Abstract
Hypothesis
The immunodeficient (severe combined immunodeficiency beige [SCID/bg]) mouse model provides a useful model for investigating vascular neotissue formation in human tissue–engineered arterial conduits (TEAC).
Design
Human aortic smooth muscle cells and endothelial cells were statically seeded on porous biodegradable polymeric scaffolds for vascular tissue engineering. These 2-cell tissue-engineered vascular conduits were implanted into immunodeficient female mice as aortic interposition grafts. Grafts were evaluated over a 30-week course to investigate their patency and structure.
Setting
In vivo animal study.
Patients
Thirteen female C.B-17 SCID/bg mice.
Intervention
The TEACs implanted as infrarenal abdominal aortic interposition grafts.
Main Outcome Measures
Selective microcomputed tomography with intra-arterial contrast revealed graft patency and structure. Histological and immunohistochemical evaluations revealed cellularity and extracellular matrix composition. Species-specific immunohistochemical evaluation determined the source of cells within TEACs.
Results
All TEACs were patent without evidence of thrombosis or rupture over the 30-week course. Histological and immunohistochemical evaluation revealed a von Willebrand factor–positive luminal monolayer surrounded by concentric collagen-rich layers of α-smooth muscle actin–positive cells.
Conclusions
The SCID/bg mouse is a useful model for investigating vascular neotissue formation in human TEACs. We see evidence that these grafts remain patent while developing into vascular neotissue histologically similar to native aorta. This chimeric animal model also enables determination of seeded cell retention, providing insight into cellular mechanisms underlying neotissue formation.
Cardiovascular Disease continues to be the leading cause of morbidity and mortality in the developed world. In addition, atherosclerosis, the major pathological feature of small and medium-sized muscular arteries, is the primary cause of death in the United States.1 It is estimated that in 2004 more than 427 000 cardiac bypasses were performed in the United States alone. Unfortunately, close to 80 000 patients are unable to undergo bypass grafting each year (both coronary and peripheral) because of a lack of adequate autologous grafts.2 While synthetic grafts have been used in large-diameter arterial bypass procedures and in the formation of arteriovenous fistulas, these graft materials have proven to be highly thrombogenic at the small diameters ("6 mm) needed for coronary and peripheral bypass grafting.3 Therefore, the production of small-caliber grafts, with better patency rates than currently available constructs, would provide additional lifesaving and limb-saving therapeutic options for patients with cardiovascular disease.
Recent achievements have shown that tissue engineering holds promise in developing functional small-caliber neovessels.4–9 Currently, most vascular tissue engineering research relies on large-animal models (ovine, porcine, and canine) for evaluation and validation of graft development and function. However, these models are limited by cost, time, and immunologic restrictions. Using a small-animal model allows these studies to be carried out in a cost-effective and rapid manner. To this end, our laboratory has already reported on the development of a severe combined immunodeficiency beige (SCID/bg) mouse model.2,10 Use of an immunodeficient mouse model that readily accepts xenotransplants enables early investigation of the use of human tissue, minimizing investigaton of biological phenomena specific to lower species. Development of a chimera (mouse/human) animal model has the added benefit of being able to perform cell tracking experiments based on species-specific immunohistochemical (IHC) evaluation.
In this study, we hypothesize that the SCID/bg mouse model is a useful model for the evaluation of human tissue–engineered arterial conduits (TEACs) produced through seeding mature human aortic smooth muscle and endothelial cells onto a biodegradable polymer scaffold. Herein, we report the successful production of a very small-diameter (<1 mm) human tissue–engineered artery via these methods.
