Abstract
Background
Hyperleukocytosis caused by acute lymphoblastic leukemia (ALL) is associated with early morbidity and mortality due to hyperviscosity arising from the excessive number of leukocytes.This study was designed to assess the incidence of hyperleukocytosis, survival outcomes, and adverse features among pediatric ALL patients with hyperleukocytosis.
Methods
Between January 2001 and December 2010, 104 children with previously untreated ALL were enrolled at the Pusan National University Hospital. All of them were initially stratified based on the National Cancer Institute (NCI) risk; 48 (46.2%) were diagnosed with high-risk ALL. The medical charts of these patients were retrospectively reviewed.
Results
Twenty (19.2%) of the 104 children with ALL had initial leukocyte counts of >100×109/L, and 11 patients had a leukocyte count of >200×109/L. Male gender, T-cell phenotype, and massive splenomegaly were positively associated with hyperleukocytosis. Common early complications during induction therapy included renal dysfunction, and central nervous system hemorrhage. The complete remission (CR) rate for the pediatric ALL patients with hyperleukocytosis (94.1%) was similar to the overall CR rate (95.6%). The estimated 3-year event free survival (EFS) and overall survival of ALL children with hyperleukocytosis were 75.0% and 81.2%, respectively. However, patients with initial leukocyte counts >200×109/L had a lower EFS than those with initial leukocyte counts 100-200×109/L (63.6% vs. 100%; P=0.046).
Conclusion
The outcome of pediatric ALL cases with an initial leukocyte count >200×109/L was very poor, probably due to early toxicity-related death during induction therapy.
Keywords: Pediatric acute lymphoblastic leukemia, Hyperleukocytosis, Central nervous system hemorrhage
INTRODUCTION
Recent advances in the treatment of pediatric acute lymphocytic leukemia (ALL) have prolonged the survival of these patients. The initial leukocyte count in these cases is a particularly strong predictor of complete remission (CR) induction and event free survival (EFS). Hyperleukocytosis caused by ALL is associated with early morbidity and mortality due to the hyperviscosity created by the high leukocyte count [1, 2, 3, 4, 5]. Pediatric ALL patients who initially present with hyperleukocytosis are at risk of early complications that lead to a mortality rate as high as 20% during remission induction therapy [3, 5]. However, very few studies from developed countries have addressed childhood ALL with hyperleukocytosis. The purpose of this study was to compare the therapeutic outcomes, incidence, clinical characteristics, and complications during induction therapy among pediatric ALL patiens according to the degree of hyperleukocytosis at presentation.
MATERIALS AND METHODS
Between January 2001 and December 2010, 104 children with previously untreated ALL were enrolled at the Pusan National University Hospital, all of whom were initially stratified by age and initial leukocyte count. Patients aged 1-10 years and with an initial leukocyte count of <50×109/L were classified as having standard-risk ALL, and patients aged >10 years or with an initial leukocyte count >50×109/L were classified as having high-risk ALL. Fifty-six (53.8%) of the children were diagnosed as having standard-risk ALL, 48 (46.2%) were diagnosed as having high-risk ALL, and 20 (19.2%) had an initial leukocyte count of >100×109/L. The treatment protocol used to treat the 48 high-risk ALL cases was a modified version of the Children's Cancer Group (CCG)-1882 and CCG-1961. The induction regimen comprised the administration of prednisolone, vincristine, daunomycin and L-asparaginase. Bone marrow samples were examined after completion of induction therapy (day 28). If CR was confirmed, patients received consolidation chemotherapy with cyclophosphamide, 6-mercaptopurine, cytosine arabinoside, and methotrexate for 5 weeks. After 8 weeks of interim maintenance chemotherapy, patients received reinduction and reconsolidation chemotherapy for 7 weeks. Finally, patients received maintenance chemotherapy consisting of 6-mercaptopurine and methotrexate for 2.5-3.0 years. Patients who sufferd from bone marrow relapse during treatment or patients with unfavorable cytogenetic abnormalities such as t(9;22), t(4;11) at first CR received stem cell transplantation, if they had a human leukocyte antigen-matched sibling donor. The medical charts of the 48 high-risk ALL patients were reviewed retrospectively. The incidence, clinical characteristics, laboratory findings, early complications, CR rates, and outcomes of ALL patients with hyperleukocytosis were compared with those of the high-risk ALL patients without hyperleukocytosis.
