Abstract
Background
Left ventricular hypertrophy (LVH) typically manifests during or after adolescence in sarcomere mutation carriers at risk for developing hypertrophic cardiomyopathy. Guidelines recommend serial imaging of mutation carriers without LVH (G+/LVH−) to monitor for phenotypic evolution, but the optimal strategy is undefined. Compared with echocardiography (echo), cardiac MRI (CMR) offers improved endocardial visualization and potential to assess scar. However, the incremental advantage offered by CMR for early diagnosis of hypertrophic cardiomyopathy is unclear. Therefore, we systematically compared echo and CMR in G+/LVH− subjects.
Methods and Results
A total of 40 sarcomere mutation carriers with normal echo wall thickness (<12 mm or z score <2.5 in children) underwent concurrent CMR. Mean age was 21.7±11.1 years, 55% were female. If left ventricular wall thickness seemed nonuniform, the size and location of relatively thickened segments were noted. Late gadolinium enhancement was assessed with CMR. Diagnostic agreement between echo and CMR was good (90%), although CMR measurements of left ventricular wall thickness were ≈19% lower than echo. Four subjects had mild hypertrophy (12.6–14 mm; ≤2 segments) appreciated by CMR but not echo. No subjects had late gadolinium enhancement. During median 35-month follow-up, 2 subjects developed overt hypertrophic cardiomyopathy, including 1 with mild LVH by CMR at baseline.
Conclusions
Echo is unlikely to miss substantial LVH; however, CMR identified mild hypertrophy in ≈10% of mutation carriers with normal echo wall thickness. CMR may be a useful adjunct in hypertrophic cardiomyopathy family screening, particularly in higher risk situations, or if echocardiographic images are suboptimal or suggest borderline LVH.
Keywords: cardiac MRI; cardiomyopathy, hypertrophic; echocardiography; genetics; humans
We now recognize that ≈60% of familial hypertrophic cardiomyopathy (HCM) is caused by sarcomere gene mutations.1,2 Although the clinical diagnosis of HCM relies on identifying unexplained left ventricular hypertrophy (LVH), LVH is not invariably present in sarcomere mutation carriers throughout life. The expression of LVH is highly age dependent, and LV wall thickness is typically normal during childhood.3,4 Overt cardiac hypertrophy often does not emerge until late adolescence or beyond. Through genetic testing, relatives who have inherited the family's pathogenic sarcomere mutation (G+) can be identified before they exhibit diagnostic clinical features of the disease (LVH−). This allows characterization of a new and important subset, denoted subclinical, preclinical, or G+/LVH− HCM.2,5,6 During this early stage, there are few, if any, clinically relevant events. However, there is evidence that G+/LVH− mutation carriers have myocardial abnormalities, including diastolic dysfunction, electrocardiographic changes, increased collagen synthesis, impaired energetics, and expanded myocardial extracellular volume even when left ventricular (LV) wall thickness is normal.7–10
Because these apparently healthy mutation carriers are at high risk for developing HCM, guidelines recommend longitudinal screening, performed most frequently during adolescence, to assess for the emergence of clinical disease.5,6 A diagnosis of HCM would in turn trigger consideration of important clinical questions relevant in the context of overt disease, such as sudden death risk stratification and exercise restrictions. The optimal strategy for evaluating the G+/LVH− population has not been established. Transthoracic echocardiography (echo) has traditionally been used but has limitations. For example, focal areas of myocardial hypertrophy may not be appreciated by echo, particularly at the inferoseptum, apex, and LV or right ventricular free walls.11–13 Consequently, a diagnosis of HCM may be missed or maximal wall thickness (MWT) underestimated.
Cardiac MRI (CMR) provides accurate assessment of regional LV wall thickness and mass.11,12,14,15 Strengths of this technique include the sharp contrast between the myocardial border and blood pool and tomographic coverage of the entire myocardium without obliquity. This allows highly reproducible and operator independent measurements of wall thickness in any segment of the left ventricle.16 Additionally, with gadolinium contrast, CMR enables tissue characterization and identification of focal myocardial fibrosis, commonly seen in patients with overt HCM.17
Recognizing the importance of making a timely and accurate diagnosis of clinically overt HCM in sarcomere mutation carriers, we undertook systematic longitudinal study, comparing echo and CMR, to examine the incremental information provided by CMR imaging in the G+/LVH– population.
Methods
Study Population
The study cohort was drawn from subjects participating in ongoing research studies and from patients receiving clinical care at Brigham and Women's Hospital and Boston Children's Hospital between 2006 and 2011. Members of HCM families with pathogenic or likely pathogenic sarcomere mutations were potential participants, focusing on G+/LVH− at-risk mutation carriers. Subjects were eligible for inclusion in this study if they (1) were confirmed to carry their family's pathogenic or likely pathogenic HCM-associated sarcomere mutation (G+); (2) did not have echocardiographic evidence of hypertrophy (LVH−; defined as maximal LV wall thickness <12 mm or z score <3 in subjects <18 years of age); and (3) had a CMR performed within 6 months of echo. The pathogenicity of sarcomere variants was determined using standard criteria as previously described.18,19 Echo, CMR, and standard 12-lead ECGs were available on all participants. These studies were performed either during participation in research studies or in the course of clinical family screening of at-risk relatives.
