Abstract
Background
Extensive neuromotor development occurs early in human life, but the time that a brain injury occurs during development has not been rigorously studied when quantifying motor impairments.
Objective
This study investigated the impact of timing of brain injury on magnitude and distribution of weakness in the paretic arm of individuals with childhood-onset hemiparesis.
Methods
Twenty-four individuals with hemiparesis were divided into time periods of injury before birth (PRE-natal, n=8), around the time of birth (PERI-natal, n=8) or after 6 months of age (POST-natal, n=8). They, along with 8 typically developing peers, participated in maximal isometric shoulder, elbow, wrist, and finger torque generation tasks using a multiple degree-of-freedom load cell to quantify torques in 10 directions. A mixed model ANOVA was used to determine the effect of group and task on a calculated relative weakness ratio between arms.
Results
There was a significant effect of both time of injury group (p<0.001) and joint torque direction (p<0.001) on the relative weakness of the paretic arm. Distal joints were more affected compared to proximal joints, especially in the POST-natal group.
Conclusions
The distribution of weakness provides evidence for the relative preservation of ipsilateral corticospinal motor pathways to the paretic limb in those individuals injured earlier, while those who sustained later injury may rely more on indirect ipsilateral cortico-bulbospinal projections during the generation of torques with the paretic arm.
Keywords: hemiplegia, childhood hemiplegia, childhood hemiparesis, arm weakness, isometric strength, cerebral palsy
Introduction
Muscle weakness has been widely observed following brain injury in children,1–3 however the distribution of weakness within the paretic limb and its relationship to the time of injury occurrence remains unknown. In this study, the distribution of weakness in the paretic arm is postulated to be dependent on the time of injury occurrence in immature brains due to profound changes that occur in the central nervous system during development. Childhood-onset hemiparesis (CH) is an ideal model for identifying the role of injury timing on neuromuscular output in childhood through young adulthood. Unique etiology of injury at different time-points (PRE-, PERI-, and POST-natal injuries) suggested by imaging studies have been related to generalized gross motor outcomes,4–8 but the impact of injury timing on strength at multiple joints has never been quantified.
During typical neural-motor system maturation, both contralaterally and ipsilaterally projecting corticospinal connections exist in fetal felines9 and rats.10 On a synaptic level, there is refinement of responses to neural activation11 due to strengthening of the most active synapses.9 During typical development, this refinement leads to reduction in ipsilateral corticospinal projections and maintenance and strengthening of contralateral corticospinal projections, which far outnumber the ipsilateral projections in adulthood. However, the activity-dependent nature of neural development is demonstrated by markedly changed distribution of corticospinal projections in cats and rats following unilateral brain injury, such that the undamaged cortex maintains typically transient ipsilateral corticospinal projections.9; 12
In humans, connectivity between the motor cortex and muscles of both upper extremities has been demonstrated at birth, proving that both contralateral and ipsilateral projections are in place. However, the strength of the ipsilateral responses decreases rapidly in early life13 and predominantly contralateral motor responses to cortical stimulation are found by early childhood. Some individuals in a subgroup of the CH population, hemiplegic cerebral palsy, also maintain fast conducting ipsilateral connectivity from the non-lesioned hemisphere to the paretic limb after the time when these type of responses would be typically seen.13 Therefore, it can be hypothesized that injury to the brain early in development inhibits ipsilateral pruning of the CS system, and subsequently preserves muscle activation throughout the paretic upper extremity, particularly at distal joints, due to a greater proportion of CS tract projections to distal muscle motoneurons.14–17 The corticomuscular connectivity studies of Eyre and colleagues18–20; 13 suggest that there may be retained ipsilateral corticospinal connectivity in both the PRE- and PERI-natal injury periods of the current study. However, the impact of apoptosis21 and myelination21; 22 during the third trimester of gestation was hypothesized to decrease the flexibility of the nervous system to respond to brain injury in the PERI-natal group. In the POST-natal group, when an injury occurs after 6–12 months of age, the ipsilateral CS connections are less likely to be available for control of the paretic arm. With respect to remaining neural resources, this group was theorized to instead rely on alternative control systems such as the reticulospinal (RS) tract for movement on the paretic side of their body. Projections from the RS pathway anatomically project to diffuse segments of the spinal cord motoneuron pools, particularly innervating proximal musculature, and to a lesser extent wrist and hand musculature23–25 in a diffuse fashion.17
In this study, all CH groups are expected to be weaker than their typically developing counterparts. Furthermore, it is postulated that the POST-natal group will demonstrate greater weakness than PRE- or PERI-natal groups, especially at the distal joints of the paretic arm and especially in extension due to innervation patterns of the RS system. The PRE-natal group is expected to have the least relative weakness throughout the paretic limb due to preserved CS influence (due to abnormally retained ipsilateral undamaged contralateral projections) on the paretic limb, resulting in the innervation of motoneuron pools activating even the more distal muscles like in the typically developing cohort. Finally, the PERI-natal group is expected to perform between the PRE- and POST-natal groups, with moderate levels of weakness, and somewhat greater distal involvement. To test these hypotheses, isometric torque generation ability was quantified at shoulder, elbow, wrist and finger joints of both upper extremities in three CH groups based on time of injury. The results confirm that timing of injury is crucial to the overall expression and distribution of weakness in the upper extremity, with different potential underlying neural mechanisms and clinical implications for timing groups.