Routine blood chemistry values were documented, as were the peak levels of potassium, creatinine, uric acid, phosphorus and trough levels of calcium during the first 14 days. Hyperkalemia was defined as a serum potassium concentration of >6 mmol/L, hyperphosphatemia as a serum phosphate concentration of >7 mg/dL, hyperuricemia as a serum uric acid concentration of >10 mg/dL, and hypocalcemia as a serum calcium concentration of <7 mg/dL. Early complications were defined as those that occurred during the first 14 days.
All patients received continuous intravenous fluids for hydration and urinary alkalinization and allopurinol for at least 5 days during induction chemotherapy. Patients with hyperphosphatemia were also administered aluminum hydroxide. None of the patients underwent prophylactic leukapheresis, cranial irradiation or urate oxidase treatment. However, patients who developed tumor lysis syndrome or renal dysfunction were treated with hemodialysis. Early mortality in this study refers to the death within 1 month of diagnosis.
This clinical data was analyzed by using SPSS 18.0 with linear by linear association, ANOVA, or Fisher exact test followed by the Bonferroni correction. EFS and overall survival (OS) were estimated for various patient subgroups by using the Kaplan-Meier method, and the log-rank test was used to compare survival curves between groups. Cox regression analysis was performed to evaluate predictors of adverse events. P-values <0.05 were considered statistically significant.
RESULTS
Clinical characteristics
Of the 48 children with high risk ALL, 20 had initial leukocyte counts of >100×109/L, and 11 had extreme leukocytosis of >200×109/L. The overall male to female ratio was 1.3:1, but this increased to 3.0:1 in ALL patients with hyperleukocytosis.
Table 1 and 2 compare the initial clinical and laboratory features of the patients with hyperleukocytosis with those of the patients with leukocyte counts of <100×109/L. Extreme leukocytosis of >200×109/L was significantly more common among the patients aged 1-10 years at diagnosis, with a T cell immunophenotype, or with massive splenomegaly. Cytogenetic studies were performed on leukemic blasts obtained from 45 patients (93.7%), 31 of which (68.9%) revealed a normal diploid chromosome. Translocations were identified in 9 patients (20.0%) among the high-risk ALL patients, although patients with t(9;22) and t(4;11), which are associated with an unfavorable prognosis, were not significantly more likely to have hyperleukocytosis. Hemoglobin and platelet counts at diagnosis were not significantly different between the patients with and without hyperleukocytosis, although serum uric acid levels at diagnosis were significantly higher in patients with extreme hyperleukocytosis (7.6 mg/dL vs. 13.6 mg/dL, P=0.027).
Table 1.
Clinical characteristics of children with high-risk ALL.
