Abstract
Background
Effects of chronic cadmium exposure on liver disease and liver-related mortality are unknown. We evaluated the association of creatinine-corrected urinary cadmium levels with hepatic necroinflammation, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), liver-related mortality, and liver cancer mortality in the US general population.
Methods
We analyzed the relationship of individuals in the top quartile for urinary cadmium measured in 12,732 adults who participated in the Third National Health and Nutrition Examination Survey in 1988–1994 (NHANES III), and hepatic necroinflammation, NAFLD, and NASH. Associations between cadmium, liver-related mortality, and liver cancer mortality were evaluated in the NHANES III mortality follow-up study.
Results
The cutoffs for highest quartile of urinary cadmium per gram of urinary creatinine were 0.65 and 0.83 μg/g for men and women, respectively (P<0.001). After multivariate adjustment for other factors including smoking, the odds ratios [95 % confidence intervals (CI)] for hepatic necroinflammation, NAFLD, and NASH associated with being in the top quartile of cadmium levels by gender, were 2.21 (95 % CI, 1.64–3.00), 1.30 (95 % CI, 1.01–1.68) and 1.95 (95 % CI, 1.11–3.41) for men and 1.26 (95 % CI, 1.01–1.57), 1.11 (95 % CI, 0.88–1.41) and 1.34 (95 % CI, 0.72–2.50) for women, respectively. The hazard ratios for liver-related mortality and liver cancer mortality for both genders were 3.42 (95 % CI, 1.12–10.47) and 1.25 (95 % CI, 0.37–4.27).
Conclusions
Environmental cadmium exposure was associated with hepatic necroinflammation, NAFLD, and NASH in men, and hepatic necroinflammation in women. Individuals in the top quartile of creatinine-corrected urinary cadmium had over a threefold increased risk of liver disease mortality but not in liver cancer related mortality.
Keywords: Liver, Cadmium, Steatosis, Survey, Mortality, NHANES
Introduction
Cadmium is a toxic heavy metal found widely in the environment. Environmental exposure to cadmium occurs primarily through smoking or industrial emissions, and the consumption of contaminated food and water.1-3 Cadmium has a whole-body half-life of between 15 and 30 years and accumulates in two target tissues: the renal cortex and the liver.3 Studies have shown an increase in all-cause and cancer mortality in general populations exposed to low level, chiefly environmental, exposure.4 Cadmium interacts with multiple aspects of liver function, chiefly through heavy metal binding proteins called metallothioneins. Acute and chronic cadmium induced hepatotoxicity is well known in animal models of liver failure. In mouse models of chronic cadmium exposure induced hepatotoxicity, nonspecific chronic inflammation, granulomatous inflammation, apoptosis, and liver cell regeneration have been noted.5 However, the effect of chronic cadmium exposure on liver-related outcomes in humans is not well characterized.
We sought to evaluate the association of creatinine-corrected urinary cadmium with hepatic necroinflammation, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH). In addition, we examine the relationship of cadmium exposure with liver-related mortality and liver cancer mortality in the general United States (US) population using data from the Third National Health and Nutrition Examination Survey (NHANES III) cross-sectional study, and the NHANES III Mortality follow-up study.
Methods
Study population
NHANES III was a stratified, multistage probability survey designed to select a representative sample of the civilian non-institutionalized US population.6 Overall, 16,115 adults 20–74 years of age completed the NHANES III interview and examination. We excluded 1,756 participants missing data for urinary cadmium, 77 participants missing data for urinary creatinine, 644 participants missing data on alanine aminotransferase (ALT), aspartate aminotransferase (AST), or ultrasonography and 906 participants for missing information on other covariates or who did not have follow-up information. These exclusions left 12,732 NHANES III participants in the present analysis.
The protocol for NHANES III was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. All participants gave written, informed consent.
