Abstract
Background
Extensive neuromotor development occurs early in human life, and the timing of brain injury may affect the resulting motor impairment. In part I of this paper series it was demonstrated that the distribution of weakness in the upper extremity depended on the timing of brain injury in individuals with childhood-onset hemiparesis.
Objective
The goal of this study was to characterize how timing of brain injury impacts joint torque synergies, or losses of independent joint control.
Method
Twenty-four individuals with hemiparesis were divided into three groups based on the timing of their injury: before birth (PRE-natal, n=8), around the time of birth (PERI-natal, n=8) and after 6 months of age (POST-natal, n=8). Individuals with hemiparesis, as well as 8 typically developing peers participated in maximal isometric shoulder, elbow, wrist, and finger torque generation tasks while their efforts were recorded by a multiple degree-of-freedom load cell. Motor output in 4 joints of the upper extremity were concurrently measured during 8 primary torque generation tasks to quantify joint torque synergies.
Results
There were a number of significant coupling patterns identified in individuals with hemiparesis that differed from the typically developing group. POST-natal differences were most noted in the coupling of shoulder abductors with elbow, wrist, and finger flexors, while the PRE-natal group demonstrated significant distal joint coupling with elbow flexion.
Conclusion
The torque synergies measured provide indirect evidence for the use of bulbospinal pathways in the POST-natal group, while those with earlier injury may utilize relatively preserved ipsilateral corticospinal motor pathways.
Keywords: childhood hemiplegia, childhood hemiparesis, independent joint control, selective motor control, cerebral palsy
Introduction
Motor impairments after pediatric brain injury resulting in hemiparesis include weakness and decreased selective motor control.1–3 In a Part I of this paper series, it was shown that there is a relationship between the timing of pediatric brain injury and the distribution of weakness in the upper extremity in childhood-onset hemiparesis (CH). The goal of this study was to ascertain if a relationship also existed between timing of the brain injury and the loss of independent joint control, or the ability to selectively control one joint of the upper extremity without influencing other joints.
The most evidence for the loss of independent joint control exists for very late onset hemiparesis due to stroke in adulthood. For this group of individuals, abnormal muscle synergies emerge4 with significant impact on functional use of the paretic upper extremity in activities of daily living. It has been demonstrated that activating shoulder abductor muscles leads to higher levels of biceps activity and elbow flexion torque,5; 6 higher involuntary wrist and finger flexor activity,7 and subsequent decreases in available work area.8 The loss of ability to independently control joints of the upper extremity is hypothesized to emerge as the result of damage to corticofugal tracts, and an increased reliance on brainstem pathways during the generation of torques in the paretic upper limb.6; 7
Despite significant advances in our understanding of reorganization following injury in adulthood, the loss of independent joint control following brain injuries much earlier in development has been largely unstudied in the upper extremity. Decreased selective motor control has been defined and described as a clinical symptom of pediatric brain injury,1–3 but whether this is the same as the loss of independent joint control reported following adult injury remains unclear. Independent joint control in the pediatric population has not been rigorously quantified in the upper extremity, nor are underlying mechanisms of this phenomenon well understood.
The state of the nervous system at the time of the lesion impacts the neural resources available, and the likely mechanisms of recovery of paretic limb function. Contralaterally and ipsilaterally projecting connections from the cortex to upper extremity muscles have been shown to be present early in development, but the ipsilateral connections are largely withdrawn by approximately 6 months of age.9–11 Childhood-onset hemiparesis (CH) occurs over a range of ages before and after withdrawal of the ipsilateral corticospinal projections, and therefore is an ideal model for studying the impact of lesion timing on motor impairments including loss of independent joint control.
To study the impact of the time of brain injury on loss of independent joint control, individuals with CH were split into groups based on the estimated time of brain injury. Participants’ ability to isolate upper extremity activity to a single joint was measured using a multiple degrees-of-freedom load cell during maximal isometric torque generation tasks in the upper extremity. Those with early (before birth, PRE-natal) injuries were hypothesized to maintain independent joint control, possibly due to access to typically transient ipsilateral pathways from the non-damaged cortical hemisphere to the paretic upper extremity. By contrast, individuals with later (after infancy, POST-natal) brain injuries were hypothesized to have a greater loss of independent joint control due to withdrawal of fast-conducting ipsilateral pathways before an injury that reduced contralateral corticospinal pathways to the paretic limb. Remaining neural resources for control of the upper extremity in this group may include brainstem pathways, which demonstrate diffuse reticulospinal innervation across multiple levels of the spinal cord.12 Finally, the group in between (injuries around the time of birth) was hypothesized to be more like the earlier injury group because the differentiation to primarily contralateral corticospinal projections does not occur until later in infancy, but apoptosis13 and myelination13; 14 of the third trimester of gestation was expected to have an impact on independent joint control.
Methods
Participants
A full description of participant recruitment and demographics is included in Part I of this paper series.15 Briefly, 24 participants with CH and 8 typically developing (TD) controls participated in this study. Participants in the PRE-natal (n=8) group sustained an injury between the late second and early third trimester of gestation, participants in the PERI-natal (n=8) group sustained an injury in the late third trimester of gestation until 6 months following birth, and participants in the POST-natal (n=8) group sustained an injury after 6 months of age. All participants, and their guardians as applicable, provided informed consent, approved by the Northwestern University Institutional Review Board, prior to enrolling in the study.
Demographic and clinical information for all participants in this study has been reported previously in Part I of this series.15 No significant differences were found in age (mean = 13.42 years across all groups), or any collected clinical score between the three CH groups.