MEHTODS
SCAFFOLD PRODUCTION
Poly-L-lactic acid (PLLA) nonwoven felts were used for the framework of the scaffold (Concordia Fibers, Coventry, Rhode Island) and coated in a 50:50 copolymer solution of ε-caprolactone and L-lactide (P[CL/LA]), as previously reported.11 The PLLA-P(CL/LA) scaffolds had the following structural characteristics: total porosity of 60.4% and mean (SD) pore size of 31.2 (16.1) µm. The biomechanical profile (in mean [SD]) was as follows: initial tensile strength of 3.7 (0.53) MPa, elastic modulus of 24 (5.9) MPa, burst pressure of 2790 (180) mm Hg, and suture retention strength of 4.37 (0.67) N.11
IN VITRO CULTURE AND CELL SEEDING
Each 4-mm P(CL/LA)-coated PLLA scaffold was sterilized via incubation, at room temperature, under UV light, in an antibiotic solution of 10% penicillin/streptomycin in sterile phosphate-buffered saline. These scaffolds were rinsed in phosphate-buffered saline prior to seeding. Human aortic smooth muscle cells between passages 6 and 8 were trypsinized and collected for seeding in SmGM-2 culture media (Lonza, Visp, Switzerland). Approximately 1×106 cells in 10 µL of media were seeded onto each scaffold via clamping one end of the scaffold and pipetting the cell suspension into the lumen of the scaffold through the opposite end. The scaffold was then gently compressed to encourage cell infiltration into the porous walls. This process of pipetting and compression was repeated 3 times at each end of the scaffold. It was then allowed to incubate for approximately 10 minutes to allow for cell adhesion. A 25-gauge needle was then gently threaded through the lumen of the graft to protect against occlusion via cellular ingrowth, and the graft was incubated for 6 days in 3 mL of SmGM-2, with media changes every 2 to 3 days. On day 6, the 25-gauge needle was removed and one end of the graft was clamped with microsurgical vascular clips. Approximately 2×105 to 2.5×105 human aortic endothelial cells in 2 to 2.5 µL of EGM-2 culture media (Lonza) were then pipetted into the lumen of the graft. Each graft was allowed to incubate for 10 minutes to allow for human aortic endothelial cell adhesion before submersion in EGM-2 for 24 hours prior to surgical implantation on day 7.
SURGICAL IMPLANTATION
Thirteen female C.B-17 SCID/bg mice aged 3 to 5 weeks underwent surgical implantation of TEACs. Animal handling was in accordance with the institutional guidelines for the use and care of animals, and the institutional review board approved the experimental procedures. Mice were anesthetized with intraperitoneal injection made by mixing 1.5 mL of 100-mg/mL xylazine (Sigma, St Louis, Missouri) and 10 mL of 100-mg/mL ketamine (Sigma) diluted in a ratio of 1:4 with 0.9% isotonic sodium chloride solution and injecting at a dose of 0.1mL/20 g of body weight. Once under general anesthesia, a dissecting microscope (original magnification ×18; Zeiss, Thornwood, New York) was used to perform a midline laparotomy and lateralize the abdominal viscera, allowing visualization of the abdominal aorta. Care was taken to separate the aorta from the inferior vena cava, and proximal and distal vascular control of the aorta was obtained between the renal vessels and iliac bifurcation. The open abdominal cavity was bathed in warmed (37°C) heparinized solution (250 U/mL). The native vessel was gently occluded with removable microvascular clamps and then transected. Anastomosis of the TEAC (approximately 3 mm in length and 0.9 mm in diameter) was performed using interrupted 10-0 monofilament nylon (Sharpoint Laboratory Sutures, Calgary, Alberta, Canada). The mice underwent placement of an end-to-end infrarenal aortic interposition graft. On completion of the distal anastomosis, vascular integrity was restored and hemostasis assured. Flow through the grafted arterial scaffold segment was confirmed, and the abdomen was flooded with warmed sterile saline before closure. Anastomosis times varied between 20 and 30 minutes. The midline incision was then closed with running 5-0 polypropylene sutures, and following laparotomy closure, animals were placed on a warm pad to avoid hypothermia. The animals recovered quickly and hind limb function was a reliable indicator of early graft patency. In this study, none of the mice displayed hind limb paralysis postoperatively. A total of 12 seeded tissue-engineering scaffolds were implanted as aortic interposition grafts. The mice were killed between 4 days and 7 months postoperatively.