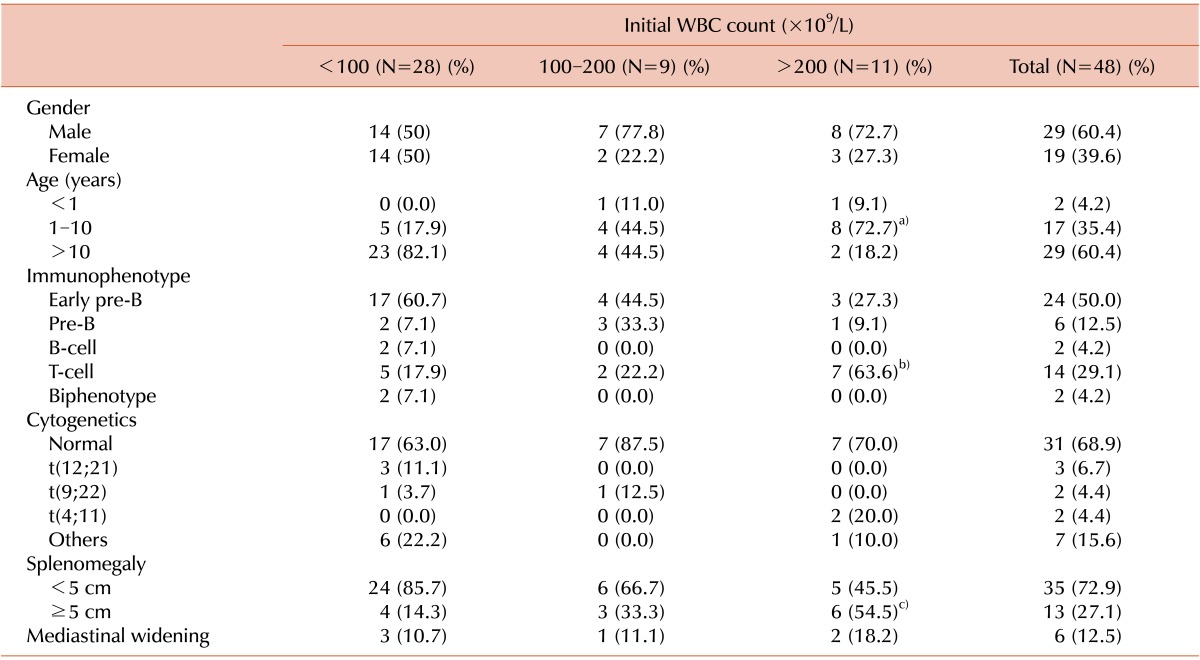
a)P=0.002, b)P=0.049, c)P=0.018.
Table 2.
Laboratory findings for children with high-risk ALL.
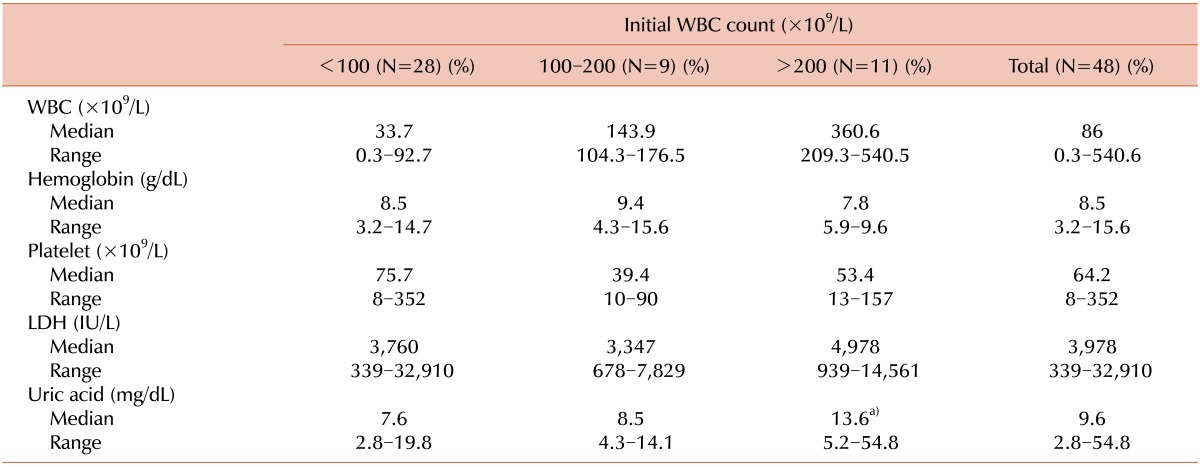
a)P=0.027.
Abbreviations: WBC, white blood cell; LDH, lactate dehydrogenase.