Baseline data collection
NHANES III baseline data were collected during an inhome interview and a subsequent visit to a mobile examination center.6 During the in-home interview, demographic and health-related information was collected using a standardized questionnaire. We classified participants into one of three groups based on cigarette smoking: never smokers, former smokers, and current smokers. Pack years were calculated for smokers, both current and former. History of cardiovascular disease was defined as a self-reported history of acute myocardial infarction, stroke, or heart failure. History of cancer was defined as a self-reported history of any cancer. We categorized people as sedentary if they answered “no” to all the questions on engaging in any of the following activities over the past month: jog or run, cycle, swim, aerobics, other dancing, calisthenics, garden or yard work, weight lifting, or other sports.7 Height and weight were measured and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Total serum cholesterol was measured enzymatically. A detailed description of the measurement of urinary cadmium levels and quality control for sample processing is available elsewhere.4,8
We performed all analyses using creatinine-corrected urinary cadmium values (urinary cadmium divided by urinary creatinine concentrations, expressed as micrograms per gram) to account for between-participant differences in urine dilution. Urinary creatinine was measured with a Beckman ASTRA automated analyzer.8
Serum biochemistries were done using the Hitachi 737 automated multichannel chemistry analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN).9 Liver tests determined levels of ALT, AST, γ-glutamyltranspeptidase (GGT), alkaline phosphatase. For analysis involving NAFLD and NASH, the levels of ALT or AST were considered raised if they were above the upper limit of normal of the National Health and Nutrition Examination Survey laboratory values.9 Hepatitis C status was determined by testing for the presence of antibodies to hepatitis C using a second generation enzyme immunoassay (Abbott Laboratories, Chicago, IL) and subsequently confirmed by the MATRIX assay (Abbott Laboratories). Presence of antibodies to hepatitis B core antigen was determined using a solid phase competitive immunoassay (Abbott Laboratories). Serum iron levels and total iron binding capacity were measured calorimetrically (Alpkem RFA analyzer, Clackamas, OR). Serum ferritin levels were measured using the BioRad Quantimmune IRMA kit (BioRad Laboratories, Hercules, CA).10,11
Hepatic necroinflammation
We considered concurrently elevated levels of both serum ALT and serum GGT levels as a marker of hepatic necroinflammation.12 The procedures for collection and analysis of the serum ALT and GGT samples are described elsewhere.12 We considered a cutoff of serum ALT level >30 U/L for men and >19 U/L for women and serum GGT level >51 U/L for men and >33 U/L for women as elevated. Because GGT was measured only in a random subsample of the NHANES III participants, all analyses with hepatic necroinflammation as an outcome were carried out on a subsample of 9909 individuals (77.8 % of the original sample for the other analyses) for whom GGT levels were available.
NAFLD and NASH
Between 2009 and 2010, 13,856 (96.6 %) of the archived ultrasound examinations on the gallbladder carried on the NHANES III participants aged 20 to 74 were reviewed to determine the presence of fat within the liver parenchyma. The details of the original examination and review are described elsewhere.13,14
We defined NAFLD and NASH using the definitions proposed by Lazo et al.15 Non-alcoholic fatty liver disease was defined as the presence of moderate to severe hepatic steatosis with normal liver enzymes levels. Non-alcoholic steatohepatitis was defined as the presence of moderate to severe hepatic steatosis with increased levels of liver enzymes, in the absence of antibodies to hepatitis B and hepatitis C and without evidence of iron overload. We excluded individuals with viral hepatitis B and hepatitis C or with iron overload from the NASH group, because these factors may be associated with increased liver enzyme levels.
Follow-up for mortality
The NHANES III-linked mortality file was used for followup analyses. Follow-up duration was calculated from the date of examination to either the date of death provided in the mortality file, in case the patient had died, or to 31st December 2006.16
Cause of death was determined from the underlying cause listed on the death certificate and reported in the mortality file. We used the publicly available linked mortality data files, which identify the cause of death using the Underlying Cause of Death-113 (UCOD 113) groups (international classification of disease, 10th revision). Cancer mortality was defined as deaths with underlying cause of death codes: ICD-10 C-00–C-97. Liver-related mortality was defined as deaths with underlying cause of death codes: ICD-10 K-70, or K-73–K-74. Liver cancer mortality was defined as deaths with underlying cause of death codes: ICD-10 C-22.
Statistical analysis
All the analyses, except for those related to liver-related and liver cancer mortality, were performed for the whole cohort and also separately for men and women because of a well-recognized difference in cadmium related outcomes by gender.4,17 The number of deaths attributed to liver-related mortality and liver cancer mortality was too small (<40 each) to allow for stable multivariable models for individual genders. We compared the baseline characteristics between individuals in the highest gender-specific quartile for creatinine-corrected urinary cadmium and those in the lower three quartiles using Pearson χ2 statistics for categorical variables or Wald test for continuous variables. For the baseline characteristics, all values, except for age and race categories, were adjusted for age and race.
In the cross-sectional NHANES III sample, multivariable logistic regression analysis was used to evaluate association of cadmium with hepatic necroinflammation, NAFLD, and NASH. Participants with NAFLD were excluded for the NASH analysis and vice versa. For the follow-up mortality cohort, Cox proportional hazards regression was used to estimate hazard ratios and 95 % confidence intervals for deaths from all liver disease and liver cancer by highest cadmium quartile status. For the cross-sectional sample, and for all-cause and all-cancer deaths we used two models with progressive degrees of adjustment: model 1 adjusted for age and race or ethnicity; model 2 further adjusted for education, smoking status, pack years of smoking, alcohol consumption, physical activity, body mass index, and serum cholesterol. For the liver-related mortality and liver cancer mortality, models were adjusted only for age, race, smoking, and alcohol use.