Protocol
Experimental setup and instructions to participants are described in detail in Part I of this series.15 In summary, participants were seated with their upper extremity in a standardized position and comfortably attached to three load cells. Forces and moments measured by the six degrees-of-freedom load cell at the radius/ulna connection were converted to torques at the elbow and shoulder using Jacobian matrices based on free body analysis of the upper limb.5 Finger and wrist torques were measured by the single degree-of-freedom load cells located distally.16
Electromyography (EMG) electrodes (Delsys, Boston, MA) were placed over the bellies of the following muscles after skin preparation by light abrasion and cleansing with alcohol prep pads: brachioradialis, biceps brachii, triceps brachii lateral head, triceps brachii long head, anterior region of the deltoid, middle region of the deltoid, posterior region of the deltoid, wrist and finger flexors, and wrist and finger extensors. Data were sampled at 1000 Hz after signals were low-pass filtered with the cut-off frequency at 500 Hz (8-pole analog Butterworth filter; Model 9064, Frequency Devices, Haverhill, MA, USA) to prevent aliasing and amplified with gains set to maximize the available input range of the analog-digital converter based on peak signal at maximal effort.
In this isometric, single-task protocol the participant was asked to generate a maximum voluntary torque in the primary torque directions of shoulder abduction (SB), shoulder adduction (SD), shoulder horizontal abduction, shoulder horizontal adduction, elbow flexion (EF), elbow extension (EE), wrist flexion (WF), wrist extension (WE), finger flexion (FF), and finger extension (FE). The tasks of shoulder horizontal adduction and abduction were removed after data collection because statistical analysis of this degree-of-freedom revealed no significant differences between groups, so for clarity this study focused on one degree of freedom (or pair of opposing torque tasks) per joint of the upper extremity. The participant started each trial in a relaxed state and received visual feedback of the primary torque to optimize performance. Participants were not provided feedback about torques generated at other joints of the upper extremity, referred to here as secondary joint torques.
For each trial, the primary and secondary joint torque data were filtered using a 250-ms moving average filter, and the maximum torque in the primary direction was identified. The maximal value across all trials of an intended primary torque direction was recorded as the maximum voluntary torque, and concurrent secondary torque at all other joints were identified for the same point in time. EMG signals were also filtered using a 250-ms moving average filter following visual inspection for artifact, rectification and baseline correction. EMG activation for each of the muscles was identified during the small time window preceding the maximal voluntary torque by 50 ms to account for the delay between muscle activation and torque output.
Several clinical examinations were performed and are listed for each participant in Table 1 of Part I of this paper series.15
Table 1.
Significant coupling patterns by groupa
| 1° | 2° | PRE
|
PERI
|
POST
|
TD | |||
|---|---|---|---|---|---|---|---|---|
| P | NP | P | NP | P | NP | |||
| SB | E | 0.023 (F) | 0.038 (F) | 0.032 (F) | 0.063 | <0.001 (F) | <0.001 (F) | 0.024 (F) |
| W | 0.366 | 0.667 | 0.912 | 0.318 | 0.015 (F) | 0.502 | 0.216 | |
| F | 0.866 | 0.684 | 0.127 | 0.219 | 0.039 (F) | 0.333 | 0.111 | |
| SD | E | 0.209 | 0.799 | 0.176 | 0.193 | 0.032 (E) | 0.001 (E) | 0.015 (E) |
| W | 0.003 (F) | 0.945 | 0.008 (F) | 0.478 | 0.022 (F) | 0.088 | 0.311 | |
| F | 0.003 (F) | 0.697 | 0.009 (F) | 0.528 | 0.024 (F) | 0.242 | 0.899 | |
| EF | S | 0.364 | 0.064 | 0.224 | 0.004 (D) | 0.010 (B) | 0.216 | 0.135 |
| W | <0.001 (F) | 0.580 | 0.071 | 0.043 (F) | <0.001 (F) | 0.024 (F) | 0.001 (F) | |
| F | <0.001 (F) | 0.888 | 0.058 | 0.094 | 0.002 (F) | 0.069 | 0.120 | |
| EE | S | 0.561 | 0.026 (B) | 0.281 | 0.063 | <0.001 (D) | 0.575 | 0.799 |
| W | 0.070 | 0.118 | 0.195 | 0.690 | 0.681 | 0.405 | 0.149 | |
| F | 0.721 | 0.125 | 0.226 | 0.978 | 0.302 | 0.608 | 0.597 | |
| WF | S | 0.063 | 0.464 | 0.005 (D) | 0.199 | 0.003 (D) | 0.134 | 0.011 (D) |
| E | 0.098 | 0.353 | 0.290 | 0.435 | 0.161 | 0.111 | 0.744 | |
| F | <0.001 (F) | 0.001 (F) | <0.001 (F) | <0.001 (F) | 0.039 (F) | 0.005 (F) | <0.001 (F) | |
| WE | S | 0.002 (B) | 0.311 | 0.104 | 0.917 | 0.037 (B) | 0.801 | 0.794 |
| E | 0.004 (F) | 0.470 | 0.538 | 0.618 | 0.665 | 0.036 (F) | 0.544 | |
| F | 0.001 (E) | 0.001 (E) | 0.007 (E) | <0.001 (E) | 0.013 (E) | 0.001 (E) | <0.001 (E) | |
| FF | S | 0.015 (D) | 0.089 | 0.055 | 0.956 | 0.005 (D) | 0.594 | 0.570 |
| E | 0.080 | 0.172 | 0.583 | 0.113 | 0.037 (E) | 0.995 | 0.133 | |
| W | 0.003 (F) | <0.001 (F) | 0.001 (F) | <0.001 (F) | 0.006 (F) | <0.001 (F) | <0.001 (F) | |
| FE | S | 0.009 (B) | 0.116 | 0.021 (B) | 0.224 | 0.229 | 0.093 | 0.179 |
| E | 0.250 | 0.564 | 0.187 | 0.849 | 0.649 | 0.091 | 0.946 | |
| W | <0.001 (E) | 0.001 (E) | 0.003 (E) | <0.001 (E) | 0.180 | <0.001 (E) | <0.001 (E) | |
CH groups’ arms are shown individually (P = paretic, NP = nonparetic), and both arms of the TD group are combined. Primary tasks (1°) are listed in the left column (SB = shoulder abduction, SD = shoulder adduction, EF = elbow flexion, EE = elbow extension, WF = wrist flexion, WE = wrist extension, FF = finger flexion, FE = finger extension). Each primary task has 3 secondary joint torques associated with it, which are listed in the second column (S = shoulder, E = elbow, W = wrist, F = fingers). Data were tested for a difference from zero using a Student’s t test, and comparisons that reached a significant level of P < .05 are highlighted in gray. For significant findings, the direction of the secondary torque is indicated in parentheses (B = abduction, D = adduction, F = flexion, E = extension).