In accordance with recommended criteria for diagnosing HCM in family members,4,20,21 a clinical diagnosis of HCM was established based on a maximal LV wall thickness ≥13 mm in subjects ≥18 years of age or z score relative to body surface area ≥3.5 in subjects <18 years of age. An echocardiographic maximal LV wall thickness of 12 to 13 mm was considered ambiguous, and such subjects (n=2) were excluded from analysis. Subjects were also excluded if they had systemic hypertension (systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg) or were on medical therapy. Follow-up studies were obtained on all available subjects during the study period. Informed consent was obtained from all participants in protocols approved by the Institutional Review Boards of Brigham and Women's Hospital and Boston Children's Hospital.
Echocardiography
Each subject underwent echo, including 2-dimensional, Doppler, and tissue Doppler imaging according to guidelines.22,23 Images were stored digitally and analyzed offline by an experienced echocardiographer blinded to CMR measurements. Standard measures of cardiac dimensions were determined from the mean of 3 cardiac cycles in accordance with guidelines of the American Society of Echocardiography.22 The end-diastolic thickness of the interventricular septum (IVS) and posterior wall (PW) was measured from 2-dimensional parasternal long-axis images and taken at the level of the mitral valve leaflet tips. In addition to measuring the IVS and PW, the uniformity of LV wall thickness was visually assessed in all LV segments by examining multiple parasternal short-axis levels (basal, mid, and apical), and the apical views, to identify regions with disproportionately increased thickness. If a relatively thickened segment was identified, the location was noted and MWT was measured. Early myocardial tissue Doppler relaxation velocities (Ea) were measured at the lateral, septal, anterior, and inferior aspects of the mitral annulus. Global Ea velocity was determined by averaging these 4 values.
Echocardiographic Image Quality Assessment
Image quality was assessed for each segment in the 17-segment model.24 Echo image grading was defined as the following:
=Excellent: endocardial and epicardial borders were clearly defined. Excellent quality measurement of wall thickness.
=Good: endocardial and epicardial borders were clearly visualized in the majority of the segment with only brief gaps requiring interpolation. Confident measurement of wall thickness.
=Fair: endocardial and epicardial borders were adequately identifiable but indistinct in some (<30%) portion of the segment, requiring minor interpolation.
=Poor: significant portions of the endocardial and epicardial borders were indistinct, requiring interpolation from visible, but indistinct, segments or more than a short segment of the boundary was outside of the imaging sector.
=Unusable: LV wall thickness was not adequately recorded or too poorly visualized for accurate measurement.
Overall image quality assessment was represented as the median score of all segments.
Cardiac MRI
All subjects underwent CMR imaging within 6 months of echocardiographic study. CMR was performed on 1 of 2 1.5-T scanners (Achieva, Philips Healthcare, Best, The Netherlands or HDX Excite II, General Electric, Milwaukee, WI) or a 3.0-T system (Magnetom Trio, Siemens, Erlangen, Germany). ECG-gated, breath-hold steady-state free precession cine images were acquired in both the long-axis and the short-axis planes from the apex to the base of the left ventricle, with slice thickness 8 to 10 mm. Images were analyzed offline using dedicated software (Medis QMass v. 7.4, Leiden, The Netherlands) by an experienced CMR cardiologist, blinded to echo results. LV systolic function was quantified using standard criteria.16 Measurements were made of the IVS wall thickness at the base (mitral valve leaflet tips) and midventricular level (papillary muscle level), and corresponding PW thickness, corresponding to the same locations used in measuring echocardiographic images. As with echocardiographic image analysis, the uniformity of LV wall thickness was visually assessed in all LV segments to identify regions with disproportionately increased thickness. If a relatively thickened segment was identified, the MWT and location were noted.
Intravenous gadolinium dimeglumine (Magnevist, Berlex Laboratories, Wayne, NJ) at a dose of 0.1 mmol/kg was administered, and rest perfusion imaging was completed. Segmented inversion-recovery fast gradient echo imaging was used to assess late gadolinium enhancement (LGE) 10 minutes after a total dose of 0.15 to 0.2 mmol/kg gadolinium dimeglumine.
CMR Image Quality Assessment
CMR image quality was graded using the following scoring system:25
=Excellent: sharply defined endocardial and epicardial borders.
=Good: minimal blurring of endocardial/epicardial borders.
=Fair: endocardial and epicardial borders visible with moderate blurring.