We carried out the following sensitivity analyses: (1) age was added to the models as a restricted quadratic spline with knots at the 10th, 50th, and 90th percentile of the overall study sample. (2) Serum cotinine was included in the models instead of pack years of smoking. These changes did not lead to any appreciable difference in the OR/HR or corresponding CI estimates. All analyses were done using the survey procedures of SAS 9.3 to account for the sampling weights and the complex survey design.
Results
Patient Characteristics
Mean age of participants in the weighted cohort was 42.2 years and most were female (51.1 %), and nonHispanic White (76.2 %); 24.4 % had less than 12 years of education. 29.3 % respondents reported being a current smoker at the time of the interview. Mean number of pack years for current or former smokers was 11.2 years. The mean BMI was 26.6 kg/m2. Mean AST, ALT, and GGT levels were 21.3±0.2 U/L, 17.9 ±0.4, and 29.2±0.6 U/L, respectively.
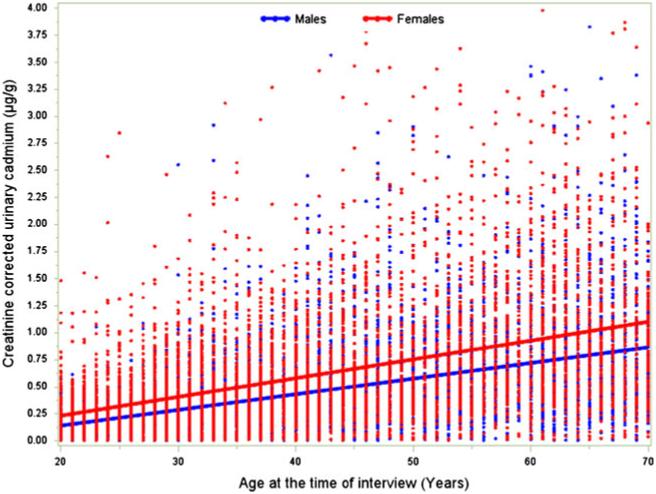
The mean urinary cadmium per gram of urinary creatinine was 0.52 ±0.01 μg/g; mean urinary cadmium per gram of urinary creatinine was lower for men (0.45 ±0.01 μg/g) than it was for women (0.63 ±0.02 μg/g) (P<0.001). The cutoffs for highest quartile of urinary cadmium per gram of urinary creatinine were 0.65 μg/g and 0.83 μg/g for men and women, respectively (P<0.001). Compared with people in the lower three quartiles of cadmium exposure, those in the top quartile were more likely to be non-Hispanic White, have <12 years of education, be sedentary, have a history of cardiovascular disease or cancer, have more pack years of smoking, and have higher total cholesterol. This was true for both men and women (Table 1). Urinary cadmium levels increased as individuals aged, and this phenomenon was noted to be similar in men versus women (Fig. 1).
Table 1.
Baseline characteristics of study participants by sex and creatinine-corrected urinary cadmium (uCd). The cutoffs for uCd separate the top quartile, and the bottom three quartiles of creatinine-adjusted urinary cadmium
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Characteristics | <0.65 μg/g (n=4478) |
≥0.65 μg/g (n=1510) |
P value | <0.83 μg/g (n=5046) |
≥0.83 μg/g (n=1698) |
P value |
| Mean (SE) age (years) | 38.6 (0.4) | 53.0 (0.4) | <0.001 | 39.1 (0.3) | 53.2 (0.5) | <0.001 |
| Race or ethnicity (%) | ||||||
| Non-Hispanic White | 76.1 (1.5) | 79.0 (2.0) | <0.001 | 75.1 (1.5) | 77.0(1.6) | <0.001 |
| Non-Hispanic Black | 9.6 (0.6) | 9.5 (0.8) | 12.2 (0.8) | 9.2 (0.9) | ||