Early complications
Early complications during remission induction chemotherapy among the 48 patients with high-risk ALL are summarized in Table 3. Metabolic complications occurred in 16 patients (33.3%) of the 48 high risks ALL patients during induction chemotherapy. Hyperphosphatemia and hypocalcemia were the most frequent metabolic complication, but they were not significantly different between ALL patients with and without hyperleukocytosis. However, hyperkalemia occurred significantly more often in patients with extreme hyperleukocytosis (P=0.033). Neurological complications such as seizures or central nervous system (CNS) hemorrhage occurred in 5 (10.4%) of the 48 high-risk ALL patients, but CNS hemorrhage occurred significantly more often in patients with extreme hyperleukocytosis (P=0.049).
Table 3.
Early complications in children with high-risk ALL.
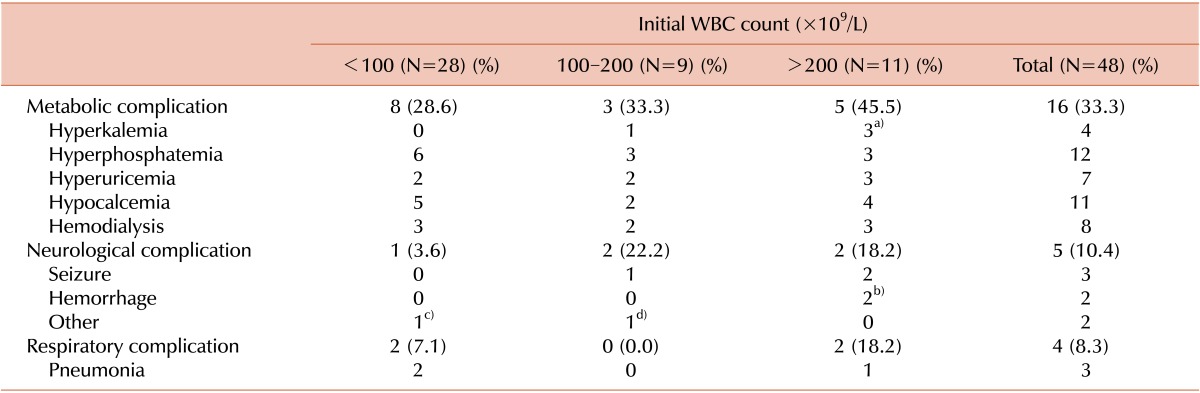
a)P=0.033, b)P=0.049. c)Cranial nerve palsy, d)Posterior reversible encephalopathy syndrome.
In 1 patient, who suffered from a complicated CNS hemorrhage, the hemorrhage had already occurred before visiting hospital and his initial leukocyte count was 328×109/L. Another patient, who had an exceptionally high initial leukocyte count (508×109/L), presented without any initial evidence of CNS hemorrhage, but intracranial hemorrhage (ICH) abruptly developed on the third day of chemotherapy.
Outcomes
Forty-three (95.6%) of 45 patients with high-risk ALL attained CR, and the CR rate on day 28 was not significantly different between the patients with and without hyperleukocytosis. Seven (15.6%) of these 45 patients suffered from relapse after CR, but the relapse rate after CR was also not different between the patients with and without hyperleukocytosis. Three (15%) of the ALL patients with hyperleukocytosis, all of whom with extreme hyperleukocytosis, died during induction chemotherapy: 2 from CNS hemorrhage and 1 from pneumonia and acute respiratory distress syndrome (ARDS) (P=0.043). Among the 48 patients with high-risk ALL, 9 (18.8%) died during treatment, although the overall death rate during treatment was not different between the patients with and without hyperleukocytosis (Table 4). The estimated 3-year EFS in patients with hyperleukocytosis was 75.0% and OS was 81.2%. Patients with leukocyte counts >200×109/L, however, demonstrated a lower EFS than patients with initial leukocyte counts between 100×109/L and 200×109/L (63.6% vs. 100%; P=0.046) (Fig. 1 and 2). Univariate analysis was used to evaluate the association between EFS and leukocyte count, age, splenomegaly, mediastinal mass, hemoglobin level, immunophenotype, and chromosomal abnormality. This demonstrated that an elevated leukocyte count and splenomegaly tended to occur in patients with a shorter survival (P=0.163 and P=0.196, respectively), but there was no significant adverse prognostic factor for EFS. Cox regression analysis was not appropriate in this study because there were no events among patients with initial leukocyte counts between 100×109/L and 200×109/L.
