Background: The control of rapid proliferation of granulosa cells during chicken oocyte growth is unknown.
Results: Reelin is expressed in theca cells and triggers disabled-1 phosphorylation in granulosa cells via ApoER2 and the VLDL receptor.
Conclusion: The Reelin signaling pathway stimulates granulosa cell proliferation during folliculogenesis.
Significance: This work demonstrates a novel function of Reelin.
Keywords: Cell Proliferation, Lipoprotein Receptor, Molecular Cell Biology, Phosphotyrosine Signaling, Signal Transduction
Abstract
Chicken oocytes develop in follicles and reach an enormous size because of a massive uptake of yolk precursors such as very low density lipoprotein and vitellogenin. Oocyte growth is supported by theca cells and granulosa cells, which establish dynamic and highly organized cell layers surrounding the oocyte. The signaling processes orchestrating the development of these layered structures are largely unknown. Here we demonstrate that the Reelin pathway, which determines the development of layered neuronal structures in the brain, is also active in chicken follicles. Reelin, which is expressed in theca cells, triggers a signal in granulosa cells via apolipoprotein E receptor 2 and the very low density lipoprotein receptor, resulting in the phosphorylation of disabled-1 and consecutive activation of the phosphatidylinositol 3-kinase/Akt pathway. This signaling pathway supports the proliferation of differentiated granulosa cells to keep up with the demand of cells to cover the rapidly increasing surface of the giant germ cell.
Introduction
In egg-laying species such as the domesticated chicken (Gallus gallus domesticus), the developing embryo depends on the egg yolk for its supply of all nutrients, vitamins, and other essential components. This physiology requires a specialized metabolism to ensure a massive deposition of yolk into the developing oocyte and special anatomical features of the follicle to support the growth and physical stability of the giant germ cell. The major components of the yolk mass are very low density lipoprotein (VLDL)3 and vitellogenin (VTG), which are synthesized in the liver, transported to the ovary, and taken up by maturing oocytes via receptor-mediated endocytosis (1, 2). The receptor is the chicken homologue of the mammalian VLDL receptor (VLDLR) (3).
The oocytes develop in follicles consisting of the oocyte proper in the center of the follicle surrounded by concentric layers of cells and acellular material (4). These structures are (from the inside to the outside) the perivitelline membrane (the equivalent of the zona pellucida in mammals), granulosa cells, a basement membrane, and the theca interna and theca externa. At later stages of development, granulosa cells differentiate and form a monolayer of epithelial cells facing the perivitelline membrane at their apical side and the basement membrane at their basal side. These cells produce progesterone, which is secreted and metabolized to androgens and estrogens by cells of the theca layers of the follicle (5). The transition of undifferentiated granulosa cells to progesterone-producing cells is supported by bone morphogenic protein 4 (6). Granulosa cells may directly contribute to oocyte growth by secreting HDL-like particles, providing the oocyte with lipids for membrane synthesis (7). The outermost layers of the follicle are the theca interna and theca externa. These cell layers are highly vascularized and contain mesenchymal cells with a fibroblastoid phenotype embedded in patches of extracellular matrix. The vascularization ensures the transport of yolk precursors from the liver to the developing follicle. Cells of the theca interna and theca externa are part of the