Methods
Participants
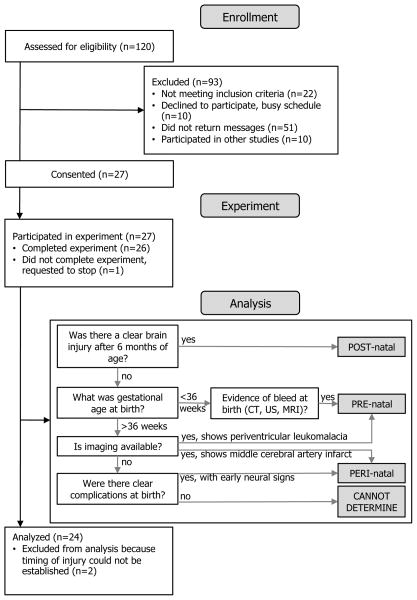
A modified CONSORT26 flow chart for the participants in this study can be found in Figure 1. A total of 120 possible childhood-onset hemiparesis (CH) candidates for the study were identified through the Cerebral Palsy Research Registry,27 local clinics, newspaper advertisements and parent support groups. After screening, 24 of these individuals were included in the final analysis. To be included, participants must have been at least 6 years of age and sustained a brain injury in the developing central nervous system that resulted in unilateral upper extremity motor impairment. Exclusion criteria included muscle tone abnormalities and motor/sensory impairment in the non-paretic limb, severe cognitive or affective dysfunction, significant concurrent medical conditions, and botulinum toxin injections to the upper extremity within 6 months of testing. Participants were divided into three groups (PRE-natal, PERI-natal, or POST-natal) based on the timing of their injury. PRE-natal was defined as timing between the late second and early third trimester of gestation (prior to approximately 34 weeks post-conceptual age,28 or 36 weeks gestational age), PERI-natal was defined from the late third trimester of gestation until 2 months following birth, and POST-natal was considered between 6 months and 10 years of age. Timing of injury was ascertained by medical record review and parent report using the decision matrix outlined in Figure 1. Those participants where the timing of injury could not be clearly ascertained (n=2) due to lack of imaging and unclear etiology were excluded from the study. Age-matched, right-hand dominant, typically developing participants with no history of neurological injury were recruited for comparison to the CH groups.
Figure 1.
Flow chart for subject inclusion and injury timing group assignment.
Twenty-four participants with clearly defined CH injuries (8 PRE-natal, 8 PERI-natal, and 8 POST-natal) and 8 typically developing (TD) controls completed the study. All participants, and their guardians as applicable, provided informed consent and/or assent prior to enrolling in the study, which was approved by the Institutional Review Board of Northwestern University. Table 1 contains demographic and clinical information for the 32 participants included in the analysis.
Table 1.
Participant characteristics
| Participant | Gender | Age (yrs) | P arm/ND arm | Clinical Assessments
|
||||
|---|---|---|---|---|---|---|---|---|
| GMFCS | MACS | FMA | QUEST (DM/G) | AH | ||||
| PRE-1 | F | 8.96 | R | I | II | 30 | 67.18/44.44 | 35 |
| PRE-2 | F | 9.27 | L | I | II | 45 | 82.81/74.07 | 33 |
| PRE-3 | M | 9.90 | L | I | II | 44 | 73.43/96.30 | 30 |
| PRE-4 | F | 11.26 | R | I | II | 37 | 73.50/37.04 | 39 |
| PRE-5 | M | 26.81 | R | I | III | 30 | 64.06/51.85 | 26a |
| PRE-6 | M | 9.48 | R | II | II | 49 | 88.37/59.26 | 28 |
| PRE-7 | M | 6.72 | R | III | III | 29 | 67.18/22.22 | 17 |
| PRE-8 | F | 6.14 | R | I | I | 60 | 100.0/96.30 | 36 |
| PERI-1 | F | 10.57 | R | I | II | 42 | 73.44/44.44 | 30 |
| PERI-2 | F | 11.99 | R | I | I | 63 | 95.31/85.18 | 31 |
| PERI-3 | M | 9.73 | R | II | II | 43 | 81.25/59.26 | 30 |
| PERI-4 | F | 9.16 | R | I | II | 38 | 50.00/51.85 | 29 |
| PERI-5 | F | 11.15 | L | I | I | 55 | 93.74/85.18 | 42 |
| PERI-6 | F | 7.63 | R | I | II | 42 | 78.12/66.67 | 25 |
| PERI-7 | M | 31.98 | R | I | III | 32 | 73.44/29.63 | 32a |
| PERI-8 | F | 12.52 | R | I | II | 37 | 79.68/66.67 | NA |
| POST-1 | M | 12.89 | R | I | III | 37 | 18.75/55.56 | 38 |
| POST-2 | M | 11.78 | L | I | III | 29 | 37.50/62.69 | 36 |
| POST-3 | F | 11.37 | L | II | III | 24 | 54.68/66.67 | 31 |
| POST-4 | M | 13.47 | R | II | II | 25 | 57.81/22.22 | 25 |
| POST-5 | M | 14.29 | L | II | I | 28 | 50.00/22.22 | 36 |
| POST-6 | M | 14.31 | L | II | II | 23 | 56.25/18.51 | 25 |
| POST-7 | M | 32.32 | R | II | I | 51 | 89.06/62.96 | 36 |
| POST-8 | M | 17.63 | L | II | I | 55 | 96.87/96.29 | 39 |
| TD-1 | M | 11.28 | L | |||||
| TD-2 | M | 10.50 | L | |||||
| TD-3 | F | 13.76 | L | |||||
| TD-4 | M | 10.85 | L | |||||
| TD-5 | F | 27.05 | L | |||||
| TD-6 | M | 12.69 | L | |||||
| TD-7 | M | 10.44 | L | |||||
| TD-8 | M | 11.75 | L | |||||
Abbreviations: P, paretic for CH timing groups; ND, nondominant for TD groups; CH, childhood-onset hemiparesis; TD, typically developing; GMFCS, Gross Motor Function Classification System; MACS, Manual Abilities Classification Scale; FMA, Fugl-Meyer Upper-Extremity Assessment; QUEST, Quality of Upper Extremity Skills Test; DM, dissociated movements section; G, grasp section; AH, ABILHAND -Kids; PRE, PRE-natal; PERI, PREI-natal; POST, POST-natal; F, female; M, male; R, right; L, left; NA, not available.
