Background: Despite a requirement for copper during meiosis, the role of copper transporters (Ctrs) in this process remains poorly understood.
Results: Ctrs exhibit temporal and dynamic cellular localization patterns during meiotic differentiation.
Conclusion: Whereas Ctr4/5 is primarily involved during early meiosis, Ctr6 is present throughout the entire program and is associated with a non-canonical cellular location.
Significance: A dynamic interplay between copper transport systems occurs during meiosis.
Keywords: Copper, Gene Regulation, Meiosis, Metalloproteins, Yeast
Abstract
Meiosis requires copper to undertake its program in which haploid gametes are produced from diploid precursor cells. In Schizosaccharomyces pombe, copper is transported by three members of the copper transporter (Ctr) family, namely Ctr4, Ctr5, and Ctr6. Although central for sexual differentiation, very little is known about the expression profile, cellular localization, and physiological contribution of the Ctr proteins during meiosis. Analysis of gene expression of ctr4+ and ctr5+ revealed that they are primarily expressed in early meiosis under low copper conditions. In the case of ctr6+, its expression is broader, being detected throughout the entire meiotic process with an increase during middle- and late-phase meiosis. Whereas the expression of ctr4+ and ctr5+ is exclusively dependent on the presence of Cuf1, ctr6+ gene expression relies on two distinct regulators, Cuf1 and Mei4. Ctr4 and Ctr5 proteins co-localize at the plasma membrane shortly after meiotic induction, whereas Ctr6 is located on the membrane of vacuoles. After meiotic divisions, Ctr4 and Ctr5 disappear from the cell surface, whereas Ctr6 undergoes an intracellular re-location to co-localize with the forespore membrane. Under copper-limiting conditions, disruption of ctr4+ and ctr6+ results in altered SOD1 activity, whereas these mutant cells exhibit substantially decreased levels of CAO activity mostly in early- and middle-phase meiosis. Collectively, these results emphasize the notion that Ctr proteins exhibit differential expression, localization, and contribution in delivering copper to SOD1 and Cao1 proteins during meiosis.
Introduction
Copper transport is essential for a number of biological processes, including respiration, antioxidant defense, xenobiotic amine metabolism, and meiosis (1, 2). Because of its property to lose and accept one electron, copper is not only required as a catalytic cofactor, but it can also participate in Fenton-like reactions that generate the highly toxic hydroxyl radical that may cause cellular damage (3). To balance the need for copper while at the same time preventing its accumulation to cytotoxic levels, organisms have developed mechanisms to control their internal copper load, including the use of specialized copper transporters.
In Schizosaccharomyces pombe, three proteins are members of the copper transporter (Ctr)4 family (4). Two of these, Ctr4 and Ctr5, form a heteroprotein complex at the plasma membrane of cells that grow mitotically (vegetative growth) under low copper conditions. Ctr4 and Ctr5 are interdependent for maturation through the secretory pathway and for copper transport activity when present at the cell surface (5). As is the case for most members of the Ctr family, Ctr4 and Ctr5 harbor extracellular N-terminal hydrophilic regions that contain methionine residues organized as MX2M and/or MXM motifs (denoted Mets motifs) (6). The primary structure of Ctr4 and Ctr5 also contains three transmembrane domains (TMDs), a MX3M motif within their second TMD and a GX3G motif within their third TMD. The last two motifs are predicted to play key roles in copper uptake and assembly of Ctr4-Ctr5 as a heterotrimeric complex, respectively (7–9). Bimolecular fluorescence complementation experiments have shown that the assembly of a functional heterotrimeric Ctr4-Ctr5 complex at the cell surface requires a combination of two Ctr4 molecules with one Ctr5 molecule (10). As expected, cells containing a deletion of ctr4+ or ctr5+ gene exhibit copper starvation phenotypes characterized by their inability to take up 64Cu and show poor cell growth on low copper medium and alterations in copper-dependent enzymes activity (e.g. SOD1, copper amine oxidase 1 (Cao1) and cytochrome c oxidase) (5, 11, 12).
A third member of the Ctr family, denoted Ctr6, localizes on the membrane of vacuoles in cells proliferating in mitosis under low copper conditions (13). The N-terminal region of Ctr6 contains a MXHCXMXM motif that is involved in the process of copper transport. The predicted topology of Ctr6 suggests that the protein possesses three TMDs and conserved motifs found in the Ctr family of copper transporters, including a MX3M motif in TMD2 and a GX3G motif in TMD3 that may be responsible for the assembly of Ctr6 as a homotrimer. The use of mutant strains has revealed that the inactivation of ctr6+ (ctr6Δ) within the context of a ctr4Δ mutant strain results in a complete loss of SOD1 activity, revealing a function for Ctr6 in providing copper to at least one cytosolic copper-dependent enzyme under low copper conditions (13). On the basis of studies on Ctr6 and its ortholog Ctr2 in S. cerevisiae (14, 15), a current model suggests that Ctr6 mediates the efflux of copper from the vacuole into the cytosol when cells undergo a transition from copper-sufficient to copper-limiting conditions.
Meiosis is a specialized mode of cell division by which precursor diploid cells produce haploid gametes (16, 17). To achieve this, diploid cells replicate their chromosomal DNA, generating pairs of homologous chromosomes that are subsequently subjected to homologous recombination. Following this process, homologous chromosomes and sister chromatids are successively separated during the first (MI) and second (MII) meiotic divisions, respectively. Once MI and MII divisions are completed, a differentiation phase occurs in order to produce four mature gametes that are competent for fertilization or germination. Studies in yeast and mice have shown that metal ions such as zinc and copper are essential for normal progression of meiosis (18–20). Insufficient concentrations of these metal ions lead to meiotic arrests as well as errors during meiosis. Despite the known essential role for copper during meiotic differentiation, copper requirement and copper transport proteins involved in this developmental process remain poorly understood.
S. pombe is a genetically amenable organism that is commonly used for identifying and characterizing cellular components that regulate molecular aspects of meiosis (21). This yeast is of special interest as growth conditions and temperature-sensitive mutants have been developed that allow synchronization of cells for their entry into the meiotic program (22, 23). Under conditions of nitrogen starvation, haploid cells arrest in the G1 phase of the cell cycle. If cells of the opposite mating type are found together under these conditions, haploid cells conjugate to form diploid cells, and the resulting zygotes usually undergo meiosis by a process called zygotic meiosis. If the resulting zygotes are transferred to a nitrogen-rich medium immediately after conjugation, cells can grow as diploids for a period of time. Over this period of time, if these diploid cells undergo a second transition from high to low nitrogen, their commitment to meiosis occurs quickly and in a more efficacious and synchronous manner in comparison with zygotic meiosis. This process is called azygotic meiosis. During the mitotic cell cycle cells express the Pat1 kinase that inhibits the initiation of meiosis by phosphorylating the meiotic transcription factor Ste11 and the meiosis-specific inducer Mei2 (21). A mutant strain carrying a temperature-sensitive pat1-114 allele produces a thermolabile Pat1 kinase. After a heat shock at 34 °C, Pat1 is readily inactivated, fostering the cell cycle switch from mitosis to meiosis in a highly efficient and synchronous manner. This latter system called pat1-induced meiosis is more synchronous than azygotic meiosis. Initiation as well as progression and conclusion of meiosis involve four successive waves of gene expression that are mainly controlled by meiosis-specific transcription factors (24, 25). A first wave of expressed genes includes those that are induced by nitrogen starvation and pheromones. Several of these genes are under the control of Ste11 and are primarily involved in initiating meiosis. Subsequently, a second wave of genes (denoted early genes) is activated by Rep1. Products of these genes are involved in pre-meiotic DNA replication and recombination. After this stage, Mei4 activates expression of middle meiotic genes (third wave) whose products are involved in meiotic divisions and early steps of spore formation. The expression of many several middle genes is subsequently repressed by the transcription factor Cuf2 (26). Late gene expression (fourth wave) is controlled by several transcription factors, including Rev2, Atf21, and Atf31. The products of these genes are required for spore maturation.
Investigation of copper starvation response during meiosis has revealed that the expression of ctr4+ is induced after 30 min of meiotic induction followed by its repression 3 h after entry into meiosis (19). Consistent with meiotic ctr4+ transcript levels, a functional GFP epitope-tagged Ctr4 protein is detected at the cell surface of zygotic cells within the first hour of meiosis and remains there until the 3-h meiotic time point is reached and then it disappears from the cell surface (19). At that stage, a meiosis-specific gene, mfc1+, is induced in response to copper starvation and remains expressed throughout the meiotic program (19). mfc1+ encodes a putative major facilitator superfamily-type transporter. At the 3-h meiotic time point, a functional Cherry epitope-tagged Mfc1 protein is first observed on membranes of small intracellular vesicles. At the 6-h meiotic time point, Mfc1-Cherry-associated fluorescence localizes at the forespore membrane where it plays an important role for copper accumulation into forespores and the production of fully active Cao1 (copper amine oxidase 1), which localized primarily in the forespores of asci (19).
In the present study proteins involved in copper transport during meiosis and the profiles of expression of ctr5+ and ctr6+ were determined and compared with that of ctr4+. Although Cuf1 was required for expression of ctr4+ and ctr5+ during meiosis, ctr6+ expression required the presence of Mei4 in addition to Cuf1. As opposed to ctr4+ and ctr5+ that are primarily expressed under low copper conditions, ctr6+ mRNA levels were detected in meiotic cells under standard, copper-depleted, and copper-replete conditions. Consistent with their gene expression profiles, Ctr4 and Ctr5 proteins co-localized at the plasma membrane shortly after induction of meiosis and then progressively disappeared after 3 h of meiotic induction. In the case of Ctr6, the protein localized to vacuolar membranes in early meiosis and then underwent redistribution in a time-dependent manner to reach forespore membranes where it persisted until sporulation. Under copper starvation conditions, meiotic ctr4Δ ctr6Δ cells were defective in SOD1 activity. Although these cells (ctr4Δ ctr6Δ) exhibited a lower level of CAO activity in early meiosis, their CAO activity significantly increased after 6 h of meiotic induction. The increase of CAO activity was due to the presence of Mfc1, as a triple mutant ctr4Δ ctr6Δ mfc1Δ strain was unable to exhibit an increase of CAO activity (6 h after induction of meiosis). Together the results revealed that during the meiotic program, copper transporters are expressed and localized in a time-dependent manner, exhibiting distinct contributions in delivering copper to two meiotic copper-dependent enzymes, SOD1 and Cao1.