Table 4.
Therapeutic responses in children with high risk ALL.
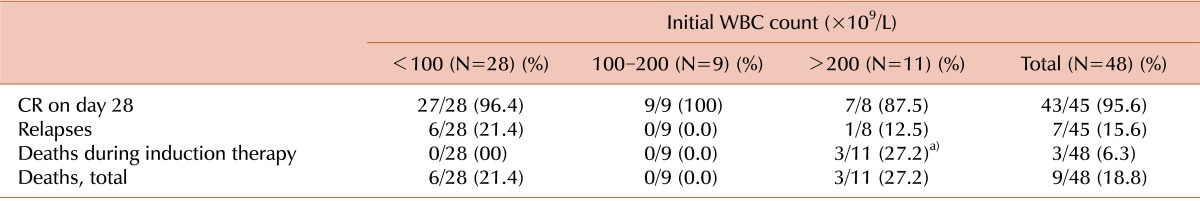
a)P=0.043.
Abbreviation: CR, complete remission.
Fig. 1.
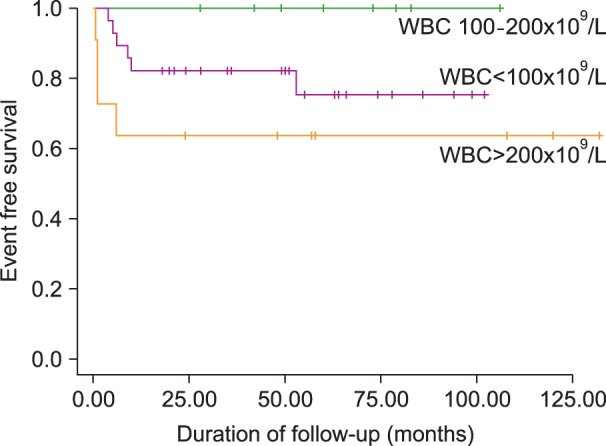
Kaplan-Meier estimates of event free survival of children with high-risk ALL (P=0.04).
Fig. 2.
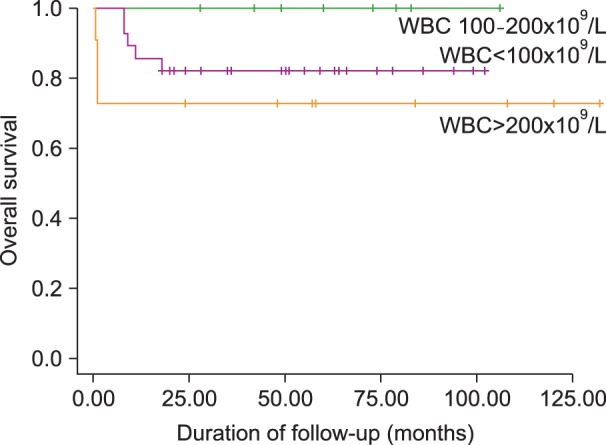
Kaplan-Meier estimate of overall survival of children with high-risk ALL (P=0.09).
DISCUSSION
The incidence of hyperleukocytosis in adult acute leukemia has been reported to be between 5% and 30%, and occurs more frequently in patients with ALL (10-30%) than in those with acute myeloid leukemia (AML) (5-13%) [6, 7, 8, 9]. Among pediatric ALL patients, hyperleukocytosis has been found to occur in 6.1-18% of cases, in which the leukocyte count was >100×109/L and in 5-8.4% of cases, in which the leukocyte count was >200×109/L [1, 2, 10, 11]. The incidence of hyperleukocytosis in our study (19.2%) was similar to those reported from previous studies.