ABILHAND used instead of ABILHAND-Kids for age appropriateness of activities.
Protocol
Participants were seated comfortably in a Biodex experimental chair (Biodex, Inc Shirley, NY), as shown in Figure 2a, with straps across the shoulders and waist to minimize trunk movement. Participants’ forearms were placed in a fiberglass cast from just distal to the elbow to just proximal to the wrist in order to be secured comfortably and rigidly to a six-degrees-of-freedom load cell (JR3, Inc., Woodland, CA) with a shoulder abduction angle of 85°, shoulder horizontal adduction angle of 40°, and elbow flexion angle of 90° (Figure 2a). Forces and moments measured by the load cell at the radius/ulna connection were converted to torques at the elbow and shoulder using Jacobian matrices based on free body analysis of the upper limb.29 Participants’ hands and fingers were strapped into a custom finger and wrist force sensor30 (Figure 2b), the casings of which were each equipped with tension-compression load cells (LC201, Omega Engineering, Stamford, CT). Wrist and finger torques were determined based on calibration curves and distance from the sensor to joint centers.30
Figure 2.
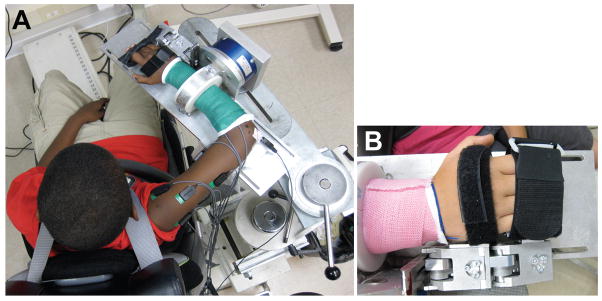
Experimental Setup. Subject was seated their arm placed in a fiberglass cast and connected rigidly to a 6 degree of freedom load cell (A). The arm was positioned such that the shoulder abduction angle was 85°, the shoulder horizontal adduction angle was 40°, and the elbow flexion angle was 90°. The subject’s hands and fingers were secured to a device to measure torques generated at the wrist and fingers (B). Placement of the finger and wrist sensors were made such that the center of rotation of the wrist was in line with the wrist sensor axis, and the center of rotation of the 2nd and 4th metacarpalphalangeal joints were in line with the axis of rotation of the finger sensor.
Each participant was asked to generate an isometric maximum voluntary torque in one of 10 randomly ordered primary directions during a 5-second trial. Primary directions included shoulder abduction (SB), shoulder adduction (SD), shoulder horizontal adduction termed here shoulder flexion (SF), shoulder horizontal abduction or shoulder extension (SE), elbow flexion (EF), elbow extension (EE), wrist flexion (WF), wrist extension (WE), finger flexion (FF), and finger extension (FE). At least 3 trials within 10% of the maximum were collected in each primary direction, the last of which could not be the greatest, to remove any possible impact of a learning curve. Rest periods were provided between trials and extended upon participant request to reduce the effects of fatigue. The participant started each trial in a relaxed state and received visual and verbal feedback to optimize performance.
For each trial, the joint torque data were filtered using a 250-ms moving average filter and the maximum torque in the primary direction was identified. The maximal value across all trials of an intended primary torque direction was recorded as the maximum voluntary torque. In order to compare across individuals, a relative weakness ratio was computed for each primary torque direction by dividing the difference between maximum voluntary torques on the paretic side and non-paretic sides by sum of the two maximum voluntary torques. A relative weakness ratio of zero indicates equivalent strength in both arms, and a relative weakness ratio greater than zero indicates greater strength on the non-paretic (or dominant in typically developing participants) side.
Several clinical assessments were used to compare upper extremity function and strength across injury timing groups. Motor function was classified clinically using the Gross Motor Function Classification System – Expanded and Revised (GMFCS-ER)31; 32 and the Manual Abilities Classification Scale (MACS).33 Evaluation of motor function in the upper extremity was completed using the Quality of Upper Extremity Skills Test (QUEST),34 the Fugl-Meyer Motor Assessment (FMA),35; 36 and 3 maximal grip strength trials (Jamar Hydraulic Hand Dynamometer, B&L Engineering, Tustin, CA).37 Parent-reported outcome measures of function were assessed using the ABILHAND-Kids instrument38 or the ABILHAND instrument39 for participants over 18 years old.
Statistics
Demographic differences between PRE-natal, PERI-natal, POST-natal and typically developing groups were assessed using a one-way analyses of variance (ANOVA) for age and Fisher’s exact tests for the binary variable of handedness. Clinical score differences were assessed using one-way ANOVAs with a factor of group for each of the following dependent variables: Fugl-Meyer score, GMFCS level, MACS level, QUEST dissociated movement section, QUEST grasp section, ABILHAND score. Grip relative weakness ratio differences were also tested with a one-way ANOVA with a 5 level factor of group, which includes a group of 8 adults following a hemiparetic stroke, published elsewhere.40
A mixed model ANOVA was performed to ascertain the effect of independent variables of group (between-subject, 4 levels) and primary torque direction, or task, (within-subject, 10 levels) on the dependent variable of relative weakness ratio. Bonferroni-corrected pairwise comparisons were made on the main effects.
To further elucidate the interaction between a joint’s distance from the trunk (shoulder, elbow, wrist, and finger joints each progressively further from the trunk) and the level of relative weakness, categorical linear regression analyses were completed in the flexion synergy (SB, EF, WF, FF) and extension synergy (SD, EE, WE, FE) muscle groups for each participant. For each set of muscle group synergies, four multiple linear regressions were completed. An ordinal predictor of joint and a nominal predictor of group was used on the dependent variable of relative weakness ratio, with full entry regression. Indicator variables were used for the 4-level categorical predictor of group to compare each of the groups (PRE, PERI, POST, and TD) with one another.