Statistics
Torque data, including primary torque direction (task) and secondary or associated torques at other joints of the upper extremity, were normalized to the maximum torque achieved over all trials for a given degree of freedom within a participant’s dataset. Subsequently, statistical analyses were completed on secondary torques to define coupling patterns and differences between groups.
Data from the typically developing participants were analyzed using two-way mixed model analyses of variance (ANOVAs), with secondary torque as the dependent variable and limb (dominant or non-dominant) and intended primary task (SB, SD, EF, EE, WF, WE, FF, and FE) as the dependent variables. The results of these tests were used to determine if the torque data for the dominant and non-dominant limb of TD participants could be grouped together, and if primary directions should be analyzed independently.
In each group, and for each task, two-tailed student’s t-tests were used to determine if the dependent variable of secondary torque at each joint was different from zero. This was done to identify joint torque coupling patterns in each group for descriptive purposes.
Subsequently, data from all subjects were analyzed for differences in magnitude or direction of torque coupling patterns between groups. The normality of distribution of secondary torque was tested using the Shapiro-Wilk test, with concurrent investigation of outliers. Differences in torque coupling were analyzed using several one-way ANOVAs, with secondary torques in each of three degrees of freedom as the dependent variables and a combined variable of limb (NP=non-paretic, P=paretic) and group as the independent variable (PRE-P, PRE-NP, PERI-P, PERI-NP, POST-P, POST-NP, TD). The Least Significant Difference (LSD) test was used to make post-hoc comparisons of the combined group-limb variable in order to indentify differences between groups. Data that was not normally distributed were analyzed using a Kruskal-Wallis non-parametric test with pairwise comparisons of the combined group-limb factor (as listed above).
The same procedure was used for the dependent variables of EMG percentage activation in each of the 9 muscles.
Statistical analysis was completed using SPSS software (version 19, SPSS, Inc.). A p-value of ≤ 0.05 was considered statistically significant for all tests.
Results
Individuals with CH and a typically developing comparison group participated in 8 torque generation tasks in the upper extremity. Secondary torques at other joints of the upper extremity were evaluated at the maximal voluntary task torque in the primary direction to characterize and compare independent joint control, or the ability to isolate activity to one joint of the upper extremity.
Typically developing group: limb and primary task
In the typically developing group, there was a significant main effect of task for all secondary directions (SB/SD, F=4.026, p=0.003; EF/EE, F=5.791, p<0.001; WF/WE, F=32.043, p<0.001; FF/FE F=23.269, p<0.001). Therefore, tasks were considered individually in subsequent comparisons with CH timing groups. The main effect of limb and the interaction of limb-by-task were not significant. Therefore, data from the dominant and non-dominant limbs of the TD group were combined for comparison with CH timing groups’ paretic and non-paretic limbs. For the EMG data, there was a significant (p<0.001) main effect of task in all muscles. Limb was also a significant main effect in the middle deltoid (F=10.140, p=0.002), posterior deltoid (F=6.238, p=0.001) and wrist/finger extensors (F=7.908, p=0.009). Therefore, the dominant and non-dominant limbs were combined for further comparison of all muscles to CH timing groups, except for middle and posterior deltoid and wrist/finger extensors. In these muscles, two levels were used to separately compare the dominant and non-dominant TD limbs. All tasks were considered individually, as were the secondary torque dependent variables.
Summary of comparative analyses
Secondary torques recorded during each task were evaluated for differences from zero, and a complete list t-test results, and the direction of significant secondary torque findings, can be found in Table 1. Overall, there was a higher number of significant secondary torques in the paretic limb of CH groups (13 in the PRE group, 9 in the PERI group, and 17 in the POST group) compared to TD group (8 significant findings), indicating more activity at joints other than the joint of the primary task direction in the CH groups. A summary of the comparisons between groups for secondary degrees of freedom associated with each task is listed in Table 2, along with post-hoc comparisons between the paretic limb of the CH groups and combined limbs of the TD group.
Table 2.