Factors related to the development of hyperleukocytosis in childhood ALL are age <1 year, male gender, T-cell phenotype, mediastinal mass, massive hepatosplenomegaly, and cytogenetic abnormalities (11q23 rearrangement, presence of the Philadelphia chromosome, and loss of p16 expression) [1, 8, 12]. It is also possible that leukemic cell ploidy of <50 chromosomes, CNS leukemia at diagnosis, and a high LDH level (>1,000 mg/dL) have a role in the development of hyperleukocytosis in ALL [1]. In this study, we found that an age 1-10 years at diagnosis, T-cell phenotype and massive splenomegaly were associated with ALL and hyperleukocytosis.
One of the immediate therapeutic problems in the management of patients with hyperleukocytosis is related to acute metabolic complications secondary to tumor lysis syndrome [1, 2, 11]. The rapid destruction of leukemic blasts because of chemotherapy may produce massive release of intracellular potassium and phosphate, which in tum results in acute renal dysfunction. Of these, hyperphosphatemia is the most common complication necessitating hemodialysis [3], although in our study, hyperkalemia was the most frequent metabolic complication in ALL patients with hyperleukocytosis.
CNS hemorrhage is the most dangerous early complication in the management of ALL patients with hyperleukocytosis. It has been reported to occur in 5-33% of AML patients with hyperleukocytosis but only in 1.5-7% of ALL patients with hyperleukocytosis [1, 4, 5, 12]. Subdural hemorrhage is a common form of CNS hemorrhage in patients without leukocytosis, but ICH is more common in patients with hyperleukocytosis [1]. In several studies that reported CNS hemorrhage in ALL cases, almost all patients had a leukocyte count of >400×109/L. No CNS hemorrhage was reported in patients whose leukocyte counts were <200×109/L at diagnosis, and the incidence of CNS hemorrhage was low in patients whose leukocyte counts were <400×109/L [1, 4, 5, 12]. Lowe et al. [5] reported that the incidence of neurological complications for children with an initial leukocyte count of >400×109/L was 17.9%, compared to 3.6% for those with an initial leukocyte count of <400×109/L. They also found that neurological complications are associated with not only the presence of hyperleukocytosis but also with its severity. In our study, 2 patients with leukocyte counts of 328×109/L and 508×109/L developed CNS hemorrhage. One of these patients had already developed CNS hemorrhage at hospital admission. He died from tumor lysis syndrome, renal failure, disseminated intravascular coagulation, and multiorgan failure. The other patient, whose initial leukocyte count was 508×109/L, presented without any initial evidence of CNS hemorrhage, but ICH abruptly developed on the third day of chemotherapy. With supportive therapy including the administration of dexamethasone and mannitol, he recovered from the CNS hemorrhage. However, during induction therapy, the patients exhibited a neutropenic state, which in turn allowed the development of pneumonia, and the patient died from complicated ARDS.
Leukocytoreduction can be achieved by performing induction chemotherapy and leukapheresis, and by using hydoxyurea [6]. The use of leukapheresis to reduce tumor burden in ALL patients with hyperleukocytosis is still controversial [3, 4]. Leukapheresis immediately removes circulating blasts and corrects metabolic abnormalities. It reduces early morbidity and mortality of newly diagnosed AML patients, but there is no evidence that it extends long-term survival [10, 13, 14]. In addition, Porcu et al. [15] did not report any difference in the early mortality of patients treated with leukocytapheresis compared with those who did not receive this treatment. Lowe et al. [5] analyzed 178 childhood ALL cases, in which the leukocyte count was above 200×109/L, 94 of which were treated with leukapheresis and exchange transfusion. Cytoreduction was not successful in reducing CNS hemorrhage or pulmonary complications, because these were mostly present before cytoreduction. The disadvantages of leukapheresis are the potential complications caused by the placement and maintenance of central venous catheters and the need for specialized equipment and trained personnel; therefore, it is not widely used [6, 16]. In this study, none of the patients underwent prophylactic leukapheresis, but all the ALL patients with hyperleukocytosis received vigorous hydration treatment as well as urinary alkalinization during induction chemotherapy.
The recommendation of the American Society for Apheresis [17] for hyperleukocytosis is category I (first-line therapy) and recommendation grade IB (strong recommendation, moderate quality evidence) for leukostasis. As the recommendation for ALL patients with leukocyte counts above 400×109/L is category II (prophylaxis) and recommendation grade IIC, cytoreduction may be reserved for patients presenting with complications resulting from leukostasis or those with very high leukocyte counts.