Statistical analysis was completed using SPSS software (SPSS, Inc. version 19.0). A p-value of ≤ 0.05 was considered statistically significant for all tests.
Results
Eight PRE-natal, 8 PERI-natal, 8 POST-natal, and 8 typically developing (TD) participants were included in the analysis. No significant difference was detected for age between the four groups of participants (F=0.723, p=0.547), and no significant differences were found in the side of paretic arms between the 3 childhood-onset hemiparesis (CH) groups.
The overall clinical presentation of individuals in each of the study groups was similar. No significant differences were found between groups of CH for clinical scores of GMFCS (F=2.660, p=0.093), MACS (F=0.223, p=0.802), Fugl-Meyer (F=1.619, p=0.222), QUEST dissociated movements section (F=3.174, p=0.062), QUEST grasp section (F=0.410 p=0.669), or ABILHAND (F=0.978, p=0.392). There was a significant difference in grip relative weakness ratio across the CH groups (F=13.541, p<0.001).
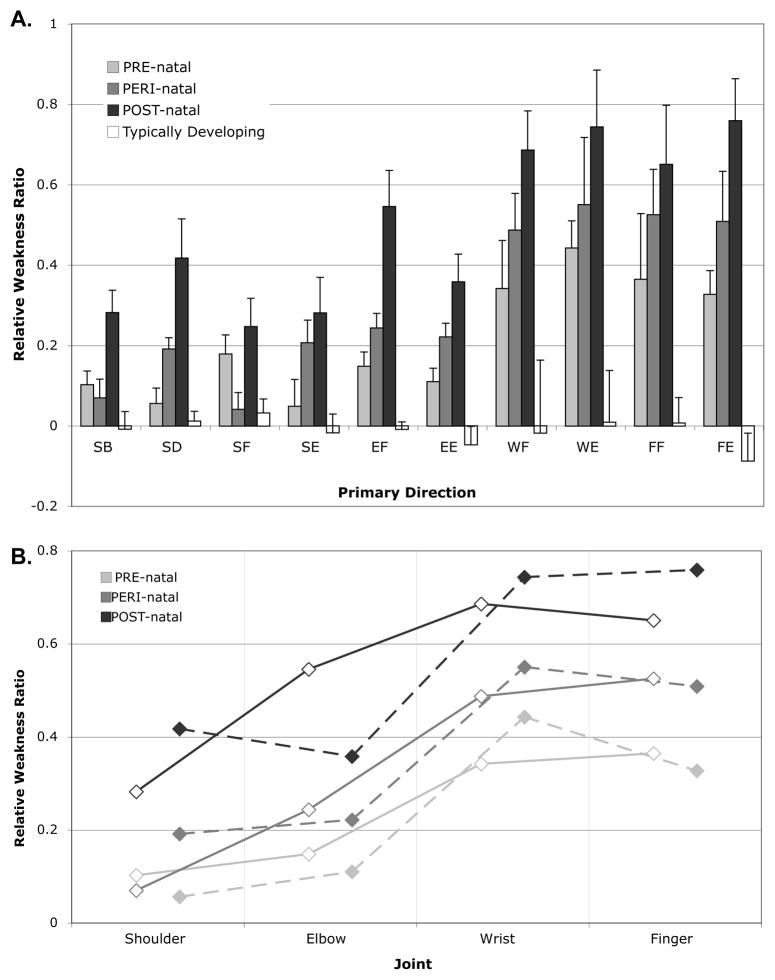
Group results for the 10 primary torque directions, or tasks, showed differences in relative weakness ratio between PRE-, PERI- and POST- natal groups (Figure 3a). The mixed model ANOVA for relative strength ratio revealed significant effects of timing group (F=16.925, p<0.001), task (F=15.614, p<0.001), and the group-by-task interaction (F=2.804, p<0.001). Post hoc testing of timing group revealed significant differences between PRE-natal and POST-natal (p=0.003), PRE-natal and TD (p=0.029), PERI-natal and TD (p=0.001), and POST-natal and TD (p<0.001). Post hoc testing of primary torque direction, or task, did not show significant differences between shoulder and elbow tasks. There were significant differences in comparisons between all shoulder tasks (SB, SD, SF, and SE) and all distal tasks (WF, WE, FF, FE) at a p≤0.001 level. Differences between EE and distal tasks were all significant at p<0.001, while EF was significantly different from WE and FF (p=0.001 and 0.028, respectively). All other comparisons did not reach significance.
Figure 3.
A. Relative weakness ratios in each of 10 primary task directions, including shoulder abduction (SB), shoulder adduction (SD), shoulder flexion (SF), shoulder extension (SE), elbow flexion (EF), elbow extension (EE), wrist flexion (WF), wrist extension (WE), finger flexion (FF), and finger extension (FE). The relative weakness ratio was calculated as the difference between maximal torques between arms divided by the sum. A value close to 0 indicates equivalent strength in the paretic and non-paretic, or dominant and non-dominant limbs, while a value closer to 1 indicates a larger non-paretic torque than paretic. Standard error bars are shown. There is a significant effect of group, task, and the group-by-task interaction (p<0.001 for all). B. Line graph of the average values for tasks used in the flexion synergy (AB, EF, WF, and FF degrees-of-freedom, solid line, open diamonds) and extension synergy (AD, EE, WE, FE degrees-of-freedom, dashed line, filled diamonds) regression analyses. There were significant effects of group in both analyses (p<0.001).