=Poor: endocardial and epicardial borders visible but markedly blurred.
=Unusable: either endocardial or epicardial borders could not be defined.
Overall image quality assessment was represented as the median score of all segments.
Statistical Methods
Measurements are reported as mean±SD. Tests of normality were performed to confirm that measures of LV wall thickness were normally distributed. The mean echocardiographic and CMR LV wall thicknesses and LV ejection fraction were compared using clustered regression to adjust for the influence of relationships between family members. An analysis of variance was performed using the GenMod procedure in SAS to account for these relationships, and a P<0.05 was considered statistically significant. Intracluster correlation coefficients were also determined to compare echo and CMR measurements. Bland–Altman plots were prepared to assess the agreement between echo and CMR measures of ventricular wall thickness.26 All analyses were performed with SAS version 9.0 (Cary, NC).
Results
Clinical Characteristics
Clinical characteristics are summarized in Table 1. A total of 40 subjects from 27 unrelated families underwent echo and CMR; 85% had both studies on the same day. Of the 6 subjects who did not have both studies performed on the same day, the interval between studies ranged from 1 to 174 days (median interval, 24.5 days), with the echo occurring first. The mean age of the cohort was 21.7±11.1 years, and there was a slight female predominance (55%). As is typical for HCM, MYH7 and MYBPC3 mutations were most common and present in 88% of subjects (all mutations are listed in the Table in the online-only Data Supplement). No subjects had cardiovascular symptoms or were receiving cardioactive medications.
Table 1. Clinical Characteristics.
| n=40 | |
|---|---|
| Age, y (range) | 21.7±11.1 (5–40) |
| Female, n (%) | 22 (55) |
| Causal gene, n | |
| MYH7 | 19 |
| MYBPC3 | 16 |
| TNNT2 | 5 |
| Body surface area | 1.67±0.36 |
| Heart rate, beats per minute | 69±14 |
| Systolic blood pressure, mm Hg | 108±20 |
| Diastolic blood pressure, mm Hg | 67±8 |
All values are mean±SD unless otherwise indicated.
Transthoracic Echocardiographic Measurements
As defined for study entry, maximal echo LV wall thickness was <12 mm (or z score <3) in all subjects. Mean IVS thickness was 8.3±1.5 mm, mean PW thickness was 7.9±1.4 mm, and LV ejection fraction was normal in all subjects (Table 2). The median overall echo image quality score was 2 (good), and 90% of subjects had fair-to-excellent image quality. Four subjects (10%) had an overall image quality score of 4 (poor). Thirteen subjects (39%) had nonuniform LV wall thickness with a myocardial segment that seemed relatively thicker, most commonly at the midventricular septum. Maximal wall thickness ranged from 6.8 to 11.9 mm (mean, 9.7±1.8 mm).
Table 2. Echo and CMR Measurements.
| Echo | CMR | Difference* | P Value† | |
|---|---|---|---|---|
| IVS, mm (range) | 8.3±1.5 (5.5 to 10.9) | 7.2±2.0 (2.7 to 11.5) | 1.1±1.5 (−1.8 to 4.2) | <0.001 |
| PWT, mm (range) | 7.9±1.4 (4.9 to 10.7) | 6.2±2.0 (2.0 to 12.6) | 17±1.5 (-2.4 to 5.2) | <0.001 |
| LVEF, % | 68±5 | 67±5 | 1±8 | 0.33 |
| Median overall image quality | Good | Excellent | ||
| Excellent, n (%) | 2 (5) | 21 (53) | ||
| Good, n (%) | 20 (50) | 13 (32) | ||
| Fair, n (%) | 14 (35) | 5 (13) | ||
| Poor, n (%) | 4 (10) | 1 (2) | ||
| Unusable, n (%) | 0 | 0 |
All values are mean±SD unless otherwise indicated. CMR indicates cardiac MRI; IVS, interventricular septum; LVEF, left ventricular ejection fraction; and PWT, posterior wall thickness.
Difference between Echo and CMR measurement (Echo-CMR).
Adjusted for family relations.
CMR Measurements
The mean CMR IVS and PW thicknesses were 7.2±2.0 and 6.2±2.0 mm, respectively (Table 2). The median overall CMR image quality score was 1 (excellent), and only 1 subject had poor overall CMR image quality. Twenty-five subjects (64%) had nonuniform LV wall thickness, with maximal thickness ranging from 5.5 to 13.9 mm (mean, 9.4±2.2 mm), typically involving the midventricular septum. Three pediatric subjects (6, 8, and 12 years of age) declined intravenous catheter insertion and did not receive gadolinium. Thirty-seven subjects (93%) received gadolinium. No subjects had resting perfusion defects or LGE.