| Mexican American | 6.5 (0.6) | 3.7 (0.3) | 5.5 (0.5) | 3.5 (0.3) | ||
| <12 years education (%) | 22.5 (0.4) | 30.0 (0.6) | <0.001 | 22.7 (0.3) | 30.7 (0.5) | <0.001 |
| Body mass index (kg/m2) | 26.4 (0.2) | 27.1 (0.3) | 0.06 | 26.4 (0.2) | 27.1 (0.3) | 0.06 |
| Sedentary (%) | 20.5 (0.3) | 23.6 (0.5) | <0.001 | 20.7 (0.3) | 24.2 (0.3) | <0.001 |
| History of CVD(%) | 2.1 (0.1) | 5.0 (0.2) | <0.001 | 2.1 (0.1) | 5.3 (0.2) | <0.001 |
| History of Cancer (%) | 5.4 (0.2) | 10.3 (0.2) | <0.001 | 5.4 (0.2) | 10.7 (0.3) | <0.001 |
| Current Smoking (%) | 30.2 (0.2) | 26.5 (0.2) | <0.001 | 30.2 (0.1) | 26.2 (0.2) | <0.001 |
| Mean (SE) Pack years | 9.7 (0.2) | 15.6 (0.2) | <0.001 | 9.9 (0.2) | 15.6 (0.2) | <0.001 |
| Mean (SE) serum Cotinine (ng/mL) | 79.7 (0.5) | 80.1 (0.5) | 0.14 | 81.2 (0.5) | 79.2 (0.5) | 0.06 |
| Consume alcohol (%) | 57.2 (0.3) | 49.6 (0.4) | <0.001 | 57.0 (0.2) | 49.6 (0.3) | <0.001 |
| Total cholesterol (mg/dL) | 199.2 (0.4) | 213.8 (0.4) | <0.001 | 199.6 (0.4) | 214.0 (0.5) | <0.001 |
| Mean (SE) alanine aminotransferase (U/L) | 17.8 (0.4) | 16.6 (0.4) | 0.06 | 17.7 (0.4) | 16.6 (0.4) | 0.06 |
| Mean (SE) aspartate aminotransferase (U/L) | 21.1 (0.3) | 21.2 (0.3) | 0.90 | 21.1 (0.2) | 21.2 (0.2) | 0.90 |
| Mean (SE) γ-glutamyl transpeptidase (U/La) | 28.2 (0.1) | 31.2 (0.2) | <0.001 | 28.5 (0.1) | 31.3 (0.2) | <0.001 |
Values are means (SE) or percentages (SE) of participants, unless otherwise specified. All values except for age and race/ethnicity were standardized for age (continuous), and race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other)
Among random subset of participants (n=9,909)
Fig. 1.
Increase in creatinine-adjusted urinary cadmium levels with age among participants in the NHANES III
Hepatic Necroinflammation, NAFLD and NASH: Impact of Cadmium
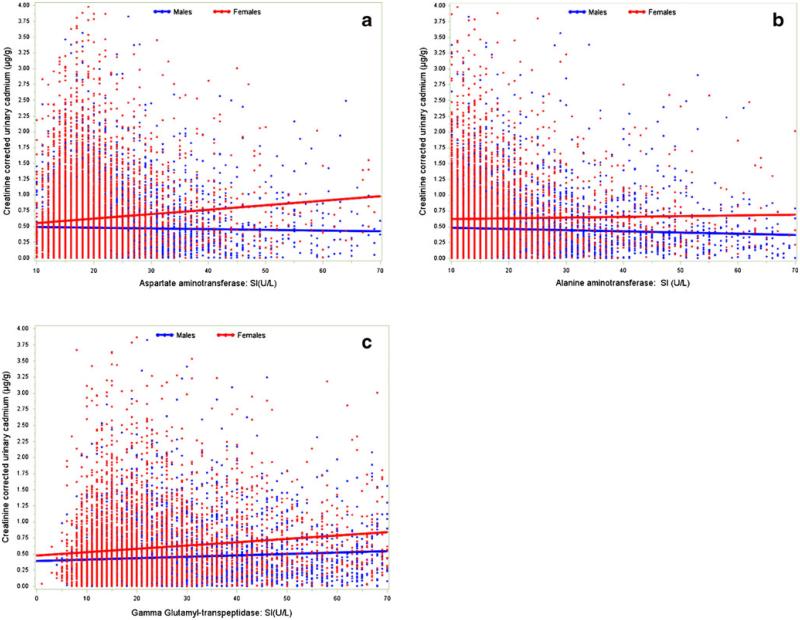
Hepatic enzyme levels were noted to increase as urinary cadmium level rose (Fig. 2). The overall incidence of hepatic necroinflammation was 22.5 %, and was no different in men versus women (21.9 % vs. 23.0 %, respectively; P=0.34). Individuals who were younger than 65 years (OR= 1.27, 95 % CI 1.12–1.43) and non-White (OR=1.42, 95 % CI 1.19–1.68) had a higher incidence of necroinflammation (both P < 0.05). O ther factors a ssociated w ith necroinflammation included education <12 years (OR = 1.22, 95 % CI 1.06–1.41), sedentary lifestyle (OR 1.59, 95 % CI 1.35–1.87), higher BMI (per unit increase in BMI; OR 1.08, 95 % CI 1.07–1.09), and higher plasma cholesterol levels (for each 10 mg/dL increase; OR 1.06, 95 % CI 1.05–1.08) (all P<0.05).
Fig. 2.