The multiple linear regression analyses further explained the nature of the interaction effect between group and primary torque direction, demonstrating significant differences in the gradient of weakness from proximal to distal joints in both regression models (flexion regression F=25.628, p<0.001; extension regression F=28.088, p<0.001). A summary of these regressions is shown in Figure 3b. As enumerated in Table 2, pairwise coefficient comparisons from each indicator variable demonstrate differences proximal to distal gradients for nearly all comparisons. The TD group showed a significant negative slope, due to the non-dominant arm being stronger distally than the dominant arm on average. The beta for the TD group was negative compared to all other groups. The PRE-natal group had a lower regression coefficient than PERI-natal group in the extension synergy regression, and lower than the POST-natal group in both regressions. The POST-natal group had positive betas compared to all other groups for both the flexion and extension synergy regressions.
Table 2.
Regression Results
| Flexion synergy regression (shoulder abduction, elbow flexion, wrist flexion, finger flexion) | |||||
|---|---|---|---|---|---|
| Indicator Variable | Constant | Comparison With
|
|||
| PRE-natal | PERI-natal | POST-natal | TD | ||
| PRE-natal | −0.005; p=0.945 | N/A | β=0.093; p=0.140 | β=0.304; p<0.001 | β=−0.244; p<0.001 |
| PERI-natal | 0.089; p=0.182 | β=−0.093; p=0.140 | N/A | β=0.211; p=0.001 | β=−0.338; p<0.001 |
| POST-natal | 0.300; p<0.001 | β=−0.304; p<0.001 | β=−0.211; p=0.001 | N/A | β=−0.548; p<0.001 |
| Typically Developing | −0.249; p<0.001 | β=0.244; p<0.001 | β=0.338; p<0.001 | β=0.548; p<0.001 | N/A |
| Extension synergy regression (shoulder adduction, elbow extension, wrist extension, finger extension) | |||||
|---|---|---|---|---|---|
| Indicator Variable | Constant | Comparison with
|
|||
| PRE-natal | PERI-natal | POST-natal | TD | ||
| PRE-natal | 0.007; p=0.917 | N/A | β=0.138; p=0.033 | β=0.338; p<0.001 | β=−0.258; p<0.001 |
| PERI-natal | 0.145; p=0.033 | β=−0.138; p=0.033 | N/A | β=0.200; p=0.002 | β=−0.396; p<0.001 |
| POST-natal | 0.345; p<0.001 | β=−0.338; p<0.001 | β=−0.200; p=0.002 | N/A | β=−0.596; p<0.001 |
| Typically Developing | −0.251; p<0.001 | β=0.258; p<0.001 | β=0.396; p<0.001 | β=0.596; p<0.001 | N/A |
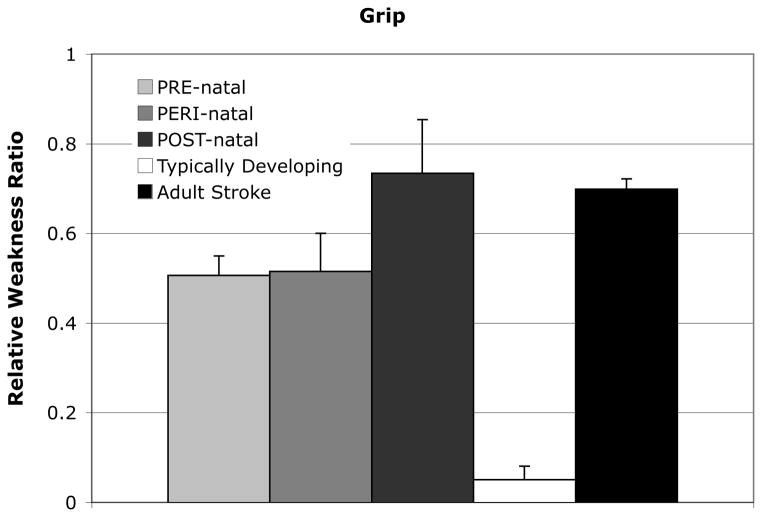
When extending the comparison to adults following a stroke, there was a main effect of group for grip strength relative weakness ratio (F=2.274, p<0.001), shown in Figure 4.
Figure 4.
Relative weakness ratio for grip in 3 CH injury timing groups, typically developing participants, and participants with an adult-onset stroke resulting in hemiparesis. The relative weakness ratio was calculated as the difference between maximal torques between arms divided by the sum. A value close to 0 indicates equivalent strength in the paretic and non-paretic, or dominant and non-dominant limbs, while a value closer to 1 indicates a larger non-paretic torque than paretic. Standard error bars are shown. There was a significant effect of group (F=2.274, p<0.001) in a one-way ANOVA. Adult stroke data published elsewhere.40
Discussion
Brain injuries early in life are likely to have a fundamental impact on subsequent neural and musculoskeletal development, but few motor studies have addressed or controlled for the timing of injury onset in childhood-onset hemiparesis (CH) despite the demonstrated impact on gross anatomy of motor pathways.4–7 Depending on the state of development of the nervous system when brain injuries occur, different avenues for neural re-organization are available. Maximal voluntary torque generation was measured for 10 tasks in the upper extremity, which included both proximal and distal joints. The results of this study demonstrate that the timing of brain injury in CH does have a significant impact on the distribution of weakness in the upper extremity. Three timing of injury groups were defined in this study based on important milestones of the motor system during typical development. Although the groups in this study were small, relative weakness of the paretic arm was found to be greater in distal joints compared to proximal joints in all groups, and most especially in the POST-natal group. The explanation for the relationship between relative paretic arm weakness and timing of injury likely lies in the sequence of typical neuromotor development, and the state of the nervous system at the time of injury.