IN VIVO MICROCOMPUTED TOMOGRAPHY
Six of the 13 SCID/bg mice were interrogated via a high-resolution (50-µm) microcomputed tomography (micro-CT) imaging system (XSPECT; Gamma Medica, Wixom, Michigan) to assess graft function. Animals were anesthetized as described earlier. The thoracic aorta was then cannulated and the animals were killed. Intra-arterial contrast (Omnipaque; Amersham Biosciences, Piscataway, New Jersey) was injected to investigate anatomical changes in the grafts. Specifically, we assessed patency, stenosis, and occlusion. Further analysis was performed through 3-dimensional reconstructions of the micro-CT data using the AMIDE software package (AMIDE is a medical image data examiner).
HISTOLOGICAL EVALUATION
Each specimen was fixed in 10% formalin overnight and then washed in 70% ethanol. Following paraffin embedding and mounting, specimens were stained with simple hematoxylineosin (Sigma), Masson trichrome (Sigma), and van Gieson (Sigma). Specimens also underwent IHC analysis for α-smooth muscle actin (α-SMA) (Dako, Glostrup, Denmark), murine isolectin (IB4; Vector Laboratories, Burlingame, California), and von Willebrand factor (Dako).
HISTOLOGICAL MORPHOMETRY
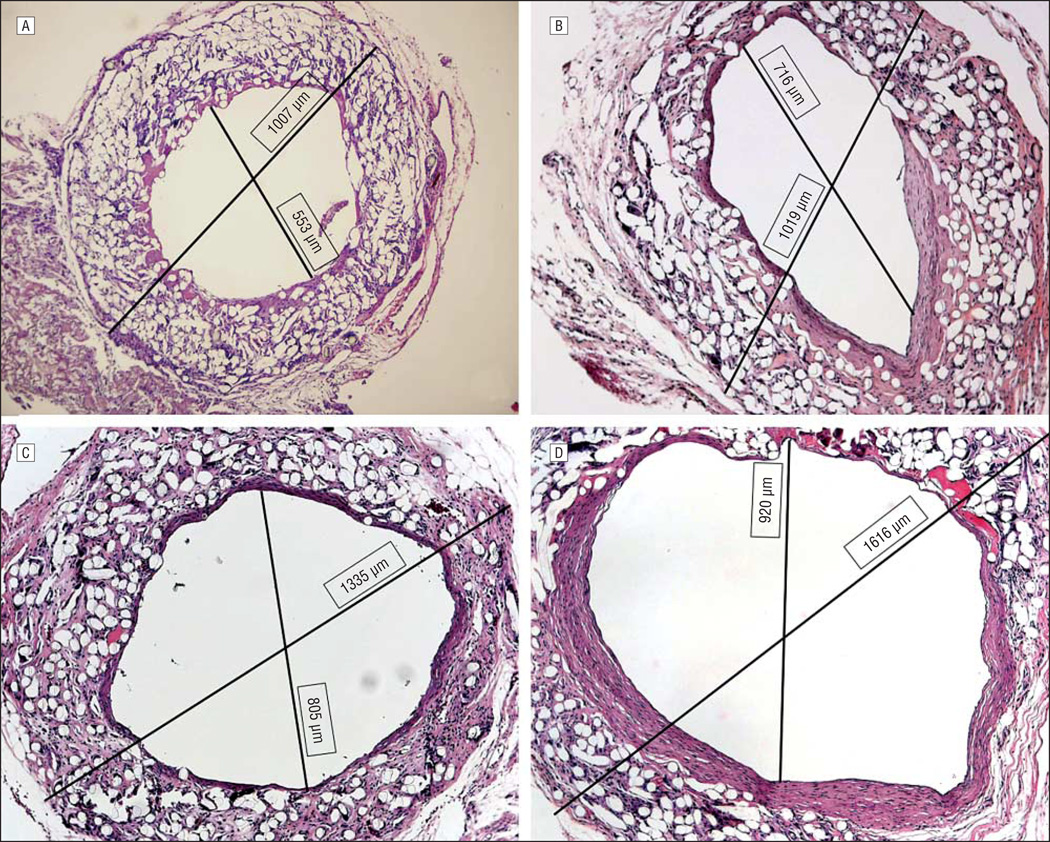
The internal and external diameters of representative grafts from 11 days, 3 weeks, 8 weeks, 20 weeks, and 30 weeks were measured using ImageJ 1.38×software (National Institutes of Health, Bethesda, Maryland, http://rsb.info.nih.gov/ij/). Luminal (internal) and total (external) cross-sectional areas were then measured by tracing the luminal and outer graft circumferences, respectively, and applying functions of the same program. Each measurement was performed 4 times to produce a mean (SD).