Liver enzyme levels according to creatinine-adjusted urinary cadmium levels among participants in the NHANES III. Panel (a) AST, (b) ALT, and (c) GGT
In examining necroinflammation relative to urinary cadmium levels, 20.8 % of men had hepatic necroinflammation in the lower three quartiles versus 26.3 % in the top quartile of urinary cadmium. The corresponding figures for women were 21.4 and 27.8 %, respectively. After adjusting for age and race/ethnicity, men in the upper quartile of urinary cadmium were noted to have a 56 % increased risk of hepatic necroinflammation (OR=1.56, 95 % CI 1.26–1.94; P<0.001), while women had a 23 % increased risk (OR= 1.23, 95 % CI 1.02–1.48; P=0.03). After adjusting for other competing risk factors in the fully adjusted multivariate model, high urinary cadmium levels remained associated with risk of hepatic necroinflammation. Specifically, men in the top quartile of urinary cadmium had over a twofold increased risk of hepatic necroinflammation (OR = 2.21, 95 % CI 1.64–3.00; P < 0.001), while the effect among women was more modest (OR =1.26, 95 % CI 1.01–1.57; P=0.04) (Table 2).
Table 2.
Multivariate logistic regression analysis of association of hepatic necroinflammation, NAFLD, and NASH with sex and highest quartile of creatinine-corrected urinary cadmium
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Characteristic | <0.65 μg/g | ≥0.65 μg/g | P value | <0.83 μg/g | ≥0.83 μg/g | P value |
| Hepatic necroinflammation | n=864 | n=328 | n=1057 | n=409 | ||
| Age and race/ethnicity adjusted | 1.00 | 1.56 (1.26–1.94) | <0.001 | 1.00 | 1.23 (1.02–1.48) | 0.03 |
| Multivariable adjusteda,b | 1.00 | 2.21 (1.64–3.00) | <0.001 | 1.00 | 1.26 (1.01–1.57) | 0.04 |
| NAFLD | n=840 | n=335 | n=835 | n=312 | ||
| Age and race/ethnicity adjusted | 1.00 | 0.98 (0.89–1.09) | 0.73 | 1.00 | 0.84 (0.71–1.01) | 0.57 |
| Multivariable adjusted | 1.00 | 1.30 (1.01–1.68) | 0.04 | 1.00 | 1.11 (0.88–1.41) | 0.37 |
| NASH | n=204 | n=74 | n=162 | n=64 | ||
| Age and race/ethnicity adjusted | 1.00 | 1.31 (0.82–2.10) | 0.25 | 1.00 | 1.06 (0.62–1.83) | 0.82 |
| Multivariable adjusted | 1.00 | 1.95 (1.11–3.41) | 0.02 | 1.00 | 1.34 (0.72–2.50) | 0.36 |
n number of events
Random subsample of 9909 individuals for whom γ-glutamyl transferase data was collected. Full sample for NAFLD and NASH
Adjustment included age (continuous), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, other), high school education, smoking category (never, former, current), pack years, sedentary lifestyle, BMI (continuous), alcohol consumption (no drinking, >0 but <1 drink/day, ≥1 but <2 drinks/day, ≥2 drinks/day), total cholesterol (continuous)
The overall incidence of NAFLD and NASH was 16.5 and 3.3 %, respectively. There was a slight predilection for men to have a higher incidence of both NAFLD and NASH (18.5 and 3.7 %) compared with women (14.5 and 2.9 %) (both P < 0.001). In looking at the entire cohort, factors associated with NAFLD included education <12 years (OR = 1.56, 95 % 1.30–1.86), sedentary lifestyle (OR = 1.43, 95 % 1.28–1.61), higher BMI (per unit increase in BMI; OR 1.14, 95 % CI 1.12-1.16), and higher plasma cholesterol levels (for each 10 mg/dL increase; OR 1.07, 95 % CI 1.05-1.09)(all P < 0.05). Several similar factors were associated with the risk of NASH, including higher BMI (per unit increase in BMI; OR 1.18, 95 % CI 1.15-1.20) and higher plasma cholesterol levels (for each 10 mg/dL increase; OR 1.11, 95 % CI 1.07-1.15) (all P< 0.05). Weighted proportion of NAFLD among men was 17.2 % in the lower three quartiles, and 22.9 % in the top quartile for cadmium. For women, the corresponding proportions were 13.8 % and 16.7 %, respectively. Weighted proportion of NASH among men was 3.6 % in the lower three quartiles, and 4.4 % in the top quartile for cadmium. For women the corresponding proportions were 2.6 and 3.6 %, respectively. After adjusting for competing risk factors, urinary cadmium levels were associated with an increased risk of NAFLD only among men. Specifically, men in the top quartile of urinary cadmium had a 30 % increased risk of NAFLD (OR = 1.30, 95 % CI 1.01–1.68; P=0.04) while no such association was noted among women (OR=1.11, 95 % CI 0.88–1.41; P=0.37). When examining urinary cadmium and NASH, a similar association was noted. While urinary cadmium levels were associated with risk of NASH among men (OR =1.95, 95 % CI 1.11–3.41; P=0.02), cadmium levels did not impact the risk of NASH among women (OR=1.34, 95 % CI 0.72–2.50; P=0.36).