Neuromotor development and impact on the distribution of upper extremity weakness
In human development, the second trimester of gestation is characterized by neuronal multiplication followed by myelination of axons starting at approximately 28 weeks,41 with the posterior limb of the internal capsule becoming largely myelinated at 33 weeks.42 Direct histological evidence from felines9 and rats10 demonstrates bilateral corticospinal projections from each of the cortical hemispheres during a comparable period of gestation. Retrograde tracing has shown ipsilateral corticospinal projections43 at birth in the monkey, with terminations including intrinsic distal muscles used for fine motor control in older animals.44 Similar histological studies are not possible in typically developing humans, but immediately following birth short latency motor evoked potentials, indicative of the corticospinal system,20; 13 have been recorded from each cortical hemisphere to muscles in both arms. Over the first months of life, there is rapid differential development between increasing contralateral and reduced ipsilateral corticospinal projections to the hand.18; 20; 13 This is an example of activity-dependent shaping of the nervous system from an early age,45 a process which leads to pruning of under-utilized synaptic connections and eventually axons. Crossed corticospinal pathways become more dominant in their connections onto spinal motor circuits9 concurrent with continued rapid myelination of axons.41 Higher level skill acquisition throughout childhood and adolescence is indirect evidence of further motor system refinement, including continued myelination albeit at a slower rate46 and increasing coherence of low frequency oscillations between the brain and distal limb muscles.47 Coherence reaches adulthood levels of prevalence and magnitude in the 15–35 Hz beta range by approximately 12–15 years of age,47 at which time motor development can be considered to have reached adult levels. In each group of the current study, there is one adult who could be considered to be at an age of mature motor development.
The interruption of typical early neural development with a unilateral brain injury has a profound impact on subsequent neural-motor development. There is evidence20; 13 to suggest that contralesional (ispilateral to the paretic limb) corticospinal connections from the non-damaged hemisphere are maintained well after the typical period of withdrawal when the injury occurs before approximately 6 months of age. Lower levels of relative weakness especially in the distal joints of the PRE-natal and PERI-natal groups found in this study are also consistent with utilization of the preserved contralesional corticospinal tracts, given corticospinal distributions and specialized innervation of the hand muscles, particularly wrist/finger extensors. Although there was not a significant post-hoc difference found between the PRE-natal and PERI-natal groups in the ANOVA with a conservative Bonferroni correction, regression analysis demonstrates a significant difference in the proximal to distal gradient when comparing the extensor muscles between the two groups, where PERI-natal becomes more weak in distal joints. This could be reflective of myelination during the third trimester strengthening more active corticospinal tract axons48 or limited apoptosis having occurred before injury in the PRE-natal group,49 but appears to indicate less effective innervations of the extensor muscles.
With unilateral brain injury occurring after pruning of the ipsilateral corticospinal projections such as in the POST-natal group, neural re-organization may involve remaining indirect contralesional pathways, similar to stroke occurring in adulthood.50; 51 A possible alternative means of neural re-organization is through the reticulospinal pathway. Shoulder abductors, wrist/elbow flexors,25 and intrinsic hand muscles24 have been activated from the reticular formation in the macaque. Our results support this hypothesis in that relatively higher levels of weakness are observed across all joints in POST-natal compared to PRE-/PERI-natal groups. In addition, regression comparisons with other timing groups showed the muscles activated by the reticulospinal tract (flexor regression – SB, EF, WF, FF) were less weak than those without demonstrated reticulospinal innervation (extensor regression – SD, EE, WE, FE). This is consistent with described innervation of the reticulospinal system, specifically diffuse proximal innervation, limited distal influence,23 and more flexor than extensor innervation.52 Previous studies investigating upper extremity isometric strength following stroke in adulthood did not find a difference in residual weakness between shoulder and elbow either,53 but reported more pronounced hand and wrist weakness,54 comparable to the POST-natal group in this study (Figure 4). These similarities indicate that findings in adult stroke may be relevant to the POST-natal CH population, but further study is warranted in the younger group to fully explore the potential impact of development.
Clinical implications
Most clinical assessments of impairment were unable to differentiate between the three CH timing groups. This study was not intended to elucidate differences in a total population, and the chance that our cohorts were statistically similar in low resolution clinical exams requiring submaximal efforts demonstrates the advantage of quantitative measures such as ones used in this study. Weakness is one aspect of impairment, and was not directly tested in the selected motor examinations with the exception of the grip strength test, which did demonstrate significant differences between groups. Of note is the statistical trend found in the dissociated movements section of the QUEST, with lower scores found in the POST-natal group. The QUEST includes assessments of abnormal movement synergies and the lower scores may indicate the presence of a loss in independent joint control in the POST-natal group. With the use of a quantitative measure with high resolution and accuracy, significant differences in the distribution of weakness were uncovered between groups of different injury timing.
There are important implications for therapeutic intervention in the injury timing groups. In the context of the current study, it could be inferred that targeting remnant corticospinal tracts would be beneficial in the POST-natal group, potentially in ways that have been described in the adult stroke literature.55 Individuals with remaining direct contralesional corticospinal projections such as the PRE-natal group are likely to benefit from different types of intervention, as their primary deficits may include high levels of obligatory mirror movements during unilateral and bilateral tasks.56 A greater understanding of mechanisms underlying neurological impairments and associated neural plasticity is required to guide the selection of appropriate, targeted interventions for each of the three CH timing groups. Lack of imaging data for every participant in this study limits our ability to draw correlations between lesion morphology and outcomes. However, it has been previously shown that lesion size is not well related to function.57
Conclusion
In summary, studies conducted to date have shown either differences in etiology based on time of injury, or grouped all children with CH together in an effort to understand complicated altered movement patterns.58–61 This study has shown, for the first time, that the distribution of weakness in the upper limb is affected by the timing of brain injury during early neural development and suggests a neuroanatomical explanation. Targeted interventions throughout the lifespan are the best opportunity for clinicians to improve motor performance in CH, and understanding the underlying neural mechanisms facilitates development of more effective training paradigms.