Results of secondary degrees of freedom comparisons, with paretic limb post-hocsa
| 1° | 2° | p-value | Post-Hoc Paretic Limb Comparisons
|
|||||
|---|---|---|---|---|---|---|---|---|
| PRE|PERI | PRE|POST | PRE|TD | PERI|POST | PERI|TD | POST|TD | |||
| SB | E | 0.016 | 0.972 | 0.028 | 0.419 | 0.030 | 0.396 | 0.001 |
| W | 0.083 | |||||||
| F | 0.010 | 0.691 | 0.022 | 0.268 | 0.159 | 0.030 | <0.001 | |
| SD | E | 0.445 | ||||||
| W | <0.001 | 0.926 | 0.997 | 0.032 | 0.966 | 0.037 | 0.041 | |
| F | 0.001 | 0.969 | 0.612 | 0.005 | 0.638 | 0.005 | 0.030 | |
| EF | S | <0.001 | 0.959 | <0.001 | 0.103 | <0.001 | 0.044 | 0.003 |
| W | 0.001 | 0.021 | 0.577 | <0.001 | 0.098 | 0.327 | 0.006 | |
| F | 0.002 | 0.149 | 0.553 | <0.001 | 0.417 | 0.044 | 0.005 | |
| EE | S | 0.001 | 0.188 | 0.001 | 0.434 | 0.042 | 0.456 | 0.003 |
| W | 0.413 | |||||||
| F | 0.080* | |||||||
| WF | S | 0.001 | 0.376 | 0.764 | 0.198 | 0.577 | 0.023 | 0.118 |
| E | 0.152 | |||||||
| F | 0.047 | 0.410 | 0.039 | 0.449 | 0.005 | 0.091 | 0.099 | |
| WE | S | 0.003* | 1.00 | 1.00 | 0.005 | 1.00 | 1.00 | 1.00 |
| E | 0.028* | 1.00 | 0.053 | 0.288 | 1.00 | 1.00 | 1.00 | |
| F | 0.676* | |||||||
| FF | S | <0.001* | 1.00 | 1.00 | 0.252 | 1.00 | 0.241 | 0.015 |
| E | 0.011* | 1.00 | 0.010 | 0.380 | 1.00 | 1.00 | 1.00 | |
| W | 0.008* | 1.00 | 1.00 | 0.070 | 1.00 | 0.015 | 0.760 | |
| FE | S | 0.003* | 1.00 | 0.006 | 0.384 | 0.096 | 1.00 | 1.00 |
| E | 0.254* | |||||||
| W | 0.064* | |||||||
Primary tasks (1°) are listed in the left column (SB = shoulder abduction, SD = shoulder adduction, EF = elbow flexion, EE = elbow extension, WF = wrist flexion, WE = wrist extension, FF = finger flexion, FE = finger extension). Each primary task has 3 secondary joint torques associated with it, which are listed in the second column (S = shoulder, E = elbow, W = wrist, F = fingers). Data were tested for differences between groups using a mixed model ANOVA, or a Kruskal-Wallis nonparametric test (indicated by an asterisk), depending on the normality of the data tested. Post hoc comparisons among the paretic arms of the CH group and combined arms of the TD group are shown, and are also nonparametric if the Kruskal-Wallis nonparametric test was used. All comparisons that reached a significant level of P < .05 are highlighted in gray.
Torque coupling patterns in the typically developing group
Patterns of significant torque coupling found in the typically developing group are listed in Table 1. Although some significant patterns appear similar between TD and CH groups, post-hoc analysis revealed several significant differences in the magnitude of the typical coupling in secondary torques. In the shoulder adduction and elbow flexion tasks, all studied groups present the same distal coupling (WF and FF), but the TD group coupled with significantly less distal flexion than the CH groups. They also demonstrated higher levels of secondary wrist flexion with the task of finger flexion than the CH groups.
The following three sections will highlight differences unique to each of the CH injury timing groups. For distinction, the latest and earliest injury timing groups will be explored first.
Unique torque coupling patterns and associated EMGs in the POST-natal group
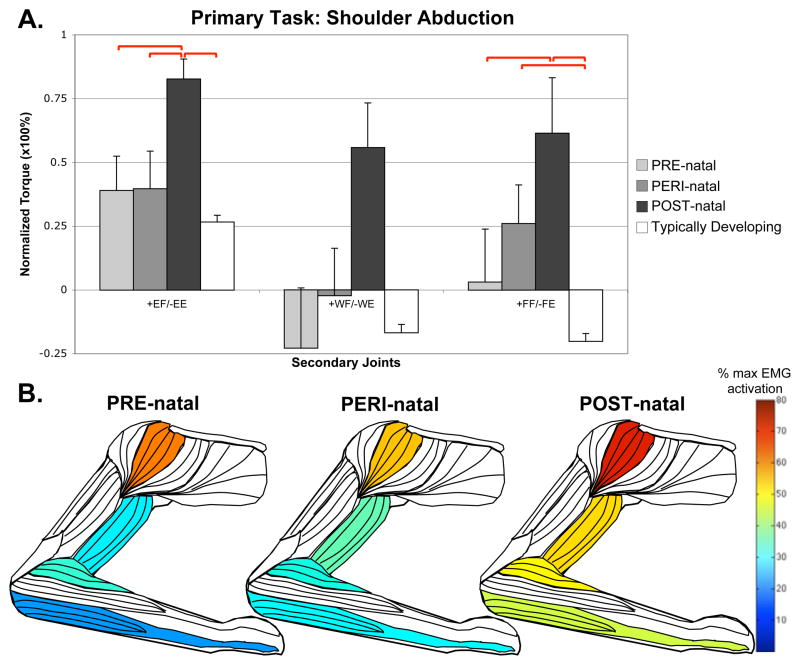
In the POST-natal injury timing group, there was a strong coupling of distal joints with proximal (elbow and shoulder) tasks. In the shoulder abduction task, the POST-natal group demonstrated significantly higher levels of coupling with elbow and finger flexion torque than other groups, with concurrently higher EMG activity in wrist/finger flexors (ANOVA, F=3.097, p=0.011) and middle deltoid (Kruskal-Wallis, H=15.979, p=0.025), with a trend seen at brachioradialis (ANOVA, F=1.923, p=0.093) and biceps (ANOVA, F=2.201, p=0.056) (Figure 1).
Figure 1.
Secondary torques associated with the primary torque direction task of shoulder abduction, with standard error bars. A significant effect of group-limb was found in the elbow (F=2.902, p=0.016) and fingers (F=3.138, p=0.010). Significant post-hoc comparisons, where p<0.05, are shown with red bars above the graph. Also shown are muscles colored to represent percentages of maximal EMG activity where differences were found between groups. These muscles include middle deltoid (Kruskal-Wallis, H=15.979, p=0.025), biceps brachii (ANOVA, F=2.201, p=0.056), brachioradialis (ANOVA, F=1.923, p=0.093) and combined wrist and finger flexors (ANOVA, F=3.097, p=0.011).
