Applied Physiology. 1980;48:139–146. doi: 10.1152/jappl.1980.48.1.139. [DOI] [PubMed] [Google Scholar]
- Wilder CN, Baken RJ. Some developmental aspects of infants cry. Journal of Genetic Psychology. 1978;132:225–230. doi: 10.1080/00221325.1978.10533334. [DOI] [PubMed] [Google Scholar]
- Winkworth AL, Davis PJ, Ellis E, Adams RD. Variability and consistency in speech breathing during reading: Lung volumes, speech intensity, and linguistic factors. Journal of Speech and Hearing Research. 1994;37:535–556. doi: 10.1044/jshr.3703.535. [DOI] [PubMed] [Google Scholar]