EXPERIMENTAL PROCEDURES
Strains and Media
S. pombe strains used in this study are listed in Table 1. Standard methods were used for growth, mating, and sporulation of cells (27). Under nonselective conditions, cells were grown on yeast extract plus supplements containing 0.5% yeast extract, 2% glucose, and 225 mg/liter adenine, histidine, leucine, uracil, and lysine. To integrate or transform plasmids into strains, Edinburgh minimal medium (EMM) supplemented with 225 mg/liter of the required amino acids was used. Unsupplemented EMM medium contained 160 nm copper. The h+/h− diploid strains used for azygotic meiosis were isolated as described previously (26), and the resulting zygotes were returned to rich medium (yeast extract plus supplements) before commitment to meiosis. Diploid cells underwent azygotic meiosis after a synchronized nitrogen-starvation shock in which EMM lacking nitrogen was supplemented with 10 mg/liter of adenine or 10 mg/liter of adenine, histidine, leucine, uracil, and lysine. Diploid strains homozygous for the mating type (h+/h+) were generated by incubating mid-logarithmic cultures of haploid cells with 20 μg/ml carbendazim (Sigma) as described previously (28). To synchronize pat1-114ts diploid cells for their entry into meiosis, they were precultured in EMM supplemented with adenine (225 mg/liter) at 25 °C for 48 h. At mid-logarithmic phase (cell titer of ∼1 × 107 cells/ml), cells were harvested, washed twice, and transferred to EMM lacking nitrogen supplemented with 10 mg/liter of adenine. After incubation for 16 h at 25 °C, NH4Cl (5 mg/liter) was added to the culture medium, and cells were split and treated with either ammonium tetrathiomolybdate (TTM) (Sigma) or CuSO4 or were left untreated. At this point the temperature was rapidly raised (to 34 °C) to induce meiosis, as described previously (19). Meiosis progression of zygotes was monitored by adding 5 μg/ml Hoechst 33342 stain (Invitrogen) at different times after meiotic induction at 34 °C.
TABLE 1.
S. pombe strain genotypes
| Strain | Genotype | Source or Reference |
|---|---|---|
| FY435 | h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 | 19 |
| FY436 | h− his7-366 leu1-32 ura4-Δ18 ade6-M216 | 19 |
| DBY6 | h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 ctr6Δ::hisG | 13 |
| SPY1 | h- his7-366 leu1-32 ura4-Δ18 ade6-M216 ctr6Δ::LoxP | This study |
| DBY11 | h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 ctr6Δ::hisG ctr4Δ::URA4 | 13 |
| SPY2 | h− his7-366 leu1-32 ura4-Δ18 ade6-M216 ctr6Δ::LoxP ctr4Δ::LoxPKANr | This study |
| SPY3 | h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 ctr4Δ::LoxP ctr5Δ::KANr | This study |
| SPY4 | h− his7-366 leu1-32 ura4-Δ18 ade6-M216 ctr4Δ::LoxP ctr5Δ::KANr | This study |
| JSY104 | h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 ctr4Δ::KANr | 19 |
| JSY204 | h− his7-366 leu1-32 ura4-Δ18 ade6-M216 ctr4Δ::KANr | 19 |
| SPY5 | h+ his7-366 leu1-32 ura4-Δ18 ade6-M210 mfc1Δ::LoxP ctr4Δ::LoxP ctr6Δ::KANr | This study |
| SPY6 | h− his7-366 leu1-32 ura4-Δ18 ade6-M210 mfc1Δ::LoxP ctr4Δ::LoxP ctr6Δ::KANr | This study |
| FY435/FY436 | h+/h- his7-366/his7-366 leu1-32/leu1-32 ura4Δ18/ura4Δ18 ade6-M210/ade6-M216 | This study |
| ctr6Δ/Δ | h+/h- his7-366/his7-366 leu1-32/leu1-32 ura4Δ18/ura4Δ18 ade6-M210/ade6-M216 ctr6Δ::HisG/ctr6Δ::LoxP | This study |
| ctr4Δ/Δ | h+/h- his7-366/his7-366 leu1-32/leu1-32 ura4Δ18/ura4Δ18 ade6-M210/ade6-M216 ctr4Δ::KAN/ctr4Δ::KAN | This study |
| ctr4Δ/Δ/ctr6Δ/Δ | h+/h- his7-366/his7-366 leu1-32/leu1-32 ura4Δ18/ura4Δ18 ade6-M210/ade6-M216 ctr6Δ::HisG/ctr6Δ::LoxP ctr4Δ::KAN/ctr4Δ::KAN | This study |
| mfc1Δ/Δ/ctr6Δ/Δ/ ctr4Δ/Δ | h+/h- his7-366/his7-366 leu1-32/leu1-32 ura4Δ18/ura4Δ18 ade6-M210/ade6-M216 mfc1Δ::LoxP/mfc1Δ::LoxP ctr4Δ::LoxP/ctr4Δ::LoxP ctr6Δ::KAN/ctr6Δ::KAN | This study |
| JSY5 | h+ pat1-114 ade6-M210 ctr4Δ::KANr | 19 |
| JSY6 | h+ pat1-114 ade6-M216 ctr4Δ::KANr | 19 |
| JB484 | h+ pat1-114 ade6-M210 | 22 |
| JB485 | h+ pat1-114 ade6-M216 | 22 |
| RAY14 | h+ pat1-114 ade6-M210 mei4Δ::KANr | 26 |
| RAY15 | h+ pat1-114 ade6-M216 mei4Δ::KANr | 26 |
| JSY3 | h+ pat1-114 ade6-M210 cuf1Δ::KANr | 19 |
| JSY4 | h+ pat1-114 ade6-M216 cuf1Δ::KANr | 19 |
| SPY7 | h+ pat1-114 ade6-M210 mei4Δ::loxP cuf1Δ::KANr | This study |
| SPY8 | h+ pat1-114 ade6-M216 mei4Δ:: loxP cuf1Δ::KANr | This study |
Plasmids
To create a plasmid that possessed the ctr6+ promoter driving expression of the ctr6+-HA4 fusion allele, the ctr6+ 5′ regulatory region (positions −650 to −1) was amplified by PCR and inserted immediately upstream of the ATG codon of the ctr6+-HA4 gene into the ApaI/XhoI-digested pctr6+-HA4 plasmid (13). Subsequently, the ApaI-BamHI DNA fragment containing the ctr6+ promoter and the coding region of ctr6+-HA4 was cloned into the corresponding sites of pJK148 (29). The integrative plasmid pJKctr4+-GFP was generated by subcloning the PstI-SpeI fragment from pBPctr4+-GFP (6) containing the entire ctr4+-GFP fusion gene under the control of its own promoter (positions −737 to −1) into the corresponding sites of pJK148. To create pJKprom-ctr5+, a PstI-XmaI PCR-amplified fragment containing the ctr5+ gene along with its promoter (up to −815 from the initiator codon) was cloned into pJK210 (29). The Cherry coding sequence derived from BM46SITmCherry (30) was isolated by PCR using primers designed to produce XmaI and SacI sites at the 5′ and 3′ termini of the Cherry gene. The resulting DNA fragment was used to clone the Cherry coding sequence into pJKprom-ctr5+ plasmid to which XmaI and SacI restriction sites had previously been introduced by PCR and placed immediately upstream of the ctr5+ stop codon. For this construct, denoted pJKctr5+-Cherry, the XmaI-SacI Cherry-encoded fragment was inserted in-frame with the C-terminal region of Ctr5. The cao1+-GFP fusion gene with its own promoter and terminator was isolated from the pSP1cao1+-GFP plasmid (12) using SpeI and ApaI. Once purified, the DNA fragment was inserted into the corresponding restriction sites of pJK148, generating pJKcao1+-GFP. ctr6+-lacZ fusion plasmids were created using similar DNA molecular cloning approaches as described previously (13).
RNA Isolation and Analysis
Total RNA was extracted by a hot phenol method as described previously (31). RNA samples were quantified by spectrophotometry, and 15 μg of RNA per sample were used for the RNase protection assays, which were performed as described previously (32). To detect ctr6+ mRNA levels, plasmid pSKctr6+-v2 was generated by inserting a 198-bp BamHI-EcoRI fragment from the ctr6+ gene into the same restriction sites of pBluescript SK (Stratagene, La Jolla, CA). The antisense RNA hybridizes to the region between positions +95 and +293 downstream of the first base of the translational start codon of ctr6+. To create pSKctr5+-v1, a 187-bp fragment from the ctr5+ gene (corresponding to the coding region between positions +301 and +482) was amplified and cloned into the BamHI-EcoRI sites of pBluescript SK. Plasmids pSKctr4+ (33), pSKcuf1+-v7 (19), pSKmei4+ (26), pKSlacZ, and pSKact1+ (34) were used to produce antisense RNA probes, allowing the detection of steady-state levels of ctr4+, cuf1+, mei4+, lacZ, and act1+ mRNAs, respectively. 32P-Labeled antisense RNA probes were produced from the above-mentioned BamHI-linearized plasmids and with the use of [α-32P]UTP and the T7 RNA polymerase. The act1+ riboprobe was used to detect act1+ mRNA as an internal control for normalization during quantification of the RNase protection products.
Direct and Indirect Immunofluorescence Microscopy
Diploid h+/h− ctr4Δ/ctr4Δ ctr5Δ/ctr5Δ cells co-expressing ctr4+-GFP/ctr4+-GFP and ctr5+-Cherry/ctr5+-Cherry were synchronously induced to enter in azygotic meiosis. At zero time point, when cells had just entered meiosis and for the subsequent time points, culture aliquots were retrieved every hour. At each time point, Hoechst 33342 stain (5 μg/ml) was added to analyze progression of meiosis of individual cells. At the indicated times, cells were subjected to microscopic analysis using a 1000× magnification and the following filters: 340–380 nm (Hoechst 33342), 465–495 nm (GFP), and 510–560 nm (Cherry). For localization of Ctr6 in mitotically growing cells, ctr6Δ mutant cells were transformed with the integrative plasmid pctr6+-HA4, which expresses a functional HA4 epitope-tagged Ctr6 protein (13). Indirect immunofluorescence microscopy was performed as described previously (6, 13), except that cells were fixed with formaldehyde (methanol-free) after a 3-h incubation in the presence of bathocuproinedisulfonic acid (BCS). In the case of Ctr6-HA4 localization during the meiotic program, h+/h− ctr6Δ/ctr6Δ diploid cells expressing ctr6+-HA4/ctr6+-HA4 were induced in azygotic meiosis and separated into different lots that were left untreated or treated with TTM and CuSO4. At the indicated meiotic phase, cells were fixed and adsorbed on poly-l-lysine-coated (0.1%) multiwall slides as described previously (13). After a 30-min block with TNB (10 mm Tris/HCl, pH 7.5, 150 mm NaCl, 1% BSA, 0.02% sodium azide), cells were incubated with anti-HA antibody (F-7) (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:250 in TNB. After an 18-h incubation at 4 °C, cells were washed with TNB and incubated for 4 h with a goat anti-mouse Alexa Fluor 546-labeled IgG antibody (Invitrogen) diluted 1:250 in TNB. After cells were washed, mounting media (Invitrogen) containing Hoechst 33342 stain was added to each well unless otherwise stated. Vacuole membrane staining using FM4–64 (Sigma) was performed as described previously (35). Briefly, mid-logarithmic copper-starved cells were harvested and resuspended in yeast extract plus supplements medium containing BCS (100 μm) and FM4–64 (16 μm) for 30 min at 30 °C. Cells were pelleted, washed, resuspended in fresh BCS-containing yeast extract plus supplements and incubated at 30 °C for an additional 30 min. Fluorescence and differential interference contrast images of the cells were obtained using a Nikon Eclipse E800 epifluorescent microscope (Nikon, Melville, NY) equipped with a Hamamatsu ORCA-ER digital cooled charge-coupled device (CCD) camera (Hamamatsu, Bridgewater, NJ). Fields shown in this study are representative of at least five independent experiments. Merged images were obtained using the Simple PCI software version 5.3.0.1102 (Compix, Sewickly, PA).