Comparison of LV Wall Thickness Determination by Echo Versus CMR
Figure 1 illustrates the moderate agreement between echo and CMR LV wall thickness measurements. Intracluster correlation coefficients were 0.62 for the IVS and 0.34 for the PW. CMR LV wall thickness measurements were on average ≈19% lower than echo. Of 40 subjects, both echo and CMR categorized 36 as without hypertrophy (LVH−), indicating clinical diagnostic agreement of 90% between these 2 modalities. In 3 of 40 subjects (7.5%), CMR detected mild, focal hypertrophy (measuring 13.1–13.9 mm) that did not reach the defined threshold for LVH and diagnosing HCM (13 mm) by echo. Subject 4 had borderline LVH by CMR (MWT=12.6 mm) that was just under the defined LVH threshold by echo (MWT=11.9 mm; Figure 2; Table 3). Echo and CMR were performed on the same day for these 4 subjects. In discordant subjects, the mean difference between CMR and echo measurements of maximal LV wall thickness was 1.9±1.2 mm (range, 0.7–3.5 mm). Table 3 also lists other subtle abnormalities present in these 4 subjects, including low global Ea velocities and mild ECG abnormalities.
Figure 1.
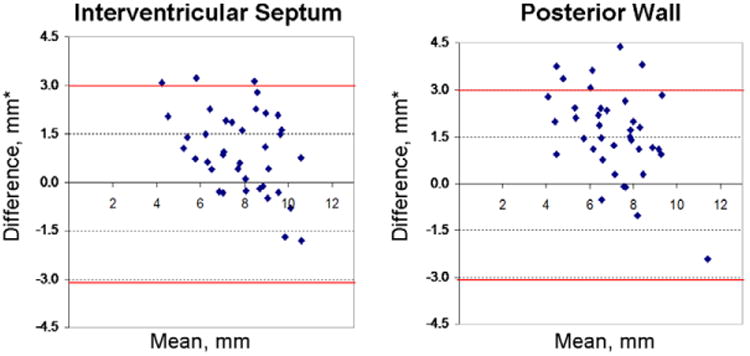
Agreement between echocardiographic and cardiac MRI (CMR) measurements of septal and posterior left ventricular (LV) wall thickness. Bland–Altman plots demonstrate that echocardiographic (echo) measurements were usually greater than CMR measurements. The red lines depict 2 SDs from the mean difference between echo and CMR measurements. *Difference between mean echo and CMR measurements of wall thickness. Mean=average of the echo and CMR measurement of LV wall thickness.
Figure 2.
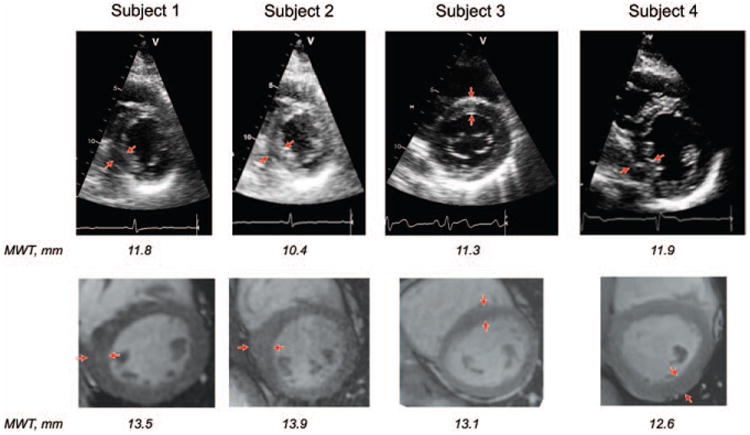
Paired echocardiography (echo) and cardiac MRI (CMR) basal and midventricular short-axis images from the 4 subjects who had maximal wall thickness (MWT) ≥12 mm identified by CMR but not by echo. Regions of nonuniform increased left ventricular wall thickness are indicated by the arrows.
Table 3. Characteristics of the 4 Subjects With Discordant Echo and CMR Determination of LVH.