Acknowledgments
We thank most especially the participants and their families. Donna Hurley, DPT, and Isabel Aguilar played vital roles in participant recruitment. Rachel Hawe, BS assisted with data collection. Alexis Kuncel, PhD, Laura Miller, PhD Candidate, Jacob McPherson, PhD and Michael Ellis, DPT provided helpful comments on the manuscript. This work was supported by the National Institutes of Health [5R01NS058667-02 and T32EB009406 to J.D.]; and the National Science Foundation [graduate student fellowship to T.M.].
References
- 1.Mockford M, Caulton JM. The pathophysiological basis of weakness in children with cerebral palsy. Pediatr Phys Ther. 2010;22:222–33. doi: 10.1097/PEP.0b013e3181dbaf96. [DOI] [PubMed] [Google Scholar]
- 2.Damiano DL, Martellotta TL, Sullivan DJ, Granata KP, Abel MF. Muscle force production and functional performance in spastic cerebral palsy: relationship of cocontraction. Arch Phys Med Rehabil. 2000;81:895–900. doi: 10.1053/apmr.2000.5579. [DOI] [PubMed] [Google Scholar]
- 3.Foran JR, Steinman S, Barash I, Chambers HG, Lieber RL. Structural and mechanical alterations in spastic skeletal muscle. Dev Med Child Neurol. 2005;47:713–7. doi: 10.1017/S0012162205001465. [DOI] [PubMed] [Google Scholar]
- 4.Carr LJ. Development and reorganization of descending motor pathways in children with hemiplegic cerebral palsy. Acta Paediatr Suppl. 1996;416:53–7. doi: 10.1111/j.1651-2227.1996.tb14278.x. [DOI] [PubMed] [Google Scholar]
- 5.Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56:854–63. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- 6.Hoon AH., Jr Neuroimaging in cerebral palsy: Patterns of brain dysgenesis and injury. J Child Neurol. 2005;20:936–9. doi: 10.1177/08830738050200120201. [DOI] [PubMed] [Google Scholar]
- 7.Feys H, Eyssen M, Jaspers E, Klingels K, Desloovere K, Molenaers G, De Cock P. Relation between neuroradiological findings and upper limb function in hemiplegic cerebral palsy. Eur J Paediatr Neurol. 2009 doi: 10.1016/j.ejpn.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Himmelmann K, Uvebrant P. Function and neuroimaging in cerebral palsy: a population-based study. Dev Med Child Neurol. 2011;53:516–21. doi: 10.1111/j.1469-8749.2011.03932.x. [DOI] [PubMed] [Google Scholar]
- 9.Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–73. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 10.Stanfield BB. The development of the corticospinal projection. Prog Neurobiol. 1992;38:169–202. doi: 10.1016/0301-0082(92)90039-h. [DOI] [PubMed] [Google Scholar]
- 11.Meng Z, Martin JH. Postnatal development of corticospinal postsynaptic action. J Neurophysiol. 2003;90:683–92. doi: 10.1152/jn.00152.2003. [DOI] [PubMed] [Google Scholar]
- 12.Martin JH, Friel KM, Salimi I, Chakrabarty S. Activity- and use-dependent plasticity of the developing corticospinal system. Neurosci Biobehav Rev. 2007;31:1125–35. doi: 10.1016/j.neubiorev.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 14.Kuypers HGJM. The descending pathways to the spinal cord, their anatomy and function. In: Eccles JC, Schade JP, editors. Organization of the spinal cord. Progress in brain research. Amsterdam: Elsevier; 1964. [DOI] [PubMed] [Google Scholar]
- 15.Asanuma H, Zarzecki P, Jankowska E, Hongo T, Marcus S. Projection of individual pyramidal tract neurons to lumbar motor nuclei of the monkey. Exp Brain Res. 1979;34:73–89. doi: 10.1007/BF00238342. [DOI] [PubMed] [Google Scholar]
- 16.Shinoda Y, Zarzecki P, Asanuma H. Spinal branching of pyramidal tract neurons in the monkey. Exp Brain Res. 1979;34:59–72. doi: 10.1007/BF00238341. [DOI] [PubMed] [Google Scholar]
- 17.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 18.Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31:1136–49. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C. Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain. 2000;123 (Pt 1):51–64. doi: 10.1093/brain/123.1.51. [DOI] [PubMed] [Google Scholar]
- 20.Eyre JA, Smith M, Dabydeen L, Clowry GJ, Petacchi E, Battini R, Guzzetta A, Cioni G. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- 21.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 22.ten Donkelaar HJ, Lammens M, Wesseling P, Hori A, Keyser A, Rotteveel J. Development and malformations of the human pyramidal tract. J Neurol. 2004;251:1429–42. doi: 10.1007/s00415-004-0653-3. [DOI] [PubMed] [Google Scholar]
- 23.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–9. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker SN. The Primate Reticulospinal Tract, Hand Function and Functional Recovery. J Physiol. 2011 doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbert WJ, Davidson AG, Buford JA. Measuring the motor output of the pontomedullary reticular formation in the monkey: do stimulus-triggered averaging and stimulus trains produce comparable results in the upper limbs? Exp Brain Res. 2010;203:271–83. doi: 10.1007/s00221-010-2231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurley DS, Sukal-Moulton T, Msall ME, Gaebler-Spira D, Krosschell KJ, Dewald JP. The Cerebral Palsy Research Registry: Development and Progress Toward National Collaboration in the United States. J Child Neurol. 2011 doi: 10.1177/0883073811408903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folkerth RD. Neuropathologic substrate of cerebral palsy. J Child Neurol. 2005;20:940–9. doi: 10.1177/08830738050200120301. [DOI] [PubMed] [Google Scholar]
- 29.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–83. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Stienen AH, Sukal-Moulton T, Miller LC, Dewald J. ICORR. Zurich, Switzerland: 2011. Wrist and Finger Torque Sensor for the Quantification of Upper Limb Motor Impairments Following Brain Injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum PL, Palisano RJ, Bartlett DJ, Galuppi BE, Russell DJ. Development of the Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol. 2008;50:249–53. doi: 10.1111/j.1469-8749.2008.02045.x. [DOI] [PubMed] [Google Scholar]
- 33.Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, Rosenbaum P. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48:549–54. doi: 10.1017/S0012162206001162. [DOI] [PubMed] [Google Scholar]
- 34.DeMatteo C, Law M, Russell D, Pollock N, Rosenbaum P, Walter S. QUEST: Quality of Upper Extremity Skills Test. Hamilton, ON: McMaster University, Neurodevelopmental Clinical Research Unit; 1992. [Google Scholar]
- 35.Fasoli SE, Fragala-Pinkham M, Haley S. Fugl-Meyer Assessment: Reliability for Children with Hemiplegia. ACRM-ASNR Joint Educational Conference.2009. [Google Scholar]
- 36.de Bode S, Firestine A, Mathern GW, Dobkin B. Residual motor control and cortical representations of function following hemispherectomy: effects of etiology. J Child Neurol. 2005;20:64–75. doi: 10.1177/08830738050200011101. [DOI] [PubMed] [Google Scholar]
- 37.Molenaar HM, Selles RW, Zuidam JM, Willemsen SP, Stam HJ, Hovius SE. Growth diagrams for grip strength in children. Clin Orthop Relat Res. 468:217–23. doi: 10.1007/s11999-009-0881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND-Kids: a measure of manual ability in children with cerebral palsy. Neurology. 2004;63:1045–52. doi: 10.1212/01.wnl.0000138423.77640.37. [DOI] [PubMed] [Google Scholar]
- 39.Penta M, Tesio L, Arnould C, Zancan A, Thonnard JL. The ABILHAND questionnaire as a measure of manual ability in chronic stroke patients: Rasch-based validation and relationship to upper limb impairment. Stroke. 2001;32:1627–34. doi: 10.1161/01.str.32.7.1627. [DOI] [PubMed] [Google Scholar]
- 40.Miller LC, Dewald J. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinney HC. Human myelination and perinatal white matter disorders. J Neurol Sci. 2005;228:190–2. doi: 10.1016/j.jns.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Fogliarini C, Chaumoitre K, Chapon F, Fernandez C, Levrier O, Figarella-Branger D, Girard N. Assessment of cortical maturation with prenatal MRI. Part I: Normal cortical maturation. Eur Radiol. 2005;15:1671–85. doi: 10.1007/s00330-005-2782-1. [DOI] [PubMed] [Google Scholar]
- 43.Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cereb Cortex. 1995;5:518–40. doi: 10.1093/cercor/5.6.518. [DOI] [PubMed] [Google Scholar]
- 44.Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, Tuszynski MH. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473:147–61. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- 45.Personius KE, Balice-Gordon RJ. Activity-dependent editing of neuromuscular synaptic connections. Brain Res Bull. 2000;53:513–22. doi: 10.1016/s0361-9230(00)00384-1. [DOI] [PubMed] [Google Scholar]
- 46.Ullen F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biol. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- 47.James LM, Halliday DM, Stephens JA, Farmer SF. On the development of human corticospinal oscillations: age-related changes in EEG-EMG coherence and cumulant. Eur J Neurosci. 2008;27:3369–79. doi: 10.1111/j.1460-9568.2008.06277.x. [DOI] [PubMed] [Google Scholar]
- 48.Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 2009;3:5. doi: 10.3389/neuro.05.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134:2197–221. doi: 10.1093/brain/awr103. [DOI] [PubMed] [Google Scholar]
- 50.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118 (Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 51.Schwerin S, Dewald JP, Haztl M, Jovanovich S, Nickeas M, MacKinnon C. Ipsilateral versus contralateral cortical motor projections to a shoulder adductor in chronic hemiparetic stroke: implications for the expression of arm synergies. Exp Brain Res. 2008;185:509–19. doi: 10.1007/s00221-007-1169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27:8053–8. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 1999;80:766–72. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- 54.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112 (Pt 3):749–63. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- 55.Ellis MD, Sukal-Moulton T, Dewald JP. Progressive shoulder abduction loading is a crucial element of arm rehabilitation in chronic stroke. Neurorehabil Neural Repair. 2009;23:862–9. doi: 10.1177/1545968309332927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sukal-Moulton T, Murray TM, Dewald JP. Loss of independent limb control in childhood hemiparesis is related to time of brain injury onset. Exp Brain Res. 2013;225:455–63. doi: 10.1007/s00221-012-3385-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Msall ME, Limperopoulos C, Park JJ. Neuroimaging and cerebral palsy in children. Minerva Pediatr. 2009;61:415–24. [PubMed] [Google Scholar]
- 58.Jaspers E, Desloovere K, Bruyninckx H, Molenaers G, Klingels K, Feys H. Review of quantitative measurements of upper limb movements in hemiplegic cerebral palsy. Gait Posture. 2009 doi: 10.1016/j.gaitpost.2009.07.110. [DOI] [PubMed] [Google Scholar]
- 59.Domellof E, Rosblad B, Ronnqvist L. Impairment severity selectively affects the control of proximal and distal components of reaching movements in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2009;51:807–16. doi: 10.1111/j.1469-8749.2008.03215.x. [DOI] [PubMed] [Google Scholar]
- 60.Ronnqvist L, Rosblad B. Kinematic analysis of unimanual reaching and grasping movements in children with hemiplegic cerebral palsy. Clin Biomech (Bristol, Avon) 2007;22:165–75. doi: 10.1016/j.clinbiomech.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Coluccini M, Maini ES, Martelloni C, Sgandurra G, Cioni G. Kinematic characterization of functional reach to grasp in normal and in motor disabled children. Gait Posture. 2007;25:493–501. doi: 10.1016/j.gaitpost.2006.12.015. [DOI] [PubMed] [Google Scholar]