SOD and CAO Activity Assays
Protein extracts were prepared from mid-logarithmic cells that had been incubated for 16 h with either BCS (100 μm) or CuSO4 (50 μm) unless otherwise stated. After treatments, cells were harvested and washed with lysis buffer containing 25 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1 mm PMSF, 1 mm DTT, 0.1 mm Na3VO4, and a protease inhibitor mixture (Sigma; P8340). Once washed, cells were suspended in 200 μl of lysis buffer and then disrupted with an equivalent volume of glass beads using a Fastprep instrument (MP Biomedicals). Lysates were centrifuged at 13,000 × g for 15 min at 4 °C, and then the supernatants that contained soluble proteins were partially purified using Spin-X centrifuge tube filters (Costar-Corning; 8161). Equal concentrations of lysates were analyzed on 10% native polyacrylamide gels as described previously (36), and SOD activity assays were carried out using in-gel nitro blue tetrazolium staining as described previously (11). Spectrophotometric determination of SOD activity was performed using a cytochrome c/xanthine oxidase method (13). CAO activity was determined by spectrophotometry as described previously (12). In these assays hydrogen peroxide that is released is quantitated using 4-aminoantipyrine and vanillic acid to generate a quinoneimine dye, which is detected at 498 nm.
Immunoblotting and ChIP
For Western blotting experiments, protein extracts were resolved on 12% or 15% sodium dodecyl sulfate-polyacrylamide gels. For protein expression analysis of SOD1, Cao1, and α-tubulin, the following primary antibodies were used for immunodetection: monoclonal anti-SOD1 antibody (SOD-100; Stressgen), monoclonal anti-His5 antibody (penta-His; Qiagen), and monoclonal anti-α-tubulin antibody (clone B-5–1-2; Sigma), respectively. After incubation with primary antibodies, membranes were washed and incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences), developed with ECL reagents (Amersham Biosciences), and visualized by chemiluminescence. Cao1 was detected by immunoblotting using an anti-His5 antibody due to the presence of an endogenous cluster that contains 5 His residues located within the N-terminal residues 10–14 of Cao1 (12). ChIP experiments were carried out as described previously (4).
RESULTS
ctr4+ and ctr5+ mRNA Levels Are Induced in Response to Low Levels of Copper in a Cuf1-dependent Manner in Early-phase Meiosis
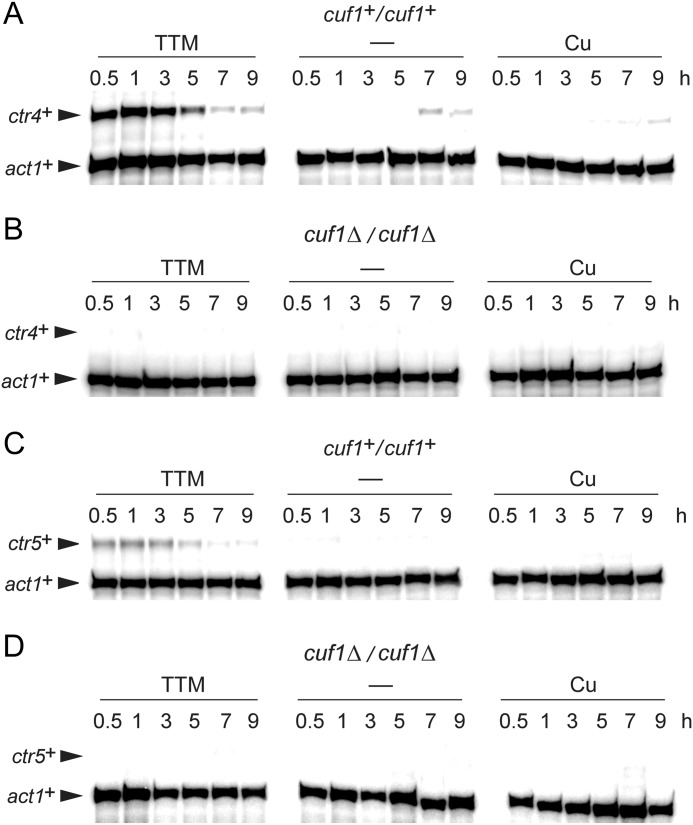
As previously observed (19) and as shown in Fig. 1, when a diploid parental strain pat1-114/pat1-114 was synchronized through meiosis under copper-deficient conditions, ctr4+ mRNA levels were induced ∼16-fold compared with basal levels observed in untreated cells after 1 h of meiotic induction (Fig. 1). After 3 h of the meiotic program, ctr4+ mRNA levels were reduced and subsequently repressed within ∼5–7 h. RNA samples prepared from a pat1-114/pat1-114 cuf1Δ/cuf1Δ mutant strain showed loss of copper starvation-dependent induction of ctr4+ gene expression (Fig. 1). A second member of the Ctr family, Ctr5, is known to assemble with Ctr4 to form the high affinity copper transport complex at the cell surface in fission yeast. Expression of the ctr5+ gene was also analyzed as a function of time during meiosis (Fig. 1). Consistently, ctr5+ mRNA levels were co-induced with the transcript levels of ctr4+, except that they were lower than ctr4+ levels. To further examine whether ctr5+ transcription was controlled by Cuf1, a pat1-114/pat1-114 cuf1Δ/cuf1Δ deletion strain was incubated in the absence or presence of the copper chelator TTM (50 μm) or CuSO4 (50 μm). As observed in the case of ctr4+, deletion of cuf1+ impaired the induction of ctr5+ during meiosis (Fig. 1). We, therefore, concluded that ctr4+ and ctr5+ genes exhibit a similar meiotic temporal expression profile and that Cuf1 transcription factor is required for induction of both genes in response to copper starvation.
FIGURE 1.
The cuf1+ gene is required for copper starvation-dependent induction of ctr4+ and ctr5+ transcripts during meiosis. Representative expression profiles of ctr4+ and ctr5+ transcripts in pat1-114/pat1-114 cuf1+/cuf1+, and pat1-114/pat1-114 cuf1Δ/cuf1Δ cells that were induced to undergo synchronous meiosis. Cells were either left untreated (−) or incubated in the presence of TTM (50 μm) or CuSO4 (50 μm). At the indicated times after meiotic induction, ctr4+, ctr5+, and act1+ (internal control) mRNA levels were analyzed in control strain (cuf1+/cuf1+) (panels A and C) and isogenic strain lacking the cuf1+ alleles (cuf1Δ/cuf1Δ) (panels B and D).
The ctr6+ Gene Is Transcriptionally Regulated by Cuf1 and Mei4 during Meiosis
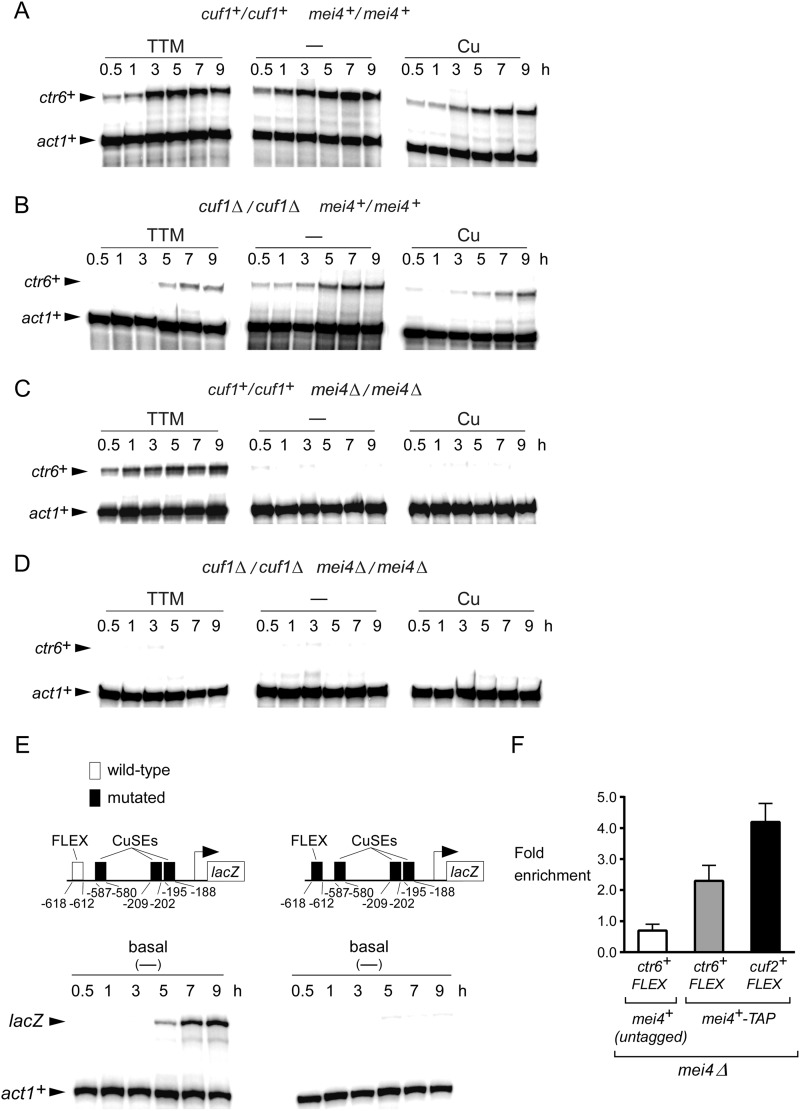
ctr6+ encodes for a third member of the Ctr family in S. pombe. In cells proliferating in mitosis, its expression is induced under low levels of copper in a Cuf1-dependent manner (13). In contrast, ctr6+ is negatively regulated when cells are exposed to exogenous copper. It is currently unknown whether ctr6+ is expressed in meiotic cells. To investigate this question, pat1-114/pat1-114 diploid cells were synchronously induced into meiosis and left untreated or were treated with either TTM (50 μm) or CuSO4 (50 μm). Aliquots of cultures were taken after meiotic induction, and the steady-state levels of ctr6+ mRNA were analyzed by RNase protection assays. Results showed that the steady-state levels of ctr6+ mRNA under basal (untreated) and copper-starved (50 μm TTM) conditions were primarily increased between 3 and 9 h after meiotic induction (Fig. 2A). Surprisingly, in the presence of exogenous copper (50 μm), ctr6+ transcript levels were detected after 5 h of meiotic induction but to a lesser extent in comparison with transcript levels observed in untreated cells (Fig. 2A). To assess whether Cuf1 was required for expression of ctr6+ during meiosis, the ctr6+ transcript was probed in a pat1-114/pat1-114 cuf1Δ/cuf1Δ mutant strain throughout meiosis. Under all conditions tested, disruption of cuf1+ resulted in strongly reduced ctr6+ mRNA levels (∼4–12-fold) in comparison with those recorded in the case of wild-type cells grown under the same conditions (Fig. 2B). Weak ctr6+ mRNA levels were primarily observed after 5 or 7 h of meiotic induction, which corresponds to the time of expression of several middle-phase genes. Because the meiotic transcription factor Mei4 is known to be required for the induction of several middle meiosis-specific genes (25), RNase protection assays were performed using a pat1-114/pat1-114 mei4Δ/mei4Δ mutant strain. Results were compared with those obtained with a pat1-114/pat1-114 control strain. In the case of mei4Δ/mei4Δ cells, ctr6+ transcript levels were only detected in response to copper starvation conditions and were absent under basal and copper-replete conditions (Fig. 2C). When a cuf1Δ/cuf1Δ mei4Δ/mei4Δ double mutant strain was examined, ctr6+ transcript was not detected under all experimental conditions, including basal, copper-starved, and copper-replete conditions (Fig. 2D). Analysis of the ctr6+ promoter revealed that it contains a putative consensus Mei4-binding FLEX sequence (40) and three Cuf1 binding CuSEs (Fig. 2E). To ascertain whether the FLEX element plays a role in ctr6+ regulation during meiosis, we constructed a ctr6+ promoter-lacZ fusion gene harboring a 620-bp fragment from the ctr6+ promoter in which the three CuSEs elements have been mutagenized. Subsequently, we used this reporter construct that contained one FLEX element or the same promoter fragment in which the FLEX box has been mutated. As shown in Fig. 2E, ctr6+-lacZ expression from the reporter plasmid containing the FLEX box exhibited a meiotic gene time-dependent expression profile, peaking ∼7–9 h after meiotic induction. In contrast, meiotic-dependent expression of lacZ mRNA was abrogated in the absence of the FLEX sequence. Furthermore, ChIP analysis showed that Mei4-TAP was enriched ∼2.3-fold at the ctr6+ promoter region encompassing the FLEX element in comparison with a non-transcribed intergenic region that served as a negative control (Fig. 2F). Untagged Mei4 immunoprecipitated only background levels of the ctr6+ promoter region (∼0.7-fold). Consistent with the fact that the Mei4 transcription factor is required for cuf2+ gene expression during meiosis (26), Mei4-TAP occupied the cuf2+ promoter with a ∼4.2-fold enrichment (used as a positive control) (Fig. 2F). Taken together, these results indicate that the meiotic-dependent expression of ctr6+ transcript requires both transcription factors, Cuf1 and Mei4, although Cuf1 has a greater contribution under copper-limiting conditions than Mei4.