RESULTS
IN VIVO MICRO-CT
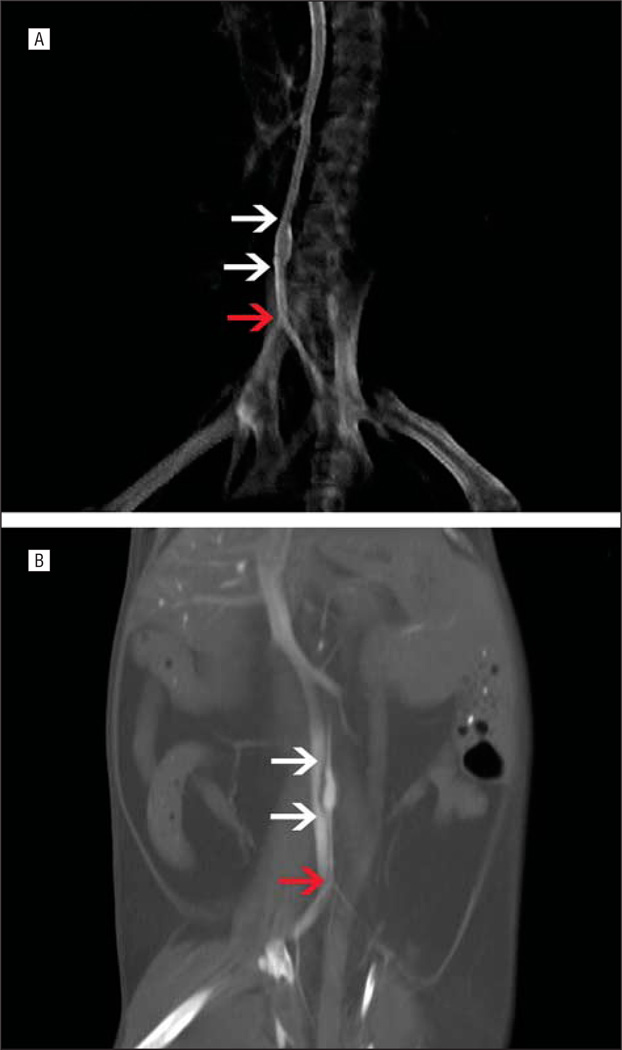
Tissue-engineered arterial conduits interrogated via micro-CT revealed 100% patency (6 of 6) without evidence of the development of stenosis or occlusion (Figure 1). These data were verified with gross and microscopic histological evaluation.
Figure 1.
Microcomputed tomography images in mice (processed in AMIDE, a medical image data examiner). A, Three-dimensional reconstruction of 8-week graft. B, Computed tomography coronal slice of 20-week graft. In each image, the white arrows mark the anastomoses of the tissue-engineered arterial conduits and the red arrow marks the iliac bifurcation.