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | |
|---|---|---|---|---|
| Age at baseline study, y | 17 | 36 | 34 | 23 |
| Sex | Male | Male | Female | Male |
| Causal gene (mutation) | MYH7 (Arg663Cys) | MYBPC3 (Arg502Trp) | MYBPC3 (Glu258Lys) | MYH7 (Arg719Gln) |
| BSA, m2 | 2.3 | 1.8 | 1.8 | 1.9 |
| IVS, mm | ||||
| Echo | 10.0 | 10.4 | 9.4 | 9.7 |
| CMR | 8.0 | 9.0 | 9.0 | 11.5 |
| PWT, mm | ||||
| Echo | 9.5 | 9.0 | 8.9 | 10.2 |
| CMR | 9.5 | 6.6 | 7.6 | 12.6 |
| LVEF, % | 73 | 70 | 61 | 60 |
| MWT, mm | ||||
| Echo | 10.4 (M-IS) | 11.8 (M-IS) | 11.3 (B-AS) | 11.9 (M-IS) |
| CMR | 13.9 (M-I) | 13.5 (M-IS) | 13.1 (M-AS) | 12.6 (M-PW) |
| Echo quality | Fair | Poor | Poor | Good |
| CMR quality | Fair | Good | Excellent | Good |
| Time interval between echo and | 0 days | 0 days | 0 days | 0 days |
| CMR | ||||
| LGE | None | None | None | None |
| Global Ea, cm/s | 12.2 | 11.8 | 13.2 | 11.1 |
| ECG | Normal | T wave Inversions | Left atrial enlargement | Normal |
| Follow-up findings | Development of overt LVH over 24 mo | Unchanged LV wall thickness (by echo) over 63 mo | Unchanged LV wall thickness (by echo and CMR) over 48 mo | Pending (baseline study performed during final year of study period) |
B-AS indicates basal anteroseptum; BSA, body surface area; CMR, cardiac MRI; Ea, global early myocardial tissue Doppler relaxation velocity; Echo, echocardiography; IVS, interventricular septum; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; M-AS, anterior septum at midventricle; M-I, inferior wall at midventricle; M-IS, inferior septum at midventricle; M-PW, midventricle posterior wall; MWT, maximal wall thickness; and PWT, posterior wall thickness.
Longitudinal Follow-up
Thirty subjects (75%) underwent follow-up imaging. A median of 35 months elapsed between baseline and follow-up studies (range, 24.5–61.5 months). Of the 10 subjects without follow-up, 7 completed baseline studies within the final year of the study period, and longitudinal evaluation is planned; 3 subjects declined further study. Serial evaluation was available in 3 of 4 mutation carriers with discordant baseline Echo−/CMR+ LVH (Table 3). One subject, a 17-year-old male with a MYH7 mutation (Arg663Cys), showed clear progression to overt HCM (Figure 3A). Baseline images showed mild asymmetry of the midinferoseptal segment by both modalities, more prominent on CMR. At 1-year follow-up, LV wall thickness was clearly increased on echo, to a maximal measurement of 14.6 mm, demonstrating clear phenotypic conversion to clinically overt HCM. LV wall thickness increased further at 2- and 3-year follow-up, but there was no further progression by echo at 4-year follow-up (17.5 mm; age 21 years).
Figure 3.

Development of overt HCM in 2 subjects during longitudinal follow-up. A, Paired echocardiography (echo) and cardiac MRI (CMR) parasternal short-axis images at the midventricular level from a 17-year-old male. Baseline images show nonuniform left ventricular (LV) wall thickness, relatively increased at the midinferoseptum, measuring 10.4 mm by echo and 13.9 mm by CMR. Subsequent imaging revealed development of obvious hypertrophy in this region of the inferoseptum. On echo follow-up, this segment measured 14.6 mm at 12 months and 15.5 mm at 24 months. At 36-month follow-up, this segment measured 17.5 mm by echo and 22.0 mm by CMR. By echo, there was no further thickening at 48 months (21 years of age). B, Paired echo and CMR parasternal short-axis images at the midventricular level from a 12-year-old male. Baseline images show normal LV wall thickness by echo and CMR. At 18 months, echo showed focally increased LV wall thickness, measuring 12.8 mm at the distal lateral wall and 12.3 mm at the midinferior septum; by CMR, this segment measured 15 mm. At 24 months, there is obvious LVH, measuring 15.5 mm both by echo and CMR. Maximal wall thickness (MWT) measurements remained stable at 36 months (15 years of age).
Of the other 3 subjects with baseline Echo−/CMR+ LVH, a 36-year-old male (41 years old at follow-up) with a MYBPC3 mutation (Arg502Trp) and a 34-year-old female (38 years old at follow-up) with a MYBPC3 mutation (Glu258Lys) did not demonstrate substantial change in LV wall thickness during 5 and 4 years of follow-up, respectively. The fourth subject, a 23-year-old male, completed baseline evaluation within the final year of the study period and will be followed longitudinally.
Twenty-seven subjects (75%) with concordant, normal LV wall thickness by echo and CMR had ≥2 years of follow-up available. One subject developed overt HCM during a 2-year period (Figure 3B). This subject is a male with a MYH7 mutation (Arg719Gln), who was 12 years of age at baseline. At 18 months, maximal LV wall thickness was 12.8 mm (z score 3.9) by echo and 15 mm by CMR at the midinferior septum. At 24 months, overt HCM was clearly apparent on echo (maximal LV wall thickness 15.5 mm, z score 8.0) at the midinferior septum, confirmed by CMR. These measurements remained stable at 36 months of follow-up (15 years of age).