In acute hyperleukocytic leukemia, the early mortality rate is reported to be 20-40% [3, 5, 12, 13]. Among the early complications of ALL, hemorrhage was common in patients with high leukocyte counts, and renal dysfunction was more common in patients with low leukocyte counts [5, 12]. Dutcher et al. [18] reported that adult acute non-lymphocytic leukemia and hyperleukocytosis was associated with a lower CR rate; these patients required more cycles of induction chemotherapy, and had significantly shorter disease-free survival and OS. Harousseau et al. [2] studied the CR rates of 141 ALL patients with hyperleukocytosis aged between 3 months and 66 years. They found that initial leukocyte count was not associated with the CR rate, but CR duration tended to be shorter when initial leukocyte counts were high. Recent studies of childhood ALL with hyperleukocytosis found that the CR rate was 91-94% and was not correlated with the actual leukocyte count [3, 4]. Eguiguren et al. [3] found that the recurrence rate in ALL patients with hyperleukocytosis was as high as 37.5%. For patients with a leukocyte count of 100-200×109/L, the recurrence rate was 30%, but for those with a leukocyte count of >200×109/L, the recurrence rate was even higher (48%). They also reported a high incidence of relapse in both the bone marrow and CNS, especially in patients whose leukocyte counts were >200×109/L. In this study, CR was achieved in 97% of cases where the initial leukocyte count was <200×109/L, and in 87.5% of cases when the leukocyte count was >200×109/L, although these differences were not statistically significant due to the small number of cases.
Maurer et al. [4] reported that the 3-year EFS of ALL patients with an initial leukocyte count of >200×109/L was 55%. They also identified high leukocyte count at diagnosis and massive splenomegaly as adverse prognostic factors for EFS in multivariate analysis. Eguiguren et al. [3] calculated that the 4-year EFS of ALL patients with an initial leukocyte count of >100×109/L was 52%. They also found that patients with a leukocyte count of >200×109/L had a poorer 4-year EFS of 34%, whilst those with a leukocyte count of 100-200×109/L, had a 4-year EFS of 64%. In this study, the estimated 3-year EFS of ALL patients with hyperleukocytosis was 75%, and the OS was 81.2%. However patients with leukocyte count of >200×109/L had a lower 3-year EFS (63.6%) than patients with initial leukocyte counts of 100-200×109/L, because there was no early toxicity-related death or recurrence in ALL patients with hyperleukocytosis having leukocyte counts in this range.
In summary, our study demonstrates that, with improved management including intensive chemotherapy, the outcome of ALL with initial leukocyte counts of 100-200×109/L can be significantly improved. Howerver, the outcomes of ALL patients with an initial leukocyte count of >200×109/L at presentation remains very poor, probably as a result of early toxicity-related death during induction.
Footnotes
This work was supported by a 2-year research grant from Pusan National University.
No potential conflicts of interest relevant to this article were reported.