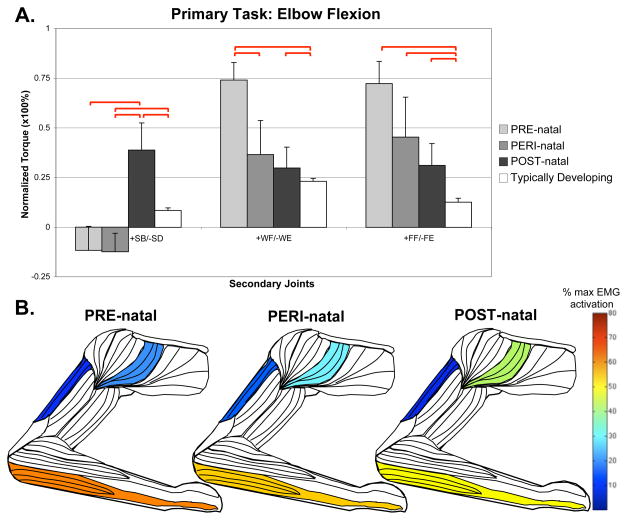
In the elbow flexion task (Figure 2), POST-natal participants demonstrated significantly higher levels of shoulder abduction coupling, with significant differences in anterior deltoid EMG activity (Kruskal-Wallis, H=14.685, p=0.023). Conversely, a significantly higher level of shoulder adduction coupling was found in the elbow extension task (ANOVA F=4.532, p=0.001; POST-natal compared to: PRE p=0.001, PERI p=0.042, TD p=0.003).
Figure 2.
Secondary torques associated with the primary torque direction task of elbow flexion, with standard error bars. A significant effect of group-limb was found in the shoulder (F=6.550, p<0.001), wrist (F=4.468, p=0.001) and fingers (F=4.179, p=0.002). Significant post-hoc comparisons, where p<0.05, are shown with red bars above the graph. Also shown are muscles colored to represent percentages of maximal EMG activity where differences were found between groups. These muscles include anterior deltoid (Kruskal-Wallis, H=14.685, p=0.023), triceps brachii long head (Kruskal-Wallis, H=13.562, p=0.035), and combined wrist and finger flexors (ANOVA, F=3.192, p=0.009).
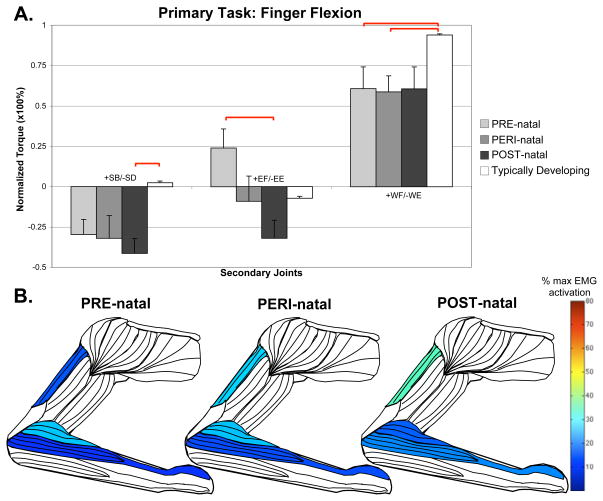
For the distal tasks at the wrist and fingers, the POST-natal group demonstrated less deviation from the TD or other CH timing groups. Notably, in wrist flexion, there was less finger flexion compared to PRE- and PERI-natal groups (ANOVA F=2.303, p=0.047; POST-natal compared to: PRE p=0.039, PERI p=0.005). In the primary task of finger flexion (Figure 4), the POST-natal group couples with higher levels of elbow extension than TD, with increased triceps long head activation (Kruskal-Wallis, H=20.537, p=0.002).
Figure 4.
Secondary torques associated with the primary torque direction task of finger flexion, with standard error bars. A significant effect of group-limb was found in the shoulder (F=25.129, p<0.001), elbow (F=16.536, p=0.011) and wrist (F=17.466, p=0.008). Significant post-hoc comparisons, where p<0.05, are shown with red bars above the graph. Also shown are muscles colored to represent percentages of maximal EMG activity where differences were found between groups. These muscles include triceps brachii long head (Kruskal-Wallis, H=20.537, p=0.002), brachioradialis (Kruskal-Wallis, H=15.433, p=0.017), and combined wrist and finger extensors (Kruskal-Wallis, H=12.035, p=0.099).
Unique torque coupling patterns and associated EMGs in the PRE-natal group
The PRE-natal group behaved more like TD during shoulder tasks, but demonstrated several differences in magnitude and direction of torque coupling during elbow, wrist and finger tasks.
In the primary task of elbow flexion, the PRE-natal group coupled with shoulder adduction, which is a significant difference from the POST-natal shoulder abduction coupling. The PRE-natal group also averaged higher levels of wrist and finger flexion coupling than any other group with significant post-hoc differences from TD (Figure 2). During the primary task of elbow flexion, EMG activity of the wrist/finger flexors was significantly greater in the PRE-natal group (ANOVA, F=3.192, p=0.009) than any other group.