Mortality and Cadmium Levels
The median follow-up was 14.6 years (range, 0.1 to 18.2 years). Table 3 presents the hazard ratios and 95 % CIs for the association of gender-specific top quartile of cadmium with all-cause and cancer mortality. There were 35 deaths attributed to liver disease. Fourteen events occurred in the lower three quartiles, while 21 deaths occurred in the top quartile of urinary cadmium levels. After adjustment for age, gender, race, and alcohol consumption, individuals in the top quartile of creatinine-corrected urinary cadmium had over threefold increased risk of liver disease mortality (HR = 3.42, 95 % CI 1.12–10.47; P=0.03). In contrast, individuals in the top quartile of creatinine-corrected urinary cadmium were not noted to have an increased risk of liver cancer related mortality (HR = 1.25, 95 % CI 0.37–4.27; P=0.52).
Table 3.
Hazard ratio (95 % CI) for all-cause and cancer related mortality associated with sex and top quartile of creatinine-corrected urinary cadmium
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Mortality | <0.65 μg/g | ≥0.65 μg/g | P value | <0.83 μg/g | ≥0.83 μg/g | P value |
| All cause | n=579 | n=626 | n=399 | n=461 | ||
| Age and race/ethnicity adjusted | 1.00 | 2.24 (1.82-2.74) | <0.001 | 1.00 | 1.80 (1.49-2.12) | <0.001 |
| Multivariable adjusteda | 1.00 | 1.77 (1.41-2.24) | <0.001 | 1.00 | 1.29 (1.02-1.62) | 0.03 |
| Cancer | n=106 | n=194 | n=105 | n=137 | ||
| Age and race/ethnicity adjusted | 1.00 | 3.68 (2.57-5.22) | <0.001 | 1.00 | 2.18 (1.57-3.04) | <0.001 |
| Multivariable adjusted | 1.00 | 2.43 (1.59-3.72) | <0.001 | 1.00 | 1.57 (1.10-2.23) | 0.01 |
n number of events
Adjustment included age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), high school education, smoking category (never, former, current), pack years, sedentary lifestyle, BMI (continuous), alcohol consumption (no drinking, >0 but <1 drink/ day, ≥1 but <2 drinks/day, ≥2 drinks/day), total cholesterol (continuous)
Discussion
Cadmium is an environmental pollutant with a long half-life that accumulates in the body with age and only a small portion of the body burden is excreted per day.18 As previously noted, urinary excretion of cadmium is commonly used as the dosimeter of lifetime exposure since it is proportional to the total body burden.8,18,19 Previous studies have examined the impact of cadmium environmental exposure and noted an increased risk of all-cause mortality and cardiovascular disease in men, but not women.4,20 Other studies have noted an association of urinary cadmium with impaired fasting glucose and diabetes.18 While limited preclinical data have suggested that liver injury may be associated with exposure to cadmium,5 no population data exist. The current study is important because, to our knowledge, it is the first study of chronic cadmium exposure and liver disease in a large population-based sample of individuals. Cadmium is one of several heavy metals, including lead, that is widely disseminated in the environment.20,21 Understanding the potential impact of cadmium on hepatic disease is relevant because the liver is one of the main biorepositories of cadmium. In the current study, we noted an association between high urinary cadmium levels and enzymatic markers of hepatic necroinflammation, NAFLD, and NASH among men. While in women, there was an association between high urinary cadmium levels and enzymatic markers of hepatic necroinflammation, no such association with NAFLD or NASH was noted. We also noted a relationship between high urinary cadmium levels and mortality.