Discussion
Genetic testing for HCM has been commercially available for nearly a decade. Such testing can identify a unique and important at-risk population: individuals who have inherited a pathogenic sarcomere mutation but have not yet developed traditional diagnostic evidence of disease (ie, LVH) on imaging. Many important issues about this early phase of HCM are unresolved, including establishing the optimal strategy for following apparently healthy sarcomere mutation carriers, understanding the absolute likelihood that clinical disease will develop, and predicting the risk of adverse outcomes irrespective of phenotypic disease expression. In this study, we take advantage of definitive genotypic identification of affected individuals to explore the incremental use of CMR over echo as a diagnostic tool in early HCM.
The best noninvasive imaging modality to assess G+/LVH− individuals has not been determined. There has been limited previous comparison of echo and CMR and limited longitudinal study of genotyped cohorts. Transthoracic echo is standardly used; however, CMR offers the prospect not only of sharp myocardial border delineation and comprehensive tomographic imaging of ventricular morphology and wall thickness, but also detection of focal myocardial scar. Together, these attributes have fostered enthusiasm for using CMR in risk stratification for sudden death and for early diagnosis of at-risk family members. Our data suggest that the incremental clinical impact of CMR in LVH-negative sarcomere mutation carriers is relatively modest. However, there may be specific situations in which CMR provides additional value.
In this systematic comparison of echo and CMR in G+/LVH− HCM, the clinical diagnostic agreement between modalities was 90%. CMR detected focal, mildly increased LV wall thickness that was not fully appreciated by echo in a small proportion of mutation carriers. This proportion of discordant echo and CMR classification of LVH is comparable with previous reports that included small numbers of G+/LVH− subjects as part of a larger cohort11,27 and also comparable with findings in patients with a clinical diagnosis of HCM (no genotyping available).12 In our investigation, the amount of hypertrophy detected by CMR and underappreciated by echo was modest, <14 mm in maximal dimension and affecting no more than 2 segments of the LV. Moreover, the absolute difference between echo and CMR measures of LV wall thickness was small in discordant subjects, with a mean difference of <2 mm between modalities. Echo image quality was fair or poor in 3 of 4 discordant subjects, potentially limiting the accuracy of wall thickness measurements. Suboptimal echo image quality was obtained despite the presence of normal body habitus and image acquisition by experienced sonographers. Collectively, these findings indicate that echo is unlikely to miss substantial hypertrophy. Notably in segments with normal wall thickness, CMR measurements were ≈19% lower than echo. This raises the question as to whether different standards to diagnose HCM should be used by echo and CMR. Obtaining more robust normative CMR data, particularly in children, is crucial to more fully address this issue.
The unique ability of CMR to identify dense, focal myocardial fibrosis did not provide additional insights in this G+/LVH− population. LGE was not seen in any of the mutation carriers in this study, including CMR LVH+ subjects and subjects that developed overt HCM during follow-up. Although there have been rare reports of LGE in G+/LVH− adults,28–31 LGE does not seem to be a characteristic feature of early/emerging disease. Nevertheless, there is evidence of increased collagen synthesis in sarcomere mutation carriers without LVH.32 Novel CMR approaches, such as T1 measurements,30–33 can quantify myocardial extracellular volume, potentially allowing detection of diffuse interstitial rather than focal fibrosis. Studies are ongoing to investigate whether such techniques may prove to be more useful in identifying myocardial abnormalities before the onset of hypertrophy in sarcomere mutation carriers.10
Although the presence of a sarcomere mutation confers high risk for developing HCM, it is not yet possible to predict when or whether disease may develop or how severe the manifestations may be in any individual mutation carrier. In this study, 2 subjects developed clinically overt HCM during follow-up, including 1 subject with mild LVH detected by CMR, but not echo, at baseline. The biological and pathophysiological changes that govern the emergence of LVH are not understood, but several features are notable. Both subjects were adolescent males with MYH7 mutations. LV wall thickness, therefore, increased during the growth spurt, a common age for LVH to emerge. Additionally, MYH7 mutations may be associated with higher penetrance and earlier development of LVH.33 In both subjects, echocardiographically overt LVH became obvious within a 2-year period, and maximal LV wall thickness did not increase the following year.
In contrast, 2 adult MYBPC3 mutation carriers with mild LVH by CMR (<14 mm) had unchanged LV dimensions and clinical features during ≥4 years of follow-up. The clinical significance of the stable, borderline LVH, detectable only by CMR, in this situation is uncertain. Recognizing the importance of making an accurate and timely clinical diagnosis, there is also a potential for false-positive CMR diagnosis (eg, inadvertent inclusion of trabeculation or right ventricular structures in LV wall thickness measurements). Moreover, our data suggest that mutation carriers who are in the process of developing overt HCM will likely display obvious disease findings and echocardiographic LVH within 12 to 18 months. It is not clear whether subtle and stable CMR findings, in isolation, should be sufficient to establish the clinical diagnosis of HCM.