HISTOLOGICAL EVALUATION
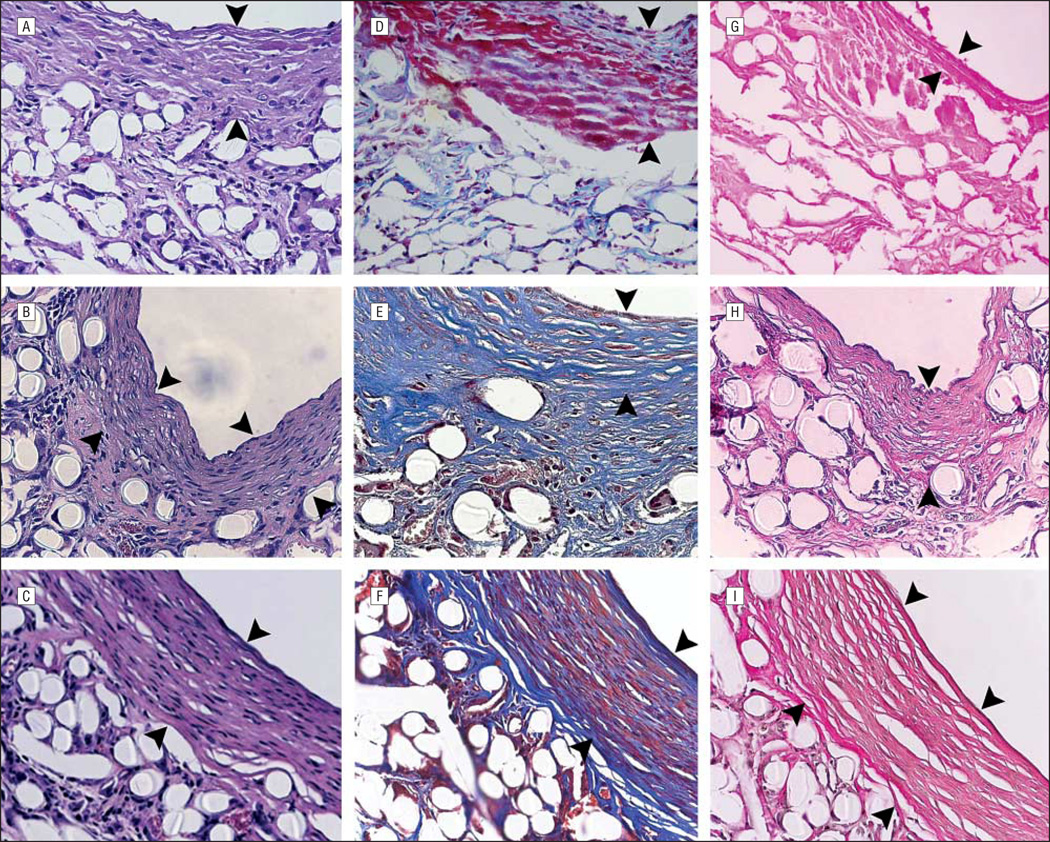
Hematoxylin-eosin and trichrome examination of 4-day and 11-day specimens revealed early deposition of sub-endothelial extracellular matrix proteins (not shown). By 3 weeks, cellular infiltration of this region was well under way (Figure 2A). Extracellular matrix stains at this time displayed a robust deposition of collagen throughout the graft but no elastin (Figure 2D and G). Between 8 and 10 weeks, remodeling of this region was a prominent feature of the microscopic structure of the TEAC, showing elongated nuclei oriented circumferentially within several slowly emerging layers of collagen fibers (Figure 2B and E). At 10 weeks, these lamellae began to resemble a neomedia with 4 or 5 distinct layers of circumferentially oriented cells surrounded by residual biodegradable polymer and tissue. At 30 weeks, there was continued remodeling and reorganization of the neomedial layers and an increase in internal diameter as evidenced on micro-CT (Figure 2C and F). This increase in graft diameter was evident on histological evaluation, with a direct relationship between graft cross-sectional area and time from implantation (Figure 3) (Table).
Figure 2.
Histological evaluation of 3-week, 8-week, and 30-week specimens (original magnification ×400, enhanced with digital color augmentation). Hematoxylin-eosin–stained specimen of 3-week (A), 8-week (B), and 30-week (C) grafts. Masson trichrome–stained specimen of 3-week (D), 8-week (E), and 30-week (F) grafts. Van Gieson–stained specimen of 3-week (G), 8-week (H), and 30-week (I) grafts. The arrowheads demarcate the thickness of the neomedial layer (ie, the layer that stains positively for α-smooth muscle actin via immunochemistry).
Figure 3.
Histological morphometry performed on hematoxylin-eosin–stained sections (original magnification ×100) reveals a progressive increase in diameter and cross-sectional area of human tissue–engineered arterial conduit over a 30-week course.
Table.