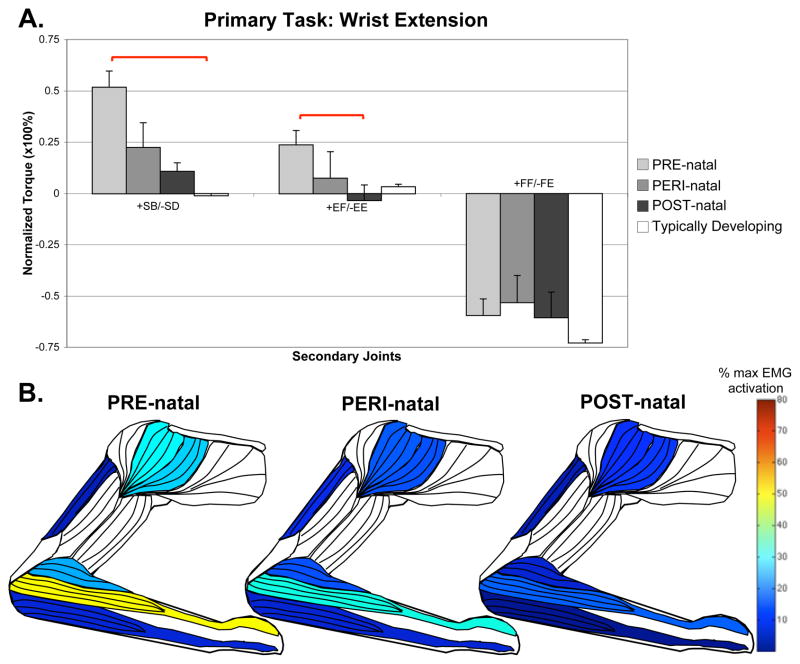
For the primary task of wrist extension, the PRE-natal group coupled with significantly higher levels of shoulder abduction than any other group, with a significant difference from the TD group (pairwise comparison p=0.005). There was a concurrent finding of higher levels of EMG activity in the anterior deltoid (Kruskal-Wallis, H=15.797, p=0.015) and middle deltoid (Kruskal-Wallis, H=18.565, p=0.010), and a trend towards higher EMG activity in the brachioradialis (Kruskal-Wallis, H=11.071, p=0.086). This supports a higher average elbow flexion torque in this group. A summary of the wrist extension task can be seen in Figure 3.
Figure 3.
Secondary torques associated with the primary torque direction task of wrist extension, with standard error bars. A significant effect of group-limb was found in the shoulder (F=19.710, p=0.003) and elbow (F=14.122, p=0.026) using the Kruskal-Wallis test. Significant post-hoc comparisons, where p<0.05, are shown with red bars above the graph. Also shown are muscles colored to represent percentages of maximal EMG activity where differences were found between groups. These muscles include middle deltoid (Kruskal-Wallis, H=18.565, p=0.010), anterior deltoid (Kruskal-Wallis, H=15.797, p=0.015), triceps brachii long head (ANOVA, F=3.847, p=0.003), brachioradialis (Kruskal-Wallis, H=11.071, p=0.086), combined wrist and finger extensors (ANOVA, F=29.952, p<0.001), and combined wrist and finger flexors (ANOVA, F=3.097, p=0.011).
In the primary task of finger flexion, secondary elbow torque is found to be significantly different between the PRE-natal group (who flexed at the elbow) and the POST-natal group (who extended at the elbow). As seen in Figure 4, there was also a concurrent effect of group with higher POST-natal triceps long head activation (Kruskal-Wallis, H=20.537, p=0.002), and higher PRE-natal brachioradialis activation (Kruskal-Wallis, H=15.433, p=0.017).
Unique torque coupling patterns and associated EMGs in the PERI-natal group
The PERI-natal group was generally statistically the same as the PRE-natal group. This includes primary tasks with secondary coupling averages very similar to the PRE-natal group (for examples, see elbow flexion during shoulder abduction, and shoulder adduction with elbow flexion in Figures 1 and 2) as well as instances where the average of the PERI-natal group falls between PRE- and POST-natal averages (for examples, see wrist and finger flexion with shoulder abduction, shoulder abduction with wrist extension, and differences in elbow activity with finger flexion in Figures 1, 3, and 4 respectively). Significant post-hoc differences can be appreciated in several degrees of freedom when comparing the PERI-natal group with POST-natal and TD, as summarized in Table 2.
Discussion
We found that individuals with childhood-onset hemiparesis (CH) demonstrated greater amounts of joint torque coupling than their typically developing peers, with significant distinctions depending on the time of brain injury. The POST-natal group showed strong coupling between shoulder abduction and elbow, wrist and finger flexion. The PRE-natal group demonstrated diffuse coupling of elbow flexion with distal tasks, such as wrist extension. These data show that the time of brain injury onset has an impact on torque coupling patterns in the upper extremity in CH. The underlying mechanisms are likely related to the neural substrates available at the time of injury.
Typical development of motor pathways and implications for joint torque patterns
During typical development, animal17–19 and human work10; 11 has shown that there is early exuberance followed by withdrawal of transient pathways connecting cortex with ipsilateral distal muscles, predominantly occurring within the first 6 months of life. Activity dependent elimination of synapses and axons is overlapped by rapid periods of myelination starting from the second trimester of gestation20 and continuing extensively for the first year of life, and beyond albeit at a slower rate. Primitive postural reflexes, including the asymmetrical and symmetrical tonic neck reflexes21 can be observed during this time period of typical development. They include patterns of muscle activation that can be replicated in animal models by stimulation of the brain stem nuclei,22 and are hallmarks of a period of typical development characterized by lack of independent joint control presumably related to a lack of maturation of corticospinal projections. They typically are no longer observed as infants begin to build a repertoire of gross and fine motor skills.