FIGURE 2.
Effect of cuf1Δ/cuf1Δ or mei4Δ/mei4Δ deletion on the expression of ctr6+. Cultures of pat1-114/pat1-114 cuf1+/cuf1+ mei4+/mei4+ (panel A), pat1-114/pat1-114 cuf1Δ/cuf1Δ mei4+/mei4+ (panel B), pat1-114/pat1-114 cuf1+/cuf1+ mei4Δ/mei4Δ (panel C), and pat1-114/pat1-114 cuf1Δ/cuf1Δ mei4Δ/mei4Δ (panel D) cells were induced to initiate and proceed through meiosis. Cells were left untreated (−) or incubated in the presence of TTM (50 μm) or CuSO4 (50 μm). Total RNA was isolated from culture aliquots taken at the indicated time points. ctr6+ and act1+ steady-state mRNA levels were analyzed by RNase protection assays. E, diagram representation of a 620-bp ctr6+ promoter DNA fragment and its mutant derivative that were fused upstream of the lacZ gene. The white box indicates the wild-type FLEX element, whereas the black ones represent mutant versions of FLEX and CuSEs elements. The nucleotide numbers refer to the positions of the cis-acting elements relative to that of the ctr6+ initiator codon. Steady-state levels of lacZ and act1+ mRNAs were analyzed at different time points after meiotic induction under basal conditions. F, ChIP assays were performed using untagged and TAP-tagged versions of Mei4. ChIP results are presented as the fold enrichment at the ctr6+ or cuf2+ promoter encompassing a FLEX element in comparison with a non-transcribed intergenic region. The data and error bars represent the average and S.D. from at least three biological replicates.
cuf1+ and mei4+ Gene Expression Profiles as a Function of Changes in Copper Levels during Meiosis
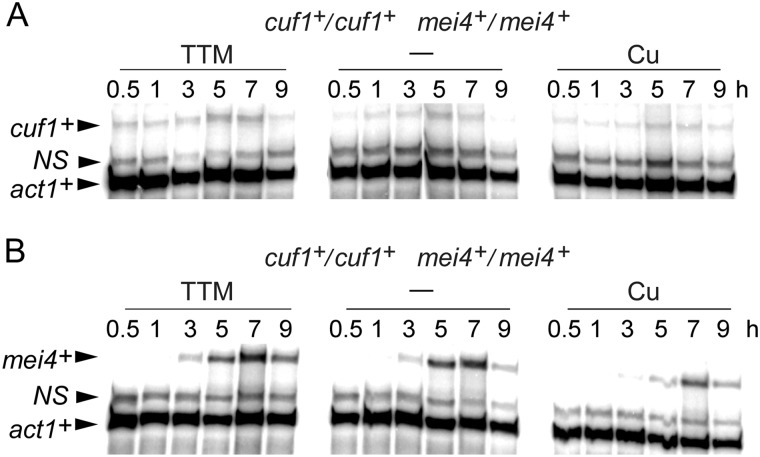
We next analyzed steady-state mRNA levels of cuf1+ and mei4+ as a function of copper availability during meiosis. A pat1-114/pat1-114 diploid strain was synchronized to initiate and proceed through the meiotic program. Before their entry into meiosis, cells were left untreated or exposed to TTM (50 μm) or CuSO4 (50 μm). Aliquots of cultures were picked up at distinct time points after meiotic induction, and steady-state levels of cuf1+ and mei4+ mRNAs were analyzed by RNase protection assays. Results showed that cuf1+ and mei4+ transcripts were detected under basal, copper-starved, or copper-replete conditions (Fig. 3). cuf1+ mRNAs were seen at each time point and were slightly increased after 5 and 7 h of meiotic induction (∼2–3-fold compared with levels observed after 1 h of meiotic induction). In the case of mei4+ mRNAs, their steady-state levels were absent after 1 h of meiotic induction. Subsequently, a slight increase of mei4+ expression was observed after 3 h, and it became more pronounced 5 and 7 h after meiotic induction (Fig. 3). After cell entrance into meiosis, the expression profile of mei4+ revealed that it reached a maximum 5–7 h after meiotic induction, indicating that its presence was primarily required during the middle meiotic phase. In the case of cuf1+, its expression profile as a function of time suggested a broader role during the differentiation process. In response to copper (50 μm), cuf1+ and mei4+ were expressed to a slightly lesser degree overtime. However, their overall expression profiles were unchanged as compared with those observed under basal and copper-depleted conditions (Fig. 3). This observation was likely related to a general effect of copper on meiosis instead of a specific effect of copper on cuf1+ and mei4+ transcripts.
FIGURE 3.
Assessment of the mRNA steady-state levels of cuf1+ and mei4+ during meiosis. pat1-114/pat1-114 cuf1+/cuf1+ mei4+/mei4+ cells underwent synchronous meiosis under basal (-), copper-depleted, and copper-replete conditions. At the indicated time points, cuf1+, mei4+, and act1+ mRNA levels were analyzed by RNase protection assays. NS, non-specific signal.
Ctr4-GFP and Ctr5-Cherry Colocalize at the Cell Surface during the First Stages of Meiosis
When cells grow mitotically under low levels of copper, Ctr4 and Ctr5 colocalize at the plasma membrane where they form a heteroprotein complex, which mediates high affinity copper transport (5, 6). Our previous studies reported location of Ctr4 at the plasma membrane shortly after induction of meiosis (19). After 3 h of meiotic induction, Ctr4-GFP-associated fluorescence progressively disappeared, a result that was consistent with decreased ctr4+ mRNA levels at the same stage of the meiotic program. To further characterize the location of Ctr4 and Ctr5 during meiosis, functional ctr4+-GFP and ctr5+-Cherry alleles were integrated into h− ctr4Δ ctr5Δ and h+ ctr4Δ ctr5Δ cells. After mating, h−/h+ ctr4Δ/ctr4Δ ctr5Δ/ctr5Δ ctr4+-GFP/ctr4+-GFP ctr5+-Cherry/ctr5+-Cherry diploid cells were cultured to undergo azygotic synchronous meiosis. Under copper-limiting conditions (50 μm TTM), Ctr4-GFP and Ctr5-Cherry fluorescent proteins were rapidly co-detected, exhibiting fluorescence in prophase I, horse tail, and metaphase I (Fig. 4). At metaphase II, the Ctr4-GFP and Ctr5-Cherry fluorescent signals progressively decreased and then virtually disappeared during forespore membrane formation and sporulation (Fig. 4). Consistent with the existence of a clear interdependence between Ctr4 and Ctr5, the Ctr4-GFP- and Ctr5-Cherry-associated fluorescent signals were consistently co-localized (Fig. 4). Because transcription of the ctr4+-GFP and ctr5+-Cherry genes were repressed under high copper conditions (50 μm CuSO4), there was an absence of fluorescent signals in copper-treated zygotic cells (data not shown). Collectively, microscopic analyses of meiotic cells revealed that Ctr4-GFP and Ctr5-Cherry fusion proteins co-localize with each other. Furthermore, these two proteins were observed at the cell surface within 30 min after meiotic induction and remained at the cell periphery until the first meiotic division occurs.
FIGURE 4.
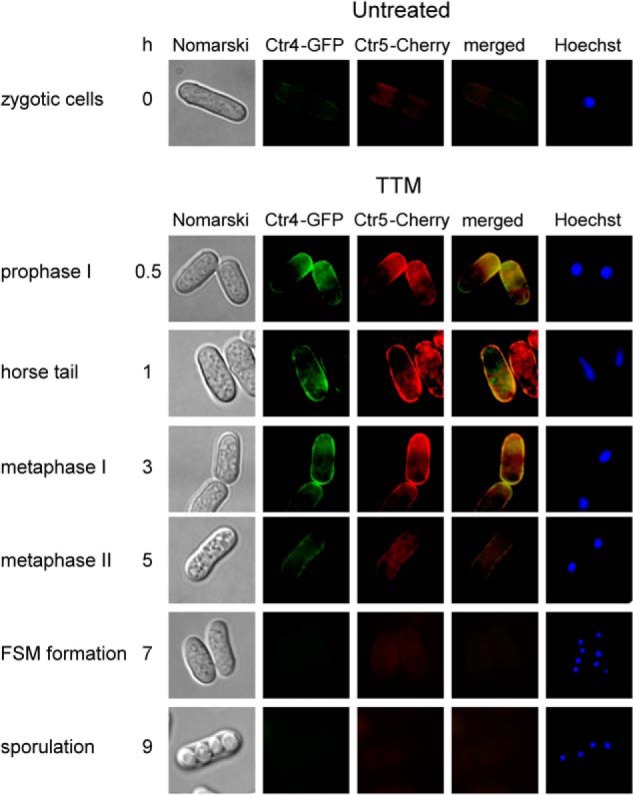
Colocalization of Ctr4-GFP and Ctr5-Cherry during meiosis. Diploid h+/h− ctr4Δ/ctr4Δ ctr5Δ/ctr5Δ cells co-expressing functional ctr4+-GFP and ctr5+-Cherry alleles were induced to undergo azygotic meiosis. Once induced, cells were differentiated in the presence of 50 μm TTM. Fluorescence signals of Ctr4-GFP and Ctr5-Cherry were observed at different stages of meiosis (center left). The merged images are shown in the center right panels. Nomarski optics (far left) were used to ascertain cell morphology and Hoechst 33342 staining to visualize DNA (far right). FSM, forespore membrane.