Histological Morphometry
| Sample | External Diameter, mm, Mean (SD) |
|---|---|
| Unseeded | 1.260 (0.164) |
| 2 wk | 0.942 (0.032) |
| 3 wk | 0.979 (0.054) |
| 8 wk | 1.276 (0.206) |
| 20 wk | 1.302 (0.046) |
| 30 wk | 1.383 (0.066) |
IHC EVALUATION
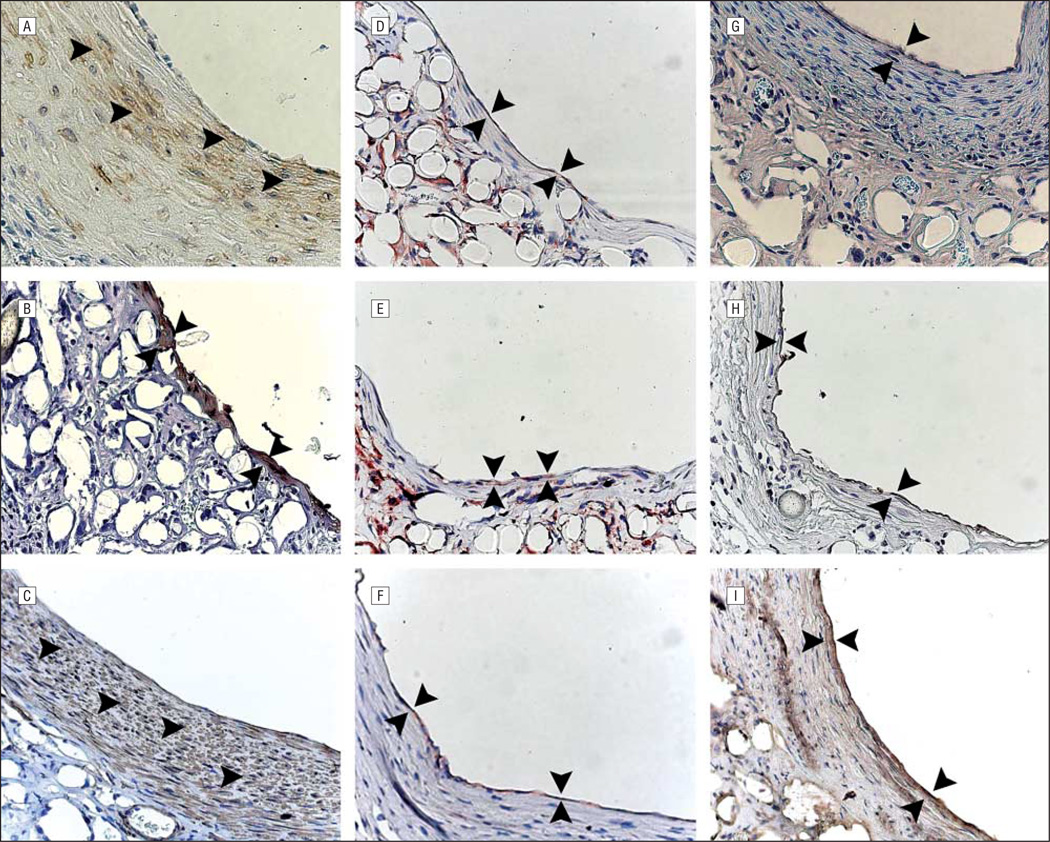
Investigation of cellular identity via IHC evaluation revealed that the region of subendothelial matrix protein deposition was infiltrated by α-SMA–positive cells by 8 weeks (Figure 4A-C). Further study showed that the human endothelial cells originally seeded within the lumen of our TEACs were replaced with murine endothelial cells beginning at 3 weeks (not shown). The TEAC endothelium became fully murine in origin by 8 weeks, as displayed by a paucity of human CD31–positive cells (not shown) and the presence of mouse-specific IB4 lectin (Figure 4D-I).
Figure 4.
Immunohistochemical evaluation of 8-week, 20-week, and 30-week specimens (original magnification ×400, enhanced with digital color augmentation). α–Smooth muscle actin at 8 weeks (A), 20 weeks (B), and 30 weeks (C) shows diffuse positive staining throughout the developing neomedia (arrowheads). Murine IB4 at 8 weeks (D), 20 weeks (E), and 30 weeks (F) shows a thin monolayer of murine endothelial cells (between arrowheads). von Willebrand factor at 8 weeks (G), 20 weeks (H), and 30 (I) weeks (between arrowheads).