Given the higher cost and logistical complexity, what is the role of CMR in evaluating early HCM? If echo image quality is good and there is no asymmetry in LV wall thickness, our data indicate that CMR is unlikely to provide substantial additional information that would importantly alter clinical management. With the exception of families with a malignant history of sudden death, asymptomatic mutation carriers with mild LVH detectable only on CMR are unlikely to qualify for primary prevention implantable cardioverter-defibrillator placement or other therapies.
Based on consensus guidelines,5 previous experience, and findings from this systematic study of a G+/LVH− population, we suggest that CMR may play a valuable role in several specific situations. Individuals whose echocardiographic studies demonstrate suboptimal image quality, borderline LVH, or nonuniform LV wall thickness may benefit from CMR to verify LV wall thickness and guide further management that would be triggered by a clinical diagnosis of overt HCM, such as initiating formal assessment of sudden cardiac death risk or recommending lifestyle changes. This may be a particular consideration in higher risk situations, such as evaluating members of families with a high prevalence of premature sudden death, or for G+/LVH− individuals engaging in competitive sports because of the higher a priori sudden cardiac death risk and potentially greater consequences of missing a diagnosis of HCM.
At a more fundamental level, the confounding influences of genetic and environmental modifiers, and the absence of rigorous outcomes data pose particular challenges in managing patients with HCM. These challenges underscore the importance of performing longitudinal studies, anchored on genotype, to better define disease. A better understanding of the clinical implications of the full phenotypic spectrum of sarcomere mutations, beyond simple assessment of LVH, is needed to optimize care of HCM.
Supplementary Material
Supplemental Table. Sarcomere Mutations Present in the Study Cohort
Clinical Perspective.
Genetic testing for hypertrophic cardiomyopathy identifies an important at-risk population: relatives who inherited the sarcomere gene mutation responsible for disease in their family (G+) but who do not yet have diagnostic clinical manifestations, that is, left ventricular hypertrophy (LVH−). The optimal management of these typically young G+/LVH− individuals has not been established. We systematically compared echocardiographic and cardiac MRI (CMR) assessment of LV wall thickness to examine whether the greater spatial resolution and tissue characterization of CMR provides advantages in evaluating early hypertrophic cardiomyopathy. Our results demonstrate 90% diagnostic agreement between the 2 modalities. Additionally, no subjects had late gadolinium enhancement. A small subset (10%) of mutation carriers had modest LVH by CMR but not echo, suggesting a potential clinical diagnosis of hypertrophic cardiomyopathy. Therefore, echo is unlikely to miss a diagnosis of clinically overt disease. However, there was only moderate agreement in absolute measurement of LV wall thickness. CMR measurements were generally ≈20% lower than echo in nonhypertrophied segments, raising the question as to whether different diagnostic criteria should be used by echo and CMR. CMR may be a helpful adjunct to family screening if echo images are ambiguous; there is a strong family history of sudden death; or for competitive athletes. In other situations, the clinical implications of earlier identification of modest LVH by CMR are less clear. The lack of outcomes data poses important limitations to guide clinical management. Better understanding of the full phenotypic spectrum, beyond LVH, will critically advance the care of patients and families with hypertrophic cardiomyopathy.
Acknowledgments
The authors wish to thank David Annese, RT, and Emily Harris for their contributions to this study.
Sources of Funding: This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health [5K23HL078901 and 1P20HL101408 to C.Y. Ho].
Footnotes
Guest Editor for this article was Jonathan W. Weinsaft, MD
The online-only Data Supplement is available at http://circgenetics.ahajournals.org/lookup/suppl/doi:10.1161/CIRCGENETICS.113.000037/-/DC1.
Disclosures: None.