A Functional Vacuolar Ctr6 Transporter Is Required for the Production of a Fully Active SOD1
As previously observed (13) and as shown in Fig. 5A, cells harboring a double deletion of ctr4+ and ctr6+ (ctr4Δ ctr6Δ) are defective in SOD1 activity under copper starvation conditions. Loss of endogenous SOD1 activity was restored to ∼40% that of wild-type parental strain (FY435) by the addition of exogenous copper (50 μm CuSO4) (Fig. 5B). Under low copper conditions, disruption of the ctr4+ gene (ctr4Δ) dramatically reduced SOD1 activity, resulting in a weak, but significant proportion of SOD1 activity that reflected the contribution of Ctr6 in activating SOD1 (Fig. 5A). This result was used as a positive control to test whether ctr4Δ ctr6Δ cells expressing an untagged or HA4-tagged ctr6+ allele could acquire levels of SOD1 that were comparable with those found in the ctr4Δ strain. Results showed that Ctr6-HA4 functionally complemented SOD1 deficiency of the double ctr4Δ ctr6Δ mutant strain at the same level as did untagged Ctr6 (Fig. 5A). We then used ctr4Δ ctr6Δ cells expressing the Ctr6-HA4 fusion protein under low copper conditions and performed indirect immunofluorescence microscopy using anti-HA antibody. Results showed that Ctr6-HA4 localized to the membrane of vacuoles in cells proliferating in mitosis under copper-limiting conditions (Fig. 5C). Conveniently, when ctr6+-HA4 was induced by copper starvation, the number and size of the vacuoles decreased and became bigger in size, respectively, as a consequence of nutrient limitation (data not shown) (13), facilitating Ctr6-HA4 localization. To further confirm that Ctr6-HA4-associated fluorescence was detected in the vacuole membranes of cells, the vacuole-staining dye FM4–64 was used as a marker. Ctr6-HA4 and FM4–64 exhibited similar subcellular localization patterns within the cells (Fig. 5C). Because the vacuole has been shown to play an important role for metal ion storage (14, 37–39), one model has proposed that Ctr6 mobilizes stores of copper from the organelle to provide it to SOD1, thereby contributing to its activation (13).
FIGURE 5.
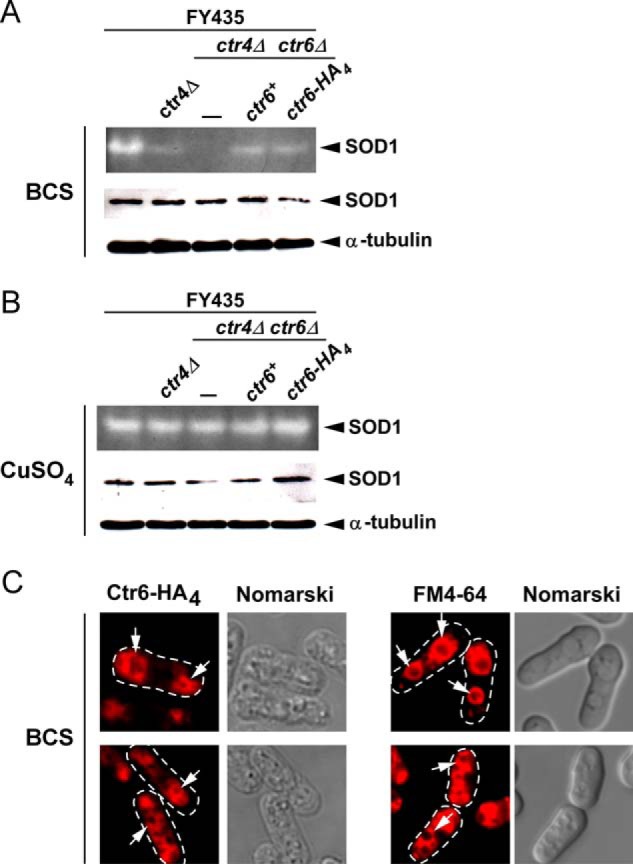
The vacuolar copper transporter Ctr6 contributes to production of active SOD1 during mitosis. A, representative in-gel activity assay of S. pombe strain (FY435) harboring a disrupted ctr4Δ allele exhibiting strong reduction of SOD1 activity under low copper conditions (100 μm BCS). Under the same conditions, a ctr4Δ ctr6Δ double mutant strain was devoid of measurable SOD1 activity unless the ctr6+ or ctr6+-HA4 allele was returned by integration. Protein extracts prepared from these strains were analyzed for steady-state levels of SOD1 by immunoblotting using anti-SOD1 and anti-α-tubulin antibodies. B, SOD1 activity and steady-state protein levels were determined in the same strains of panel A in the presence of excess CuSO4 (50 μm). C, cells were analyzed by indirect immunofluorescence microscopy for vacuolar localization of a functional Ctr6-HA4 fusion protein that was expressed in ctr4Δ ctr6Δ cells grown under copper-limiting conditions (100 μm BCS) (left). FM4–64 staining (right) was visualized by fluorescence microscopy as a marker of vacuolar membranes. Nomarski optics were used to examine cells under fixed (left) and non-fixed (right) conditions. White arrows indicate examples of vacuolar membranes.
Analysis of Ctr6 Localization during Meiotic Program
To further investigate the role of Ctr6 during meiosis, a functional ctr6+-HA4 allele was integrated into h− ctr6Δ and h+ ctr6Δ cells. After mating, h−/h+ ctr6Δ/ctr6Δ ctr6+-HA4/ctr6+-HA4 diploid cells were cultured under conditions to induce azygotic synchronous meiosis. Under basal or low copper conditions, Ctr6-HA4 was detected in the vacuole membranes of cells at prophase I (data not shown) and metaphase I (3-h time point) (Fig. 6). At the 3-h time point, Ctr6-HA4-associated fluorescence was more prominent in cells starved for copper compared with cells incubated under basal conditions. When azygotic meiosis was synchronously induced in the presence of copper, Ctr6-HA4 fluorescent signal was barely detected during the first 3 h of meiosis (Fig. 6). During meiotic metaphase II (5-h time point) and forespore membrane formation (7-h time point), zygotic cells displayed Ctr6-HA4 fluorescence in smaller vacuole or vesicle membranes. At these stages the strength of the fluorescent signals was strong regardless of copper conditions. After 9 h of meiotic induction (sporulation), Ctr6-HA4 generated a fluorescent signal at the spore membrane, suggesting a novel role for the Ctr6 transporter at the spore surface. To validate that Ctr6-HA4 located at the forespore membrane during late meiosis, we analyzed (under the same conditions) cells expressing a GFP-Psy1 protein, an intrinsic component of forespore membrane. Both proteins exhibited similar fluorescent patterns (Fig. 6). Additional evidence supporting the presence of Ctr6 at the membrane of spores was obtained by findings that Ctr6-HA4-associated fluorescence was detected at the surface of spores that had been newly released from asci after 12 h of meiotic induction (Fig. 6). Taken together, data from microscopic analysis of meiotic cells revealed that a functional Ctr6-HA4 protein localizes in the vacuole membranes of cells that had undergone meiotic divisions and that it becomes resident of the spore membrane during the forespore membrane formation.
FIGURE 6.
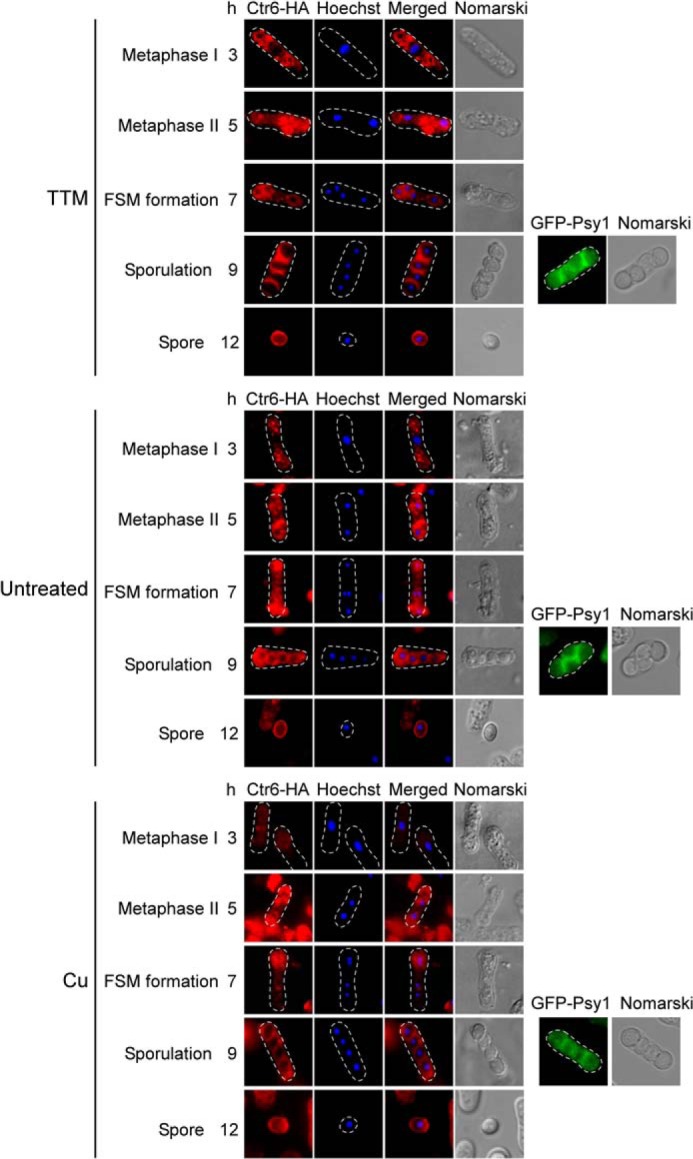
Subcellular localization of Ctr6-HA4 during meiosis and sporulation. h−/h+ ctr6Δ/ctr6Δ mutant cells expressing Ctr6-HA4 were synchronously induced to undergo azygotic meiosis and then were left untreated or were treated with TTM (50 μm) or CuSO4 (Cu) (50 μm) for the indicated time points. Hoechst 33342 staining was used to visualize DNA (center left). The merged images of the Ctr6-HA4 and the Hoechst dye are shown (center right). Fixed cells observed by indirect immunofluorescence (Ctr6-HA4; far left) were also examined by Nomarski optics (far right). After 9 h of meiotic induction, the GFP-Psy1 fluorescent protein was examined and used as a forespore membrane resident marker. At the 12-h time point, the Ctr6-HA4 fluorescent signal was observed on the membrane of spores that were newly released from asci. FSM, forespore membrane.