References
- 1.Wald BR, Heisel MA, Ortega JA. Frequency of early death in children with acute leukemia presenting with hyperleukocytosis. Cancer. 1982;50:150–153. doi: 10.1002/1097-0142(19820701)50:1<150::aid-cncr2820500128>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Harousseau JL, Tobelem G, Schaison G, et al. High risk acute lymphocytic leukemia: a study of 141 cases with initial white blood cell counts over 100,000/cu mm. Cancer. 1980;46:1996–2003. doi: 10.1002/1097-0142(19801101)46:9<1996::aid-cncr2820460917>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Eguiguren JM, Schell MJ, Crist WM, Kunkel K, Rivera GK. Complications and outcome in childhood acute lymphoblastic leukemia with hyperleukocytosis. Blood. 1992;79:871–875. [PubMed] [Google Scholar]
- 4.Maurer HS, Steinherz PG, Gaynon PS, et al. The effect of initial management of hyperleukocytosis on early complications and outcome of children with acute lymphoblastic leukemia. J Clin Oncol. 1988;6:1425–1432. doi: 10.1200/JCO.1988.6.9.1425. [DOI] [PubMed] [Google Scholar]
- 5.Lowe EJ, Pui CH, Hancock ML, Geiger TL, Khan RB, Sandlund JT. Early complications in children with acute lymphoblastic leukemia presenting with hyperleukocytosis. Pediatr Blood Cancer. 2005;45:10–15. doi: 10.1002/pbc.20178. [DOI] [PubMed] [Google Scholar]
- 6.Majhail NS, Lichtin AE. Acute leukemia with a very high leukocyte count: confronting a medical emergency. Cleve Clin J Med. 2004;71:633–637. doi: 10.3949/ccjm.71.8.633. [DOI] [PubMed] [Google Scholar]
- 7.Porcu P, Farag S, Marcucci G, Cataland SR, Kennedy MS, Bissell M. Leukocytoreduction for acute leukemia. Ther Apher. 2002;6:15–23. doi: 10.1046/j.1526-0968.2002.00402.x. [DOI] [PubMed] [Google Scholar]
- 8.Porcu P, Cripe LD, Nq EW, et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000;39:1–18. doi: 10.3109/10428190009053534. [DOI] [PubMed] [Google Scholar]
- 9.Hoelzer D, Thiel E, Loffler H, et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults. Blood. 1988;71:123–131. [PubMed] [Google Scholar]
- 10.Ganzel C, Becker J, Mintz PD, Lazarus HM, Rowe JM. Hyperleukocytosis, leukostasis and leukapheresis: practice management. Blood Rev. 2012;26:117–122. doi: 10.1016/j.blre.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni KP, Marwaha RK. Childhood acute lymphoblastic leukemia with hyperleukocytosis at presentation: perspective and lessons from a tertiary care institution in India. Asia Pac J Clin Oncol. 2011;7:185–187. doi: 10.1111/j.1743-7563.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- 12.Bunin NJ, Pui CH. Differing complications of hyperleukocytosis in children with acute lymphoblastic or acute nonlymphoblastic leukemia. J Clin Oncol. 1985;3:1590–1595. doi: 10.1200/JCO.1985.3.12.1590. [DOI] [PubMed] [Google Scholar]
- 13.Bug G, Anargyrou K, Tonn T, et al. Impact of leukapheresis on early death rate in adult acute myeloid leukemia presenting with hyperleukocytosis. Transfusion. 2007;47:1843–1850. doi: 10.1111/j.1537-2995.2007.01406.x. [DOI] [PubMed] [Google Scholar]
- 14.Giles FJ, Shen Y, Kantarjian HM, et al. Leukapheresis reduces early mortality in patients with acute myeloid leukemia with high white cell counts but does not improve long-term survival. Leuk Lymphoma. 2001;42:67–73. doi: 10.3109/10428190109097677. [DOI] [PubMed] [Google Scholar]
- 15.Porcu P, Danielson CF, Orazi A, Heerema NA, Gabig TG, McCarthy LJ. Therapeutic leukapheresis in hyperleucocytic leukaemias: lack of correlation between degree of cytoreduction and early mortality rate. Br J Haematol. 1997;98:433–436. doi: 10.1046/j.1365-2141.1997.1943011.x. [DOI] [PubMed] [Google Scholar]
- 16.Holig K, Moog R. Leukocyte depletion by therapeutic leukocytapheresis in patients with leukemia. Transfus Med Hemother. 2012;39:241–245. doi: 10.1159/000341805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher. 2010;25:83–177. doi: 10.1002/jca.20240. [DOI] [PubMed] [Google Scholar]
- 18.Dutcher JP, Schiffer CA, Wiernik PH. Hyperleukocytosis in adult acute nonlymphocytic leukemia: impact on remission rate and duration, and survival. J Clin Oncol. 1987;5:1364–1372. doi: 10.1200/JCO.1987.5.9.1364. [DOI] [PubMed] [Google Scholar]