Timing of brain injury and implications for joint torque patterns
Brain reorganization following stroke in adulthood has been widely studied, which has led to increased understanding of potential causal factors of abnormal joint torque synergies. One mechanism that has been postulated to underlie the expression of synergistic movement patterns at the shoulder and elbow following stroke is an increased reliance on medial bulbospinal pathways such as the reticulospinal tract.6; 23; 8; 7 This can be substantiated by studies that demonstrate patterns of muscle activation with stimulation of the reticular formation in the macaque24–26 that match the muscle co-activation patterns seen following stroke.6
The results of the current study indicate that the POST-natal group has shoulder and elbow torque coupling patterns similar to adults following hemiparetic stroke.7; 6; 23; 8 These patterns include grouping of shoulder abductors with elbow, wrist and finger flexors activated with all proximal movements.7 As in adults, brain injury following the typical period of intact ipsilateral corticospinal projections (in this study, the POST-natal group) could result in re-organization via ipsilateral, polysynaptic, cortico-bulbar and bulbo-spinal pathways. One study observed that MEPs could be elicited from the ipsilateral hemisphere in 2 patients with POST-natal injury, but at a longer latency and reduced amplitude than was seen in those with earlier injuries.27 Patterns observed in the current study are consistent with innervation patterns of the reticulospinal tract, which is known to terminate diffusely across multiple levels of the spinal cord,26 to shoulder abductors, elbow flexors and with distal influence limited to the flexors of the hand and forearm.28; 29 Interestingly, the tasks with the lowest levels of torque coupling and muscle activation across all tested muscles including prime movers were seen in the primary tasks of wrist and finger extension. These muscles have not been shown to receive input from reticular sources, implying that the corticospinal tract plays a predominant role in the activation of distal extensors. Following damage to the corticospinal system in CH, profound weakness is seen in wrist and finger extension.15 In this study, there was very low activation in the combined wrist and finger extensor EMG in addition to limited activation of other muscles in the upper extremity during this task, presumably due to inability to use the reticulospinal output for distal extension.
The interruption of typical development before complete in-utero gestation by a unilateral brain injury (the PRE-natal group in this study) has been linked to maintenance of fast conducting cortico-motor connections, indicative of direct corticospinal projections, from the undamaged hemicortex to both the non-paretic and paretic upper extremities.9; 30 Several abnormal torque coupling patterns (compared to TD) were found in the PRE-natal group. Elbow and wrist/finger joints were activated together to a greater extent than in other groups, potentially demonstrating a decrease in resolution of the cortical connections to the paretic limb. It is possible that the adjacent distal joint coupling results from a net loss of cortical neural territory available to activate the paretic limb, or decreased specificity of the typically transient ipsilateral connections, now presumably projecting to both arms. Nearly half of the subjects in one study demonstrated reorganization of an intrinsic hand muscle outside of the hand-knob area on the contra-lesional side using motor evoked potentials elicited with MRI guidance.31 Wrist and finger extension tasks in the PRE-natal group demonstrated relatively preserved strength,15 but also resulted in high levels of shoulder abduction. As noted previously, the distal extensors are primarily driven by the corticospinal system, so the concurrent activation of more proximal muscles could be further indicative of a lower resolution corticospinal control system in the PRE-natal group, although we did not perform TMS during this study to confirm the presence of ipsilateral connectivity to the paretic limb. It was also noted that there was submaximal middle deltoid activity in this group during shoulder abduction (Figure 1), suggesting altered muscle recruitment despite relatively preserved strength.15 The impact of very early brain unilateral brain injury on the emergence of muscle co-activation patterns warrants further investigation.
Finally, the PERI-natal group in this study typically represented a mid-point in the continuum between PRE- and POST-natal. Statistically, they tended to perform more like the PRE-natal group, probably due to similar availability of corticospinal projections. However, the impacts of myelination20 during the third trimester seem to play a role in the activity dependent plasticity following injury, which can be observed in the averages and trends of secondary coupling patterns when comparing to PRE- and POST-natal cohorts.
Clinical and scientific implications
These findings also have important implications for therapeutic interventions, as intervention efficacy for a specific patient is inherently linked to their source of impairment. For example, patients with CH and ipsilateral cortical-motor connectivity to the paretic limb (which has been shown in the PRE- and PERI-natal injury groups10) responded differently to an intervention than those without ipsilateral connectivity.32 In the adult stroke literature, where torque patterns resemble the POST-natal group in this study, elbow extension/ shoulder flexion repetitions while abducting the arm against resistance resulted in improvements in independent joint control,33 suggesting that this impairment can be improved with appropriate practice. To fully characterize impairments in CH, further information is required about how restricted each group is to the spontaneous patterns measured in this study, using a protocol that requires efforts outside of the patterns measured here. Finally, it should be considered that participants in this study had a range of early motor experiences and therapies, but nonetheless retained stereotypical patterns of joint torque synergies.
Although medical records were reviewed, one potential limitation of this study is that brain imaging results were not available for all participants. However, participants’ data were analyzed only if the timing of their injury could be confidently ascertained based on available information. Abnormal structural MRIs are not always associated with clinical diagnosis,34 but more sensitive diffusivity measures obtained from diffusion tensor imaging may have the potential improve understanding of the CH population,35 although even with this technology several methodological issues36 require further refinement.
Conclusions
Loss of independent joint control may impact all CH injury groups, but the expression of this loss is different with regard to the measured joint torque coupling patterns depending on the time of injury. We have suggested a plausible underlying neural mechanism behind these differences, but further study is required especially in the early injury groups to fully understand the neural response and functional consequences of brain injury superimposed upon neuro-motor development.
In this paper series, timing of brain injury has been shown to impact both the relative weakness distribution (Part I)15 and independent joint control (Part II) in CH. Injury timing must be considered in future studies evaluating motor dysfunction and underlying neural mechanisms in this population.
Acknowledgments
We thank most especially the participants and their families. Donna Hurley, DPT, and Isabel Aguilar played vital roles in participant recruitment. Rachel Hawe, PhD Candidate assisted with data collection. Alexis Kuncel, PhD, Laura Miller, PhD Candidate, Jacob McPherson, PhD and Michael Ellis, DPT provided helpful comments on the manuscript. This work was supported by the National Institutes of Health [5R01NS058667-02 and T32EB009406 to J.D.]; and the National Science Foundation [graduate student fellowship to T.S-M.].