Ctr4 and Ctr6 Are Required for the Synthesis of Fully Active SOD1, whereas an Additional Contribution by Mfc1 Is Necessary for Full Activation of CAO in Late-phase Meiosis
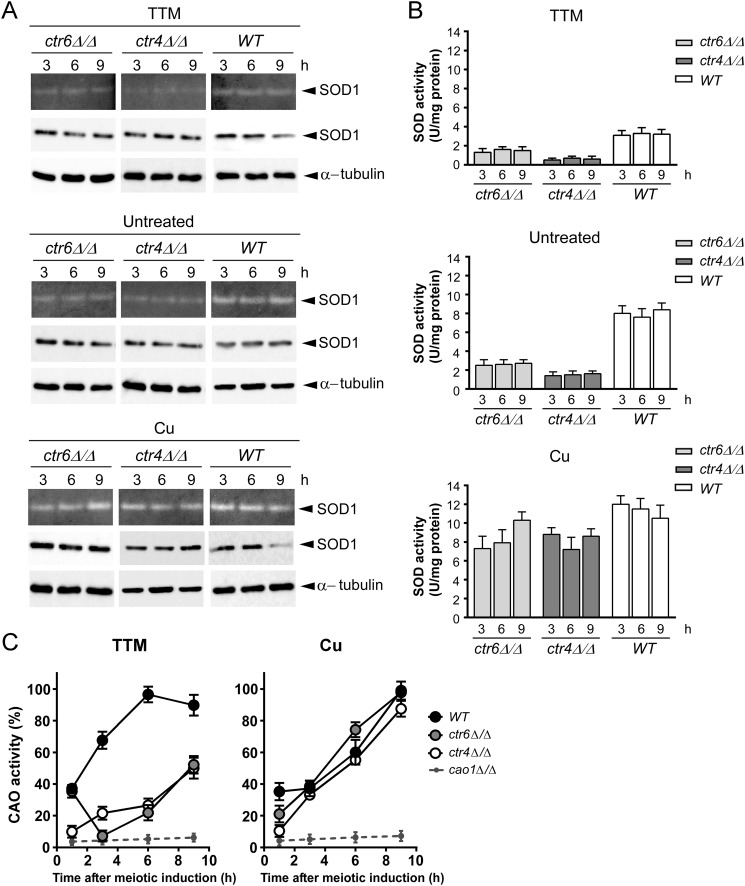
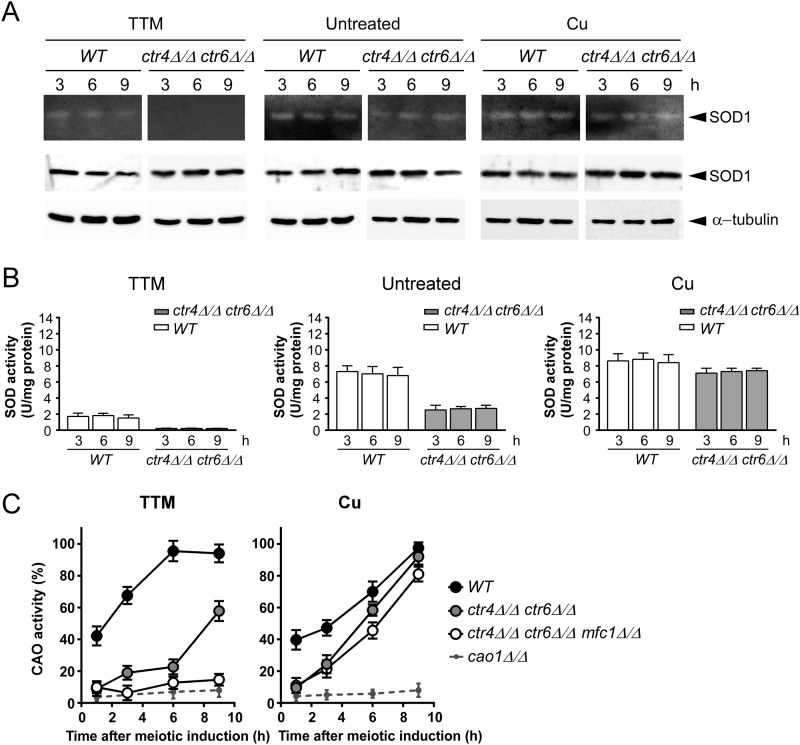
To determine whether Ctr4 or Ctr6 were required for providing copper to SOD1 during meiosis, we used isogenic diploid strains harboring wild-type genes or insertionally inactivated ctr4Δ/ctr4Δ, ctr6Δ/ctr6Δ (Fig. 7) or ctr4Δ ctr6Δ/ctr4Δ ctr6Δ alleles (Fig. 8). In the presence of TTM (25 μm), ctr4Δ/ctr4Δ cells exhibited SOD1 activity that was significantly lower (∼5.3-fold) than that of wild-type cells (Fig. 7). In the case of ctr6Δ/ctr6Δ cells, SOD1 activity was less (∼2.2-fold) than that of the wild-type cells but higher (∼2.4-fold) than that of ctr4Δ/ctr4Δ cells (Fig. 7). Under copper starvation conditions (25 μm TTM), no measurable SOD1 activity was observed in the case of ctr4Δ ctr6Δ/ctr4Δ ctr6Δ double mutant strain (Fig. 8), revealing a critical role for both Ctr4 and Ctr6 in conferring SOD1 activity during meiosis. To verify that the SOD1 protein was present in wild-type and mutant strains, total protein extracts were analyzed by immunoblotting at the indicated meiotic time points (Figs. 7 and 8). Results showed that detectable levels of SOD1 protein were present in all strains, indicating that the decrease or lack of activity was not due to the absence of SOD1 expression. As expected, SOD1 activity could be restored by the addition of high exogenous copper concentrations (25 μm) to the cell cultures (Figs. 7 and 8). This effect was likely due to the fact that copper ions were taken up by way of a low-affinity copper transport system, thereby bypassing requirements for high-affinity copper transporters.
FIGURE 7.
Effect of ctr4Δ/ctr4Δ and ctr6Δ/ctr6Δ disruptions on SOD1 and CAO activities during meiosis. A, wild-type diploid (h−/h+ ctr4+/ctr4+ ctr6+/ctr6+), h−/h+ ctr6Δ/ctr6Δ, and h−/h+ ctr4Δ/ctr4Δ mutant strains were synchronously induced to undergo meiosis under basal (untreated), copper-depleted (25 μm TTM), and copper-replete (25 μm CuSO4) conditions. At the indicated time points total extracts from aliquots of cultures were analyzed for SOD1 activity using an in-gel activity assay. Protein extracts were analyzed for steady-state protein levels of SOD1 and α-tubulin by immunoblotting using anti-SOD1 and anti-α-tubulin antibodies, respectively. B, spectrophotometric determination of SOD activity was also performed from these strains using a cytochrome c/xanthine oxidase method. Data are the averages of triplicate determinations ± S.D. C, the above-mentioned strains plus a h−/h+ cao1Δ/cao1Δ mutant were analyzed for CAO activity by spectrophotometric assays using 4-aminoantipyrine and vanillic acid. Error bars indicate S.D. of activities from samples analyzed in triplicate.
FIGURE 8.
SOD1 and Cao1 enzymes require different combinations of copper transporters for their activation during meiosis. Cultures of h−/h+ ctr4+/ctr4+ ctr6+/ctr6+ (WT) and h−/h+ ctr4Δ/ctr4Δ ctr6Δ/ctr6Δ diploid cells were synchronously induced into meiosis under basal (untreated), copper-starved (25 μm TTM), or copper-replete (25 μm CuSO4) conditions. Total extracts were prepared from culture aliquots taken at the indicated time points. SOD1 activity was determined using an in-gel assay. Aliquots of total-extract preparations were also examined by immunoblotting using either anti-SOD1 or anti-α-tubulin antibody. B, cell extracts were prepared from strains used in A, and analyzed by a cytochrome c/xanthine oxidase method. Results are the averages of triplicate determinations ± S.D. C, FY435/FY436 (h+/h−) (WT), h+/h− ctr4Δ/ctr4Δ ctr6Δ/ctr6Δ, h+/h− ctr4Δ/ctr4Δ ctr6Δ/ctr6Δ mfc1Δ/mfc1Δ, and h+/h− cao1Δ/cao1Δ strains were synchronously induced into azygotic meiosis under copper-starved or copper-replete conditions. CAO activity was quantitated in cell lysates at the indicated time after meiotic induction. Data are the averages of triplicate determinations ± S.D.
The above-mentioned strains were also used to monitor CAO activity during meiosis. Two genes encode members of the CAO family in S. pombe. However, only one gene (cao1+) encodes an active enzyme (12). Diploid wild-type cells were synchronized through meiosis in the presence of either TTM (25 μm) or CuSO4 (25 μm). Under copper-limiting conditions, results showed that levels of CAO activity increased as a function of time to reach maximal activity within 6 h, at which point CAO activity levels remained high until the end of the meiotic program (Figs. 7 and 8). In the case of ctr4Δ/ctr4Δ mutant cells they exhibited an activity ∼3–3.7-fold weaker than wild-type cells after 1 h of meiotic induction under low copper conditions (Fig. 7). Although CAO activity slightly increased after 3 and 6 h of meiotic induction, ctr4Δ/ctr4Δ null cells displayed an activity that was ∼3.1–3.6-fold weaker than that of wild-type cells. After the 6-h time point, we observed that CAO activity levels were significantly up-regulated (∼27%), although this increase was insufficient to reach levels observed in the case of wild-type cells (Fig. 7). After 1 h of meiotic induction, copper-starved ctr6Δ/ctr6Δ mutant cells exhibited CAO activity comparable with that of the wild-type strain (Fig. 7). However, levels of CAO activity were quickly reduced ∼4.9-fold within the next 3 h (Fig. 7). Results showed that CAO activity increased after 6 h of meiotic induction (∼3-fold compared with levels observed after 3 h of meiotic induction). This increased activity was followed by a second increase (∼2.4-fold compared with levels after 6 h of meiotic induction) within 9 h of TTM treatment (Fig. 7). CAO activity of ctr4Δ/ctr4Δ and ctr6Δ/ctr6Δ cells was restored by supplementing the medium with 25 μm exogenous copper (Fig. 7). This effect was likely due to mobilization of copper via a low-affinity copper uptake system, which bypasses the requirement for Ctr4 and Ctr6 transport systems. To ensure that the decrease of CAO activity in the ctr4Δ/ctr4Δ and ctr6Δ/ctr6Δ mutant strains grown under low copper conditions was not due to a defect in Cao1 expression, we analyzed the steady-state protein levels of Cao1 by immunoblotting. We found that Cao1 was produced at similar steady-state levels in wild-type and mutant strains (data not shown).