COMMENT
Despite the success of current surgical therapies for end-stage cardiovascular disease, many patients remain without therapeutic options because of a lack of appropriate grafting material. The use of synthetic grafts at the necessary diameters (<6 mm) is hampered by high rates of thrombo-occlusion. Small-diameter autologous grafts have performed relatively well here, but because of graft scarcity, concomitant peripheral vascular disease with poor-quality autologous vessels, intimal hyperplasia, and advanced rates of atherosclerotic plaque formation, the use of autologous grafts has remained limited. Webelieve that vascular tissue engineering holds promise for providing an improved small-caliber arterial conduit12,13 that will adequately meet clinical demands for such constructs.
To this end, we previously validated the use of SCID/bg mice in vascular tissue engineering research.2,10 Building on that work, this study demonstrates the successful in vivo development of small-diameter (<1-mm) human TEACs. We achieved high rates of patency with intra-abdominal aortic replacement grafts, without evidence of stenosis, occlusion, or ischemia of the hind limbs. Immunohistochemical assessment revealed a luminal endothelial lining composed of von Willebrand factor–positive cells at each point assessed. Staining for human CD31, a human endothelial cell marker, and for isolectin IB4, a murine endothelial cell surface marker, further differentiated the endothelial cells into species-specific cell type. In doing so, data obtained revealed that the seeded human aortic smooth muscle cells were completely replaced by host cells. A limitation of our study is the use of an immunocompromised host. The immune system likely plays an important role in vascular neotissue formation. Further validation of these findings using an immunocompetent animal model will be essential to understanding the clinical significance of human TEACs.
In addition, our histological data also revealed a highly cellularized graft built around a biodegradable poly-l-lactide polymer backbone. Though the majority of polymer was retained within the developing graft at 8 weeks, an organized extracellular matrix rich in collagen, but lacking elastin, was deposited. At this time, α-SMA–positive cells infiltrated the collagen-rich extracellular matrix, enacting a robust remodeling process that resulted in well-organized concentric circumferential layers. Studies are currently under way to determine the effect of residual polymer on the biomechanical properties of TEACs and to assess the contractile and relaxation properties of these conduits.
Imaging of the implanted grafts was conducted via micro-CT at various points throughout the study. Analyses over the 30-week study period revealed remodeling as evidenced by changes in graft diameter. Additionally, histological evaluation of the grafts on explantation confirmed those findings over the 30-week study. Although all implanted grafts remained intact without evidence of rupture throughout the study period, further investigation of this potential aneurysmal dilation is warranted.
Building on the work of other investigators in the field,14–17 we sought to accelerate the evaluation of tissue-engineered grafts by using an in vivo model. While others have already proven the feasibility of producing tissue-engineered vessels from porcine, canine, or ovine sources,4–6 it is well known that human cells behave differently in in vitro systems. Thus, there is a great need to investigate the behavior of human cells in these experimental systems before the translation of these technologies to the clinic setting. We did this by exploiting an immunocompromised mouse model, as previously described. 18 Because of the lack of specific immune response, we were able to avoid the difficulties of xenogeneic transplantation and rejection encountered by other investigators8 while simultaneously studying the behavior of human vascular cells on a tissue-engineering scaffold. Using this chimeric system will hopefully minimize the challenges of translating findings across species. Certainly, the in vivo milieu of SCID/bg mice cannot be immediately generalized and applied to human subjects. However, there are fundamental biological similarities that make our findings translatable to future clinical applications.
In conclusion, the SCID/bg mouse model appears to be a promising animal model for evaluating human vascular tissue–engineering technologies. In fact, using a chimeric system in which the genetic sequencing of both species is complete provides investigators with many tools to understand the mechanisms underlying tissue engineering and vascular biology. For these reasons, we anticipate this model will continue to contribute to the translation of vascular tissue engineering into clinical practice.
Acknowledgments
Funding/Support: This work was supported by National Institutes of Health grants K08HL83980-2, R01 EB006494-01, and P01-HL070295, the Howard Hughes Medical Institute, American Surgical Association, the Yale University School of Medicine Office of Student Research, and the Yale University Department of Surgery Ohse Grant Program.