References
- 1.Ho CY, Seidman CE. A contemporary approach to hypertrophic cardiomyopathy. Circulation. 2006;113:e858–e862. doi: 10.1161/CIRCULATIONAHA.105.591982. [DOI] [PubMed] [Google Scholar]
- 2.Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell. 2001;104:557–567. doi: 10.1016/s0092-8674(01)00242-2. [DOI] [PubMed] [Google Scholar]
- 3.Hagège AA, Dubourg O, Desnos M, Mirochnik R, Isnard G, Bonne G, et al. Familial hypertrophic cardiomyopathy: cardiac ultrasonic abnormalities in genetically affected subjects without echocardiographic evidence of left ventricular hypertrophy. Eur Heart J. 1998;19:490–499. doi: 10.1053/euhj.1997.0735. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Seidman JG, Seidman CE. Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004;44:2125–2132. doi: 10.1016/j.jacc.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 5.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2761–2796. doi: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the European Society of Cardiology Committee for practice guidelines. J Am Coll Cardiol. 2003;42:1687–1713. doi: 10.1016/s0735-1097(03)00941-0. [DOI] [PubMed] [Google Scholar]
- 7.Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003;41:1776–1782. doi: 10.1016/s0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- 8.Ho CY. New paradigms in hypertrophic cardiomyopathy: insights from genetics. Prog Pediatr Cardiol. 2011;31:93–98. doi: 10.1016/j.ppedcard.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakdawala NK, Thune JJ, Maron BJ, Cirino AL, Havndrup O, Bundgaard H, et al. Electrocardiographic features of sarcomere mutation carriers with and without clinically overt hypertrophic cardiomyopathy. Am J Cardiol. 2011;108:1606–1613. doi: 10.1016/j.amjcard.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho CY, Abbasi SA, Neilan TG, Shah RV, Chen Y, Heydari B, et al. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:415–422. doi: 10.1161/CIRCIMAGING.112.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germans T, Wilde AA, Dijkmans PA, Chai W, Kamp O, Pinto YM, et al. Structural abnormalities of the inferoseptal left ventricular wall detected by cardiac magnetic resonance imaging in carriers of hypertrophic cardiomyopathy mutations. J Am Coll Cardiol. 2006;48:2518–2523. doi: 10.1016/j.jacc.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 12.Rickers C, Wilke NM, Jerosch-Herold M, Casey SA, Panse P, Panse N, et al. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation. 2005;112:855–861. doi: 10.1161/CIRCULATIONAHA.104.507723. [DOI] [PubMed] [Google Scholar]
- 13.Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:220–228. doi: 10.1016/j.jacc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Maron MS. The current and emerging role of cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2:415–425. doi: 10.1007/s12265-009-9136-3. [DOI] [PubMed] [Google Scholar]
- 15.Olivotto I, Maron MS, Autore C, Lesser JR, Rega L, Casolo G, et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2008;52:559–566. doi: 10.1016/j.jacc.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 16.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 17.Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, et al. Clinical profle and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008;1:184–191. doi: 10.1161/CIRCHEARTFAILURE.108.768119. [DOI] [PubMed] [Google Scholar]
- 18.Hershberger RE, Siegfried JD. Update 2011: clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol. 2011;57:1641–1649. doi: 10.1016/j.jacc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakdawala NK, Funke BH, Baxter S, Cirino AL, Roberts AE, Judge DP, et al. Genetic testing for dilated cardiomyopathy in clinical practice. J Card Fail. 2012;18:296–303. doi: 10.1016/j.cardfail.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKenna WJ, Spirito P, Desnos M, Dubourg O, Komajda M. Experience from clinical genetics in hypertrophic cardiomyopathy: proposal for new diagnostic criteria in adult members of affected families. Heart. 1997;77:130–132. doi: 10.1136/hrt.77.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michels M, Soliman OI, Phefferkorn J, Hoedemaekers YM, Koffard MJ, Dooijes D, et al. Disease penetrance and risk stratification for sudden cardiac death in asymptomatic hypertrophic cardiomyopathy mutation carriers. Eur Heart J. 2009;30:2593–2598. doi: 10.1093/eurheartj/ehp306. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- 24.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 25.Garg R, Powell AJ, Sena L, Marshall AC, Geva T. Effects of metallic implants on magnetic resonance imaging evaluation of Fontan palliation. Am J Cardiol. 2005;95:688–691. doi: 10.1016/j.amjcard.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 27.Moon JC, Mogensen J, Elliott PM, Smith GC, Elkington AG, Prasad SK, et al. Myocardial late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy caused by mutations in troponin I. Heart. 2005;91:1036–1040. doi: 10.1136/hrt.2004.041384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuster V, van der Zee S, Miller MA. Evolving anatomic, functional, and molecular imaging in the early detection and prognosis of hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2:398–406. doi: 10.1007/s12265-009-9133-6. [DOI] [PubMed] [Google Scholar]
- 29.Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation. 2011;124:40–47. doi: 10.1161/CIRCULATIONAHA.110.985812. [DOI] [PubMed] [Google Scholar]
- 30.Strijack B, Ariyarajah V, Soni R, Jassal DS, Greenberg CR, McGregor R, et al. Late gadolinium enhancement cardiovascular magnetic resonance in genotyped hypertrophic cardiomyopathy with normal phenotype. J Cardiovasc Magn Reson. 2008;10:58. doi: 10.1186/1532-429X-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowin EJ, Maron MS, Lesser JR, Maron BJ. CMR with late gadolinium enhancement in genotype positive-phenotype negative hypertrophic cardiomyopathy. J Am Coll Cardiol Cardiovasc Imaging. 2012;5:119–122. doi: 10.1016/j.jcmg.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010;363:552–563. doi: 10.1056/NEJMoa1002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niimura H, Bachinski LL, Sangwatanaroj S, Watkins H, Chudley AE, McKenna W, et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338:1248–1257. doi: 10.1056/NEJM199804303381802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Sarcomere Mutations Present in the Study Cohort