Once early-phase meiosis has been initiated under conditions of copper starvation (25 μm TTM), ctr4Δ ctr6Δ/ctr4Δ ctr6Δ double mutant cells exhibited a decrease in CAO activity that was reminiscent of cells bearing a single ctr4Δ deletion (∼4.2-fold weaker activity compared with wild-type strain) (Fig. 8). This was followed by a modest increase of CAO activity after 3 h (∼2.1-fold) and 6 h (∼1.2-fold) of meiotic induction in comparison with double mutant cells at the 1- and 3-h time points, respectively. As observed in the case of ctr4Δ/ctr4Δ mutant strain, CAO activity levels of ctr4Δ ctr6Δ/ctr4Δ ctr6Δ mutant cells were markedly up-regulated (∼39%) between 6 and 9 h, suggesting that another protein participated in delivering copper to Cao1. Because previous results had shown that Mfc1 was required for production of fully active CAO during meiosis (19), a ctr4Δ ctr6Δ mfc1Δ/ctr4Δ ctr6Δ mfc1Δ triple mutant was generated and induced to undergo synchronous meiosis. After 9 h of meiotic induction, results showed that inactivation of mfc1+ (mfc1Δ) in the context of ctr4Δ ctr6Δ resulted in ∼43 and ∼79% less CAO activity as compared with ctr4Δ ctr6Δ/ctr4Δ ctr6Δ double mutant and wild-type cells, respectively. Consistent with the restoration of CAO activity after the addition of exogenous copper (25 μm), double and triple mutant strains displayed an increase in CAO activity up to ∼93 and ∼82% of the levels observed in the case of wild-type strain, respectively (Fig. 8). To ensure that the decrease of CAO activity in mutant strains grown under copper-limiting conditions was not due to a defect in CAO expression, steady-state levels of Cao1 were analyzed and found to remain present at similar levels throughout the entire period of meiosis in all strains (data not shown). Taken together, the results revealed a distinct requirement of copper transporters for activation of SOD1 and CAO enzymes under conditions of copper starvation during meiosis. Whereas Ctr4 and Ctr6 transporters are required to produce a fully active SOD1 enzyme, the presence of the additional transporter Mfc1 is needed to ensure maximal CAO activity throughout the entire meiotic program.
DISCUSSION
The biosynthesis of copper transporters Ctr4 and Ctr5 is dependent on the presence of the copper-sensing transcription factor Cuf1, which itself is activated under low-copper conditions during the mitotic cell cycle (5, 11, 33). Under these conditions, Ctr4 and Ctr5 are co-expressed and are assembled as a heteromeric complex at the cell surface where it mediates high affinity copper transport. When cells switch from mitosis to meiosis, expression profiles of ctr4+ and ctr5+ revealed that the two genes are still co-expressed and co-localized at the cell surface but only during the early steps of the meiotic program. Once pre-meiotic DNA replication and recombination are completed, both transcript and protein levels of Ctr4 and Ctr5 decrease progressively, suggesting that high affinity copper transport mediated by the Ctr4-Ctr5 complex is primarily needed in early meiosis. Possible explanations may be that one or more copper-dependent enzyme(s) is required for early steps of meiosis or copper ions need to be transported with very high efficacy from the environment during early meiosis, thereby allowing storage for subsequent usage during middle and late meiosis. As observed in the case of mitotic cells, expression of ctr4+ and ctr5+ in meiotic cells was copper starvation- and Cuf1-dependent. During meiosis, ccc2+ that encodes a putative copper-transporting P-type ATPase exhibited a constitutive transcription as a function of time or copper needs during meiosis (data not shown), eliminating any correlation between changes in expression profiles of ctr4+/ctr5+ genes and transcriptional levels of ccc2+.
In the case of cuf1+, its meiotic expression profile differed from that of ctr4+ and ctr5+. Although cuf1+ mRNA levels were detected in early meiosis, its transcript levels remained expressed throughout the meiotic division and spore maturation processes. They were slightly increased 5 and 7 h after meiotic induction, which corresponds to the end of the second meiotic division and the biogenesis of forespore membrane. The fact that the expression of cuf1+ was sustained throughout the meiotic program suggested the existence of additional Cuf1-dependent target genes along with ctr4+ and ctr5+. One candidate gene was ctr6+, as its mitotic copper-dependent expression is regulated by Cuf1 (13). Furthermore, as opposed to the expression profiles of ctr4+ and ctr5+, ctr6+ transcripts were detected during early, middle, and late phases of meiosis. The nature of the mechanism by which Cuf1 stops to activate ctr4+ and ctr5+ after early meiosis but still activates ctr6+ during middle and late phases is unknown. Nonetheless, deletion of cuf1+ (cuf1Δ/cuf1Δ) led to a substantial decrease of ctr6+ mRNA levels. Surprisingly, its expression was not completely abolished and remained detectable during and after meiotic divisions. On the basis of this observation, ctr6+ expression was analyzed in a mei4Δ/mei4Δ mutant strain, which lacks the transcription regulator Mei4 that is required for the induction of several middle meiosis-specific genes (25). The results showed that ctr6+ transcription relies on Mei4 during the meiotic divisions and the process of spore formation, especially under basal and copper-replete conditions. These observations led to the conclusion that meiotic differentiation requires a dual regulation of ctr6+ by two distinct transcription factors and that ensures the continuous presence of Ctr6 during the developmental program.
In cells undergoing vegetative growth (mitosis), Ctr6 localizes to the vacuolar membrane under conditions of low copper availability. After cell entry into meiosis, microscopic analyses revealed that Ctr6 localized to the vacuolar membrane before completion of the first meiotic division. After completion of meiosis I, Ctr6 intracellular distribution had the appearance of cytoplasmic vesicular staining within the zygote. After the second meiotic division, zygotic cells exhibited Ctr6 fluorescence as large vesicular structures that appeared to progressively surround the chromosomal material. After forespore membrane closure, Ctr6 resided at the prespore membrane in which the chromosomal DNA is packed. On the basis of these observations, we propose that Ctr6 moves from the vacuolar membrane to the forespore membrane over the course of the meiotic program. Another S. pombe protein, Psy1, has been reported to be differentially located in cells proliferating in mitosis as opposed to meiosis (41). In the case of Psy1, it localizes to the plasma membrane in mitotic cells but to the forespore membrane of developing asci (42). Previous experiments using the fluorescent copper binding tracker Coppersensor-1 (43, 44) have shown that forespores accumulate labile cellular copper in sporulating cells (19). This observation was consistent with a need for translocation of Ctr6 from the vacuolar membrane to the forespore membrane. Ctr6 may transport stored copper from the prespore to the cytosol where meiotic copper-dependent enzymes can be found such as the SOD1 (data not shown). It is known that the forespore membrane expands by membrane vesicle fusion. Studies have reported that these vesicles are derived from the endoplasmic reticulum via the Golgi network (41, 46–48). However, recent studies have shown that vacuolar proteins participate in forespore membrane assembly and may involve vacuolar membranous structures for forespore membrane formation (49, 50). This mechanism may contribute to relocation of Ctr6 to the nascent forespore membrane where it would serve to mobilize intracellular pools of labile copper. The localization of Ctr6 on the spore membrane at the end of sporulation may also suggest a role for Ctr6 in spore germination. In agreement with this possibility, we have observed that isolated spores from ctr6Δ/ctr6Δ asci exhibited a decrease in viability as compared those of ctr6+/ctr6+ when returned for germination under low-copper conditions (data not shown).
To characterize the functional importance of the presence of Ctr4 and Ctr6 during meiosis, copper-dependent SOD1 and CAO activities were monitored during spore formation. Results showed that under conditions of copper starvation, cells lacking Ctr4 (ctr4Δ) exhibited a dramatic decrease in SOD1 activity compared with that of wild-type cells. Under the same conditions, a ctr6Δ single mutant displayed less SOD1 activity, but the decrease was less pronounced compared with a ctr4Δ single mutant. In copper-starved ctr4Δ ctr6Δ double mutant cells, SOD1 activity was undetectable, suggesting that both Ctr4 and Ctr6 represent two key players for delivering copper to SOD1 during meiosis. The fact that Ctr4 and Ctr6 exhibited a distinct meiotic temporal expression profile may help to ensure a constant source of copper for SOD1. In S. cerevisiae it has been reported that sporulation in homozygous sod1Δ/sod1Δ diploids is decreased ∼100-fold in comparison with homozygous wild-type (SOD1/SOD1) diploids (45), suggesting an important role of SOD1 in meiosis. In addition to scavenging reactive superoxide anions that are produced during meiosis, SOD1 may participate in other functions that are required during meiotic differentiation. On the basis of the results, we conclude that SOD1 must already be active or rapidly activated after induction of meiosis and that Ctr4 and Ctr6 play an important role in delivering copper to SOD1 for its activation.
To further analyze the relative contribution of Ctr4 and Ctr6 during meiosis, CAO activity was analyzed in wild-type and ctr4Δ/ctr4Δ, ctr6Δ/ctr6Δ and ctr4Δ ctr6Δ/ctr4Δ ctr6Δ mutant cells. After induction of meiosis under low-copper conditions, we observed that the presence of Ctr4 was required for providing copper to Cao1 (the sole active CAO in S. pombe) at the initial stage of meiosis. Although the presence of Ctr6 was also important, its contribution was not detected at the same time in comparison with that of Ctr4, and was mainly seen after 3 h of meiotic induction. Intriguingly, but consistently with its meiotic temporal expression profile (19), the meiosis-specific copper transporter Mfc1 participated in the production of active CAO but only after 6 h of meiotic induction. Taken together, our results argue in favor of a model in which the three copper transporters Ctr4, Ctr6, and Mfc1 participate in the delivery of copper to CAO in a time-dependent manner, thereby ensuring that an active CAO is present throughout the entire meiotic program. One can envision that Ctr4, which is a major component of the heteromeric Ctr4-Ctr5 complex at the cell surface during the early stages of meiosis, plays a critical role for the acquisition of the primary pool of copper from the environment. When the Ctr4-Ctr5 heterocomplex is no longer present at the cell surface, Ctr6 serves to mobilize intravacuolar stores of copper, participating in the delivery of copper to cytosolic copper-dependent enzymes. After forespore membrane closure, Mfc1 would mediate copper accumulation into prespores, participating in the activation of Cao1 that localizes to the spore at this meiotic stage. During the sporulation process, a proportion of Ctr6 migrates from the vacuole to the forespore membrane where its presence may be required to counterbalance the action of Mfc1, therefore, ensuring that a fraction of copper could be delivered in the cytoplasmic region of the ascus. Although future studies will be required to demonstrate this model, results presented here are consistent with the existence of a dynamic interplay between the copper transport systems during meiosis of S. pombe.
Acknowledgments
We are grateful to Dr. Gilles Dupuis for critically reading the manuscript and for valuable comments. We thank Raymund Wellinger for the use of the micromanipulator in the tetrad dissections.
This work was supported by Canadian Institutes of Health Research Grant MOP-CP-243929 (to S. L.).