Footnotes
Author Contributions: Mr Nelson had full access to all of the data in the study and, as first and submitting author, takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Nelson, Brennan, Roh, and Breuer. Acquisition of data: Nelson, Mirensky, Brennan, Roh, Yi, and Wang. Analysis and interpretation of data: Nelson, Mirensky, Brennan, Roh, and Breuer. Drafting of the manuscript: Nelson, Brennan, Yi, Wang, and Breuer. Critical revision of the manuscript for important intellectual content: Mirensky, Roh, and Breuer. Obtained funding: Nelson and Breuer. Administrative, technical, and material support: Nelson, Brennan, Mirensky, Roh, and Breuer. Study supervision: Breuer.
Financial Disclosure: None reported.
Previous Presentations: This paper was presented at the Annual Resident and Fellows’ Research Day (First Place Basic Science Presentation); May 11, 2007; Boston, Massachusetts, and at the 88th Annual Meeting of the New England Surgical Society; September 30, 2007; Burlington, Vermont, and is published after peer review and revision.
References
- 1.Majesky MW, Benditt EP, Schwartz SM. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez-Soler RI, Brennan MP, Goyal A, et al. Development of a mouse model for evaluation of small diameter vascular grafts [published ahead of print March 2, 2007] J Surg Res. 2007;139(1):1–6. doi: 10.1016/j.jss.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Sayers RD, Raptis S, Berce M, Miller JH. Long-term results of femorotibial by-pass with vein or polytetrafluoroethylene. Br J Surg. 1998;85(7):934–938. doi: 10.1046/j.1365-2168.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- 4.Kaushal S, Amiel GE, Guleserian KJ, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7(9):1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe M, Shin’oka T, Tohyama S, et al. Tissue-engineered vascular auto-graft: inferior vena cava replacement in a dog model. Tissue Eng. 2001;7(4):429–439. doi: 10.1089/10763270152436481. [DOI] [PubMed] [Google Scholar]
- 6.Niklason LE, Gao J, Abbott WM, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 7.Shum-Tim D, Stock U, Hrkach J, et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg. 1999;68(6):2298–2304. doi: 10.1016/s0003-4975(99)01055-3. discussion 2305. [DOI] [PubMed] [Google Scholar]
- 8.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. Completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 9.Teebken OE, Haverich A. Tissue engineering of small diameter vascular grafts. Eur J Vasc Endovasc Surg. 2002;23(6):475–485. doi: 10.1053/ejvs.2002.1654. [DOI] [PubMed] [Google Scholar]
- 10.Goyal A, Wang Y, Su H, et al. Development of a model system for preliminary evaluation of tissue-engineered vascular conduits. J Pediatr Surg. 2006;41(4):787–791. doi: 10.1016/j.jpedsurg.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Roh JD, Nelson GN, Brennan MP, et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model [published online ahead of print December 27, 2007] Biomaterials. 2008;29(10):1454–1463. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte MS. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998;12(1):43–45. doi: 10.1096/fasebj.12.1.43. [DOI] [PubMed] [Google Scholar]
- 13.Kakisis JD, Liapis CD, Breuer CK, Sumpio BE. Artificial blood vessel: the Holy Grail of peripheral vascular surgery. J Vasc Surg. 2005;41(2):349–354. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Naito Y, Imai Y, Shin’oka T, et al. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J Thorac Cardiovasc Surg. 2003;125(2):419–420. doi: 10.1067/mtc.2003.134. [DOI] [PubMed] [Google Scholar]
- 15.Shin’oka T, Matsumura G, Hibino N, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129(6):1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 16.Shin’oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344(7):532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura G, Hibino N, Ikada Y, Kurosawa H, Shin’oka T. Successful application of tissue engineered vascular autografts: clinical experience. Biomaterials. 2003;24(13):2303–2308. doi: 10.1016/s0142-9612(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 18.Yacoub-Youssef H, Marcheix B, Calise D, et al. Engraftment of human T, B and NK cells in CB. 17 SCID/beige mice by transfer of human spleen cells. Transpl Immunol. 2005;15(2):157–164. doi: 10.1016/j.trim.2005.07.002. [DOI] [PubMed] [Google Scholar]