While the overall prevalence of NASH were relatively low ( 3.3 % ), the p revalence o f NAFLD and necroinflammation were considerably higher (16.5 and 22.5%, respectively). As expected, certain factors were associated with an increased risk of liver disease. Specifically, higher BMI and hypercholesterolemia were each associated with NAFLD and NASH. While these factors are well-established risk factors for liver disease, we report the novel finding that high cadmium exposure was also associated with liver disease. On multivariable analyses, men in the highest quartile of creatinine-corrected urinary cadmium had a higher risk of necroinflammation, NAFLD, as well as NASH. While women with high cadmium exposure were also at higher risk of necroinflammation, the risk was much less pronounced and no such association between cadmium levels and NAFLD or NASH was noted. Sex-specific differences in the effect of cadmium exposure on other non-liver specific outcomes have been previously reported.4 While such differences are undoubtedly multifactorial, some investigators have speculated that the differences may be due to the interaction of cadmium and menopause status. Cadmium may be redistributed in bone after menopause.22 In addition, cadmium may have estrogen- and androgen-like activities with differential effects in pre- versus post-menopausal women, as well as men.23
There is limited evidence available on the mechanisms through which chronic cadmium exposure affects the liver. Irrespective of the source, cadmium is normally taken up from the bloodstream by the liver and complexed to metallothionein (MT). This Cd-MT complex neutralizes the toxic effects of cadmium.19 Following glomerular filtration of this complex, renal tubular cells destroy the MT portion and re-complex the Cd with MT secreted in the renal tubular cell cytoplasm. It has been noted that higher levels of cadmium exposure can exceed the production capacity of MT in the renal tubular cytoplasm leading to free cadmium.24 The effects of cadmium on the liver may be through a similar mechanism of cadmium levels exceeding hepatic MT production. In mouse models of chronic cadmium exposure induced hepatotoxicity, inflammation and apoptosis of hepatocytes have been noted in both wild-type (mice with intact MT) and MT-null mice, but were more pronounced in the latter.5 A threshold effect of cadmium exposure and depletion of MT may potentially explain our finding of an association between cadmium and adverse liver outcomes only in the top quartile for cadmium exposure, but not at lower levels.
Cadmium exposure was associated with an increased risk of mortality. Specifically, patients in the upper quartile of cadmium exposure had over a threefold increased risk of liver disease mortality (HR =3.42, 95 % CI 1.12–10.47; P= 0.03) even after adjusting for major competing risk factors. Other investigators have similarly noted an increase in overall and cancer-specific mortality among individuals with high cadmium exposure.4,25 Menke and colleagues noted a 28 and 55 % increased risk of all-cause and cancer-specific death among men with high urinary cadmium levels.4 Menke et al. did not find a similar association among women. In the current study, while we did note an increase in adjusted all-cause mortality with higher cadmium exposure levels among all patients, the effect was much more pronounced in men (Table 3). Unlike any previous study, we also investigated liver disease related mortality as an outcome measure for cadmium exposure. Of note, we found a strong association between high cadmium urinary levels and the risk of liver-related mortality, with high-exposure patients having more than 240 % increase in risk of liver-related mortality even after adjusting for other factors. We did not, however, find an association between high urinary cadmium and liver cancer mortality. Indeed, there were only six patients whose death was attributable to liver cancer. Given the relatively rarity of liver cancer deaths, the current analyses may have been susceptible to a type II statistical error. Given the population-based nature of the NHANES III dataset, any association between cadmium exposure and liver cancer mortality is likely, however, to be small.
The current study had several limitations. Ultrasonography in combination with liver enzyme levels was utilized to assess liver status (e.g., necroinflammation, NAFLD, NASH). While other authors have reported that such methods are reasonably accurate,26 such classification schemes may have led to some misclassification. However, the definitions for hepatic necroinflammation, NAFLD, and NASH were established and have been utilized by previous investigators using similar datasets.12,15 In addition, any misclassification owing to a reliance on measurements of liver enzymes and liver fat would most likely have been non-systematic and would have, if anything, biased the results towards the null. Other limitations of the current study include the fact that many covariates included in NHANES III were self-reported. These limitations are offset, however, by the strength of the NHANES III being one of the only nationally representative datasets that contains information on liver imaging, hepatic enzymes, cadmium exposure as well as long-term mortality. In addition, data from the NHANES III were collected by trained staff following rigorous, standardized protocols to minimize measurement errors.
In conclusion, we report an association between high urinary cadmium levels and enzyme markers of hepatic necroinflammation, NAFLD, and NASH primarily among men in the US population. The effect of cadmium exposure on hepatic necroinflammation, NAFLD, and NASH among women was less pronounced. After adjustment, individuals in the top quartile of creatinine-corrected urinary cadmium had over threefold increased risk of liver disease mortality but not in liver cancer related mortality. These findings have important implications considering the relatively high prevalence of cadmium in the environment. Further study on the effect of chronic exposure to cadmium on liver-related conditions in the general population is needed so that adverse outcomes from hepatic cadmium accumulation can be better defined and obviated.