- Ctr
- copper transporter
- BCS
- bathocuproinedisulfonic acid
- Cao1
- copper amine oxidase 1
- CAO
- copper amine oxidase
- Cherry
- red fluorescent protein
- Cuf1
- copper factor 1
- EMM
- Edinburgh minimal medium
- Mfc1
- major facilitator copper transporter 1
- TTM
- ammonium tetrathiomolybdate
- TMD
- transmembrane domain.
REFERENCES
- 1. Nevitt T., Ohrvik H., Thiele D. J. (2012) Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta 1823, 1580–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beaudoin J., Ekici S., Daldal F., Ait-Mohand S., Guérin B., Labbé S. (2013) Copper transport and regulation in Schizosaccharomyces pombe. Biochem. Soc. Trans. 41, 1679–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halliwell B., Gutteridge J. M. (1992) Biologically relevant metal ion-dependent hydroxyl radical generation. An update. FEBS Lett. 307, 108–112 [DOI] [PubMed] [Google Scholar]
- 4. Labbé S., Beaudoin J., Ioannoni R. (2013) in Metals in Cells (Culotta V. C., Scott R. S., eds) pp. 163–174, John Wiley & Sons, Chichester, UK [Google Scholar]
- 5. Zhou H., Thiele D. J. (2001) Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 276, 20529–20535 [DOI] [PubMed] [Google Scholar]
- 6. Beaudoin J., Laliberté J., Labbé S. (2006) Functional dissection of Ctr4 and Ctr5 amino-terminal regions reveals motifs with redundant roles in copper transport. Microbiology 152, 209–222 [DOI] [PubMed] [Google Scholar]
- 7. Puig S., Lee J., Lau M., Thiele D. J. (2002) Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem. 277, 26021–26030 [DOI] [PubMed] [Google Scholar]
- 8. Aller S. G., Eng E. T., De Feo C. J., Unger V. M. (2004) Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J. Biol. Chem. 279, 53435–53441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beaudoin J., Thiele D. J., Labbé S., Puig S. (2011) Dissection of the relative contribution of the Schizosaccharomyces pombe Ctr4 and Ctr5 proteins to the copper transport and cell surface delivery functions. Microbiology 157, 1021–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ioannoni R., Beaudoin J., Mercier A., Labbé S. (2010) Copper-dependent trafficking of the Ctr4-Ctr5 copper transporting complex. PLoS ONE 5, e11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Labbé S., Peña M. M., Fernandes A. R., Thiele D. J. (1999) A copper-sensing transcription factor regulates iron uptake genes in Schizosaccharomyces pombe. J. Biol. Chem. 274, 36252–36260 [DOI] [PubMed] [Google Scholar]
- 12. Peter C., Laliberté J., Beaudoin J., Labbé S. (2008) Copper distributed by Atx1 is available to copper amine oxidase 1 in Schizosaccharomyces pombe. Eukaryot. Cell. 7, 1781–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellemare D. R., Shaner L., Morano K. A., Beaudoin J., Langlois R., Labbe S. (2002) Ctr6, a vacuolar membrane copper transporter in Schizosaccharomyces pombe. J. Biol. Chem. 277, 46676–46686 [DOI] [PubMed] [Google Scholar]
- 14. Rees E. M., Lee J., Thiele D. J. (2004) Mobilization of intracellular copper stores by the Ctr2 vacuolar copper transporter. J. Biol. Chem. 279, 54221–54229 [DOI] [PubMed] [Google Scholar]
- 15. Rees E. M., Thiele D. J. (2007) Identification of a vacuole-associated metalloreductase and its role in Ctr2-mediated intracellular copper mobilization. J. Biol. Chem. 282, 21629–21638 [DOI] [PubMed] [Google Scholar]
- 16. Marston A. L., Amon A. (2004) Meiosis. Cell-cycle controls shuffle and deal. Nat. Rev. Mol. Cell Biol. 5, 983–997 [DOI] [PubMed] [Google Scholar]
- 17. Handel M. A., Schimenti J. C. (2010) Genetics of mammalian meiosis. Regulation, dynamics, and impact on fertility. Nat. Rev. Genet. 11, 124–136 [DOI] [PubMed] [Google Scholar]
- 18. Kim A. M., Vogt S., O'Halloran T. V., Woodruff T. K. (2010) Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 6, 674–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beaudoin J., Ioannoni R., López-Maury L., Bähler J., Ait-Mohand S., Guérin B., Dodani S. C., Chang C. J., Labbé S. (2011) Mfc1 is a novel forespore membrane copper transporter in meiotic and sporulating cells. J. Biol. Chem. 286, 34356–34372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernhardt M. L., Kong B. Y., Kim A. M., O'Halloran T. V., Woodruff T. K. (2012) A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol. Reprod. 86, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harigaya Y., Yamamoto M. (2007) Molecular mechanisms underlying the mitosis-meiosis decision. Chromosome Res. 15, 523–537 [DOI] [PubMed] [Google Scholar]
- 22. Bähler J., Schuchert P., Grimm C., Kohli J. (1991) Synchronized meiosis and recombination in fission yeast. Observations with pat1-114 diploid cells. Curr. Genet. 19, 445–451 [DOI] [PubMed] [Google Scholar]
- 23. Doll E., Molnar M., Cuanoud G., Octobre G., Latypov V., Ludin K., Kohli J. (2008) Cohesin and recombination proteins influence the G1-to-S transition in azygotic meiosis in Schizosaccharomyces pombe. Genetics 180, 727–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mata J., Lyne R., Burns G., Bähler J. (2002) The transcriptional program of meiosis and sporulation in fission yeast. Nat. Genet. 32, 143–147 [DOI] [PubMed] [Google Scholar]
- 25. Mata J., Wilbrey A., Bähler J. (2007) Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol. 8, R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ioannoni R., Beaudoin J., Lopez-Maury L., Codlin S., Bahler J., Labbe S. (2012) Cuf2 is a novel meiosis-specific regulatory factor of meiosis maturation. PLoS ONE 7, e36338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabatinos S. A., Forsburg S. L. (2010) Molecular genetics of Schizosaccharomyces pombe. Methods Enzymol. 470, 759–795 [DOI] [PubMed] [Google Scholar]
- 28. Zhang M. M., Wu P. Y., Kelly F. D., Nurse P., Hang H. C. (2013) Quantitative control of protein S-palmitoylation regulates meiotic entry in fission yeast. PLoS Biol. 11, e1001597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Keeney J. B., Boeke J. D. (1994) Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics 136, 849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nevitt T., Thiele D. J. (2011) Host iron withholding demands siderophore utilization for Candida glabrata to survive macrophage killing. PLoS Pathog. 7, e1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen D., Toone W. M., Mata J., Lyne R., Burns G., Kivinen K., Brazma A., Jones N., Bähler J. (2003) Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14, 214–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mercier A., Watt S., Bähler J., Labbé S. (2008) Key function for the CCAAT-binding factor Php4 to regulate gene expression in response to iron deficiency in fission yeast. Eukaryot. Cell. 7, 493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beaudoin J., Labbé S. (2001) The fission yeast copper-sensing transcription factor Cuf1 regulates the copper transporter gene expression through an Ace1/Amt1-like recognition sequence. J. Biol. Chem. 276, 15472–15480 [DOI] [PubMed] [Google Scholar]
- 34. Mercier A., Pelletier B., Labbé S. (2006) A transcription factor cascade involving Fep1 and the CCAAT-binding factor Php4 regulates gene expression in response to iron deficiency in the fission yeast Schizosaccharomyces pombe. Eukaryot. Cell 5, 1866–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pouliot B., Jbel M., Mercier A., Labbé S. (2010) abc3+ encodes an iron-regulated vacuolar ABC-type transporter in Schizosaccharomyces pombe. Eukaryot. Cell 9, 59–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Laliberté J., Whitson L. J., Beaudoin J., Holloway S. P., Hart P. J., Labbé S. (2004) The Schizosaccharomyces pombe Pccs protein functions in both copper trafficking and metal detoxification pathways. J. Biol. Chem. 279, 28744–28755 [DOI] [PubMed] [Google Scholar]
- 37. Li L., Chen O. S., McVey Ward D., Kaplan J. (2001) CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 276, 29515–29519 [DOI] [PubMed] [Google Scholar]
- 38. MacDiarmid C. W., Milanick M. A., Eide D. J. (2002) Biochemical properties of vacuolar zinc transport systems of Saccharomyces cerevisiae. J. Biol. Chem. 277, 39187–39194 [DOI] [PubMed] [Google Scholar]
- 39. MacDiarmid C. W., Gaither L. A., Eide D. (2000) Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae. EMBO J. 19, 2845–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abe H., Shimoda C. (2000) Autoregulated expression of Schizosaccharomyces pombe meiosis-specific transcription factor Mei4 and a genome-wide search for its target genes. Genetics. 154, 1497–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakamura T., Nakamura-Kubo M., Hirata A., Shimoda C. (2001) The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1(+)-encoding syntaxin-like protein. Mol. Biol. Cell 12, 3955–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakamura T., Asakawa H., Nakase Y., Kashiwazaki J., Hiraoka Y., Shimoda C. (2008) Live observation of forespore membrane formation in fission yeast. Mol. Biol. Cell 19, 3544–3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller E. W., Zeng L., Domaille D. W., Chang C. J. (2006) Preparation and use of Coppersensor-1, a synthetic fluorophore for live-cell copper imaging. Nat. Protoc. 1, 824–827 [DOI] [PubMed] [Google Scholar]
- 44. Zeng L., Miller E. W., Pralle A., Isacoff E. Y., Chang C. J. (2006) A selective turn-on fluorescent sensor for imaging copper in living cells. J. Am. Chem. Soc. 128, 10–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu X. F., Elashvili I., Gralla E. B., Valentine J. S., Lapinskas P., Culotta V. C. (1992) Yeast lacking superoxide dismutase. Isolation of genetic suppressors. J. Biol. Chem. 267, 18298–18302 [PubMed] [Google Scholar]
- 46. Nakase Y., Nakamura T., Hirata A., Routt S. M., Skinner H. B., Bankaitis V. A., Shimoda C. (2001) The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol. Biol. Cell 12, 901–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakamura-Kubo M., Nakamura T., Hirata A., Shimoda C. (2003) The fission yeast spo14+ gene encoding a functional homologue of budding yeast Sec12 is required for the development of forespore membranes. Mol. Biol. Cell 14, 1109–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakamura T., Kashiwazaki J., Shimoda C. (2005) A fission yeast SNAP-25 homologue, SpSec9, is essential for cytokinesis and sporulation. Cell Struct. Funct. 30, 15–24 [DOI] [PubMed] [Google Scholar]
- 49. Takegawa K., Iwaki T., Fujita Y., Morita T., Hosomi A., Tanaka N. (2003) Vesicle-mediated protein transport pathways to the vacuole in Schizosaccharomyces pombe. Cell Struct. Funct. 28, 399–417 [DOI] [PubMed] [Google Scholar]
- 50. Ohtaka A., Okuzaki D., Nojima H. (2008) Mug27 is a meiosis-specific protein kinase that functions in fission yeast meiosis II and sporulation. J. Cell Sci. 121, 1547–1558 [DOI] [PubMed] [Google Scholar]