References
- 1.Sanger TD, Delgado MR, Gaebler-Spira D, Hallett M, Mink JW. Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111:e89–97. doi: 10.1542/peds.111.1.e89. [DOI] [PubMed] [Google Scholar]
- 2.Tedroff K, Knutson LM, Soderberg GL. Synergistic muscle activation during maximum voluntary contractions in children with and without spastic cerebral palsy. Dev Med Child Neurol. 2006;48:789–96. doi: 10.1017/S0012162206001721. [DOI] [PubMed] [Google Scholar]
- 3.Fowler EG, Staudt LA, Greenberg MB. Lower-extremity selective voluntary motor control in patients with spastic cerebral palsy: increased distal motor impairment. Dev Med Child Neurol. 2010;52:264–9. doi: 10.1111/j.1469-8749.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 4.Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. New York: Medical Dept; 1970. [Google Scholar]
- 5.Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–83. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118 ( Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 7.Miller LC, Dewald JP. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012;123:1216–25. doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukal TM, Ellis MD, Dewald JP. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007;183:215–23. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31:1136–49. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Eyre JA, Smith M, Dabydeen L, Clowry GJ, Petacchi E, Battini R, Guzzetta A, Cioni G. Is hemiplegic cerebral palsy equivalent to amblyopia of the corticospinal system? Ann Neurol. 2007;62:493–503. doi: 10.1002/ana.21108. [DOI] [PubMed] [Google Scholar]
- 11.Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 12.Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 13.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–29. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 14.ten Donkelaar HJ, Lammens M, Wesseling P, Hori A, Keyser A, Rotteveel J. Development and malformations of the human pyramidal tract. J Neurol. 2004;251:1429–42. doi: 10.1007/s00415-004-0653-3. [DOI] [PubMed] [Google Scholar]
- 15.Sukal-Moulton T, Krosschell K, Gaebler-Spira D, Dewald J. Motor impairments related to brain injury timing in early hemiparesis Part I: expression of upper extremity weakness. 2012 doi: 10.1177/1545968313500564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stienen AH, Sukal-Moulton T, Miller LC, Dewald J. ICORR. Zurich; Switzerland: 2011. Wrist and Finger Torque Sensor for the Quantification of Upper Limb Motor Impairments Following Brain Injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–73. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 18.Stanfield BB. The development of the corticospinal projection. Prog Neurobiol. 1992;38:169–202. doi: 10.1016/0301-0082(92)90039-h. [DOI] [PubMed] [Google Scholar]
- 19.Galea MP, Darian-Smith I. Postnatal maturation of the direct corticospinal projections in the macaque monkey. Cereb Cortex. 1995;5:518–40. doi: 10.1093/cercor/5.6.518. [DOI] [PubMed] [Google Scholar]
- 20.Kinney HC. Human myelination and perinatal white matter disorders. J Neurol Sci. 2005;228:190–2. doi: 10.1016/j.jns.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Capute AJ, Shapiro BK, Accardo PJ, Wachtel RC, Ross A, Palmer FB. Motor functions: associated primitive reflex profiles. Dev Med Child Neurol. 1982;24:662–9. doi: 10.1111/j.1469-8749.1982.tb13677.x. [DOI] [PubMed] [Google Scholar]
- 22.Herbert WJ, Davidson AG, Buford JA. Measuring the motor output of the pontomedullary reticular formation in the monkey: do stimulus-triggered averaging and stimulus trains produce comparable results in the upper limbs? Exp Brain Res. 2010;203:271–83. doi: 10.1007/s00221-010-2231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis MD, Acosta AM, Yao J, Dewald JP. Position-dependent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp Brain Res. 2007;176:594–602. doi: 10.1007/s00221-006-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–9. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. J Neurophysiol. 2004;92:83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker SN. The Primate Reticulospinal Tract, Hand Function and Functional Recovery. J Physiol. 2011 doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nezu A, Kimura S, Takeshita S, Tanaka M. Functional recovery in hemiplegic cerebral palsy: ipsilateral electromyographic responses to focal transcranial magnetic stimulation. Brain Dev. 1999;21:162–5. doi: 10.1016/s0387-7604(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 28.Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- 29.Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci. 2007;27:8053–8. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–37. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- 31.Vandermeeren Y, Davare M, Duque J, Olivier E. Reorganization of cortical hand representation in congenital hemiplegia. Eur J Neurosci. 2009;29:845–54. doi: 10.1111/j.1460-9568.2009.06619.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol. 2008;50:898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 33.Ellis MD, Sukal-Moulton T, Dewald JP. Progressive shoulder abduction loading is a crucial element of arm rehabilitation in chronic stroke. Neurorehabil Neural Repair. 2009;23:862–9. doi: 10.1177/1545968309332927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Msall ME, Limperopoulos C, Park JJ. Neuroimaging and cerebral palsy in children. Minerva Pediatr. 2009;61:415–24. [PubMed] [Google Scholar]
- 35.Son SM, Ahn YH, Sakong J, Moon HK, Ahn SH, Lee H, Yu IK, Shin YJ, Jang SH. Diffusion tensor imaging demonstrates focal lesions of the corticospinal tract in hemiparetic patients with cerebral palsy. Neurosci Lett. 2007;420:34–8. doi: 10.1016/j.neulet.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 36.Nagae LM, Hoon AH, Jr, Stashinko E, Lin D, Zhang W, Levey E, Wakana S, Jiang H, Leite CC, Lucato LT, van Zijl PC, Johnston MV, Mori S. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol. 2007;28:1213–22. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]