Financial Support
No financial support.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NHANES
National Health and Nutrition Examination Survey
- CI
Confidence intervals
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BMI
Body mass index
- GGT
γ-Glutamyltranspeptidase
- OR
Odds ratio
- HR
Hazard ratio
- MT
Metallothionein
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environmental health perspectives. 2004;112(10):1099–103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verougstraete V, Lison D, Hotz P. Cadmium, lung and prostate cancer: a systematic review of recent epidemiological data. Journal of toxicology and environmental health. Part B, Critical reviews. 2003;6(3):227–55. doi: 10.1080/10937400306465. [DOI] [PubMed] [Google Scholar]
- 3.Bernard A. Renal dysfunction induced by cadmium: biomarkers of critical effects. Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine. 2004;17(5):519–23. doi: 10.1023/b:biom.0000045731.75602.b9. [DOI] [PubMed] [Google Scholar]
- 4.Menke A, Munter P, Silbergeld EK, Platz EA, Guallar E. Cadmium levels in urine and mortality among U.S. adults. Environmental health perspectives. 2009;117(2):190–96. doi: 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habeebu SS, Liu J, Liu YP, Klaassen CD. Metallothionein-null mice are more sensitive than wild-type mice to liver injury induced by repeated exposure to cadmium. Toxicol Sci. 2000;55(1):223–32. doi: 10.1093/toxsci/55.1.223. [DOI] [PubMed] [Google Scholar]
- 6.Statistics NCfH Service DoHaH., editor. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. 1994 [Google Scholar]
- 7.Ioannou GN, Morrow OB, Connole ML, Lee SP. Association between dietary nutrient composition and the incidence of cirrhosis or liver cancer in the United States population. Hepatology. 2009;50(1):175–84. doi: 10.1002/hep.22941. [DOI] [PubMed] [Google Scholar]
- 8.Paschal DC, Burt V, Caudill SP, Gunter EW, Pirkle JL, Sampson EJ, et al. Exposure of the U.S. population aged 6 years and older to cadmium: 1988–1994. Archives of environmental contamination and toxicology. 2000;38(3):377–83. doi: 10.1007/s002449910050. [DOI] [PubMed] [Google Scholar]
- 9.Statistics NCfH Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994: Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Environmental Health. 1996 [Google Scholar]
- 10.Edwards CQ, Griffen LM, Goldgar D, Drummond C, Skolnick MH, Kushner JP. Prevalence of hemochromatosis among 11,065 presumably healthy blood-donors. New Engl J Med. 1988;318(21):1355–62. doi: 10.1056/NEJM198805263182103. [DOI] [PubMed] [Google Scholar]
- 11.Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A population-based study of the clinical expression of the hemochromatosis gene. N Engl J Med. 1999;341:718–24. doi: 10.1056/NEJM199909023411002. [DOI] [PubMed] [Google Scholar]
- 12.Afzali A, Weiss NS, Boyko EJ, Ioannou GN. Association between serum uric acid level and chronic liver disease in the United States. Hepatology. 2010;52(2):578–89. doi: 10.1002/hep.23717. [DOI] [PubMed] [Google Scholar]
- 13.Statistics NCfH Hepatic steatosis assessment procedure manual. 2010 [Google Scholar]
- 14.Westat Third National Health and Nutritional Survey: gallbladder ultrasonography procedure manual. 1988 [Google Scholar]
- 15.Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. Brit Med J. 2011:343. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, Guallar E. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US National Health and Nutrition Examination Survey. American journal of epidemiology. 2010;172(6):671–81. doi: 10.1093/aje/kwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferraro PM, Costanzi S, Naticchia A, Sturniolo A, Gambaro G. Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999–2006. BMC public health. 2010;10:304. doi: 10.1186/1471-2458-10-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care. 2003;26(2):468–70. doi: 10.2337/diacare.26.2.468. [DOI] [PubMed] [Google Scholar]
- 19.Nordberg GF, Piscator M, Nordberg M. On the distribution of cadmium in blood. Acta Pharmacol Toxicol. 1971;30:289–95. doi: 10.1111/j.1600-0773.1971.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S, Zaman T, Tuzcu EM, Kapadia SR. Heavy metals and cardiovascular disease: results from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Angiology. 2011;62(5):422–9. doi: 10.1177/0003319710395562. [DOI] [PubMed] [Google Scholar]
- 21.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Nash D, Magder LS, Sherwin R, Rubin RJ, Silbergeld EK. Bone density-related predictors of blood lead level among peri- and postmenopausal women in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. American journal of epidemiology. 2004;160(9):901–11. doi: 10.1093/aje/kwh296. [DOI] [PubMed] [Google Scholar]
- 23.Vahter M, Akesson A, Liden C, Ceccatelli S, Berglund M. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Andujar P, Bensefa-Colas L, Descatha A. Acute and chronic cadmium poisoning. Rev Med Interne. 2010;31(2):107–15. doi: 10.1016/j.revmed.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Adams SV, Passarelli MN, Newcomb PA. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occupational and environmental medicine. 2012;69(2):153–6. doi: 10.1136/oemed-2011-100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver : a meta-analysis. Hepatology. 2011;54(3):1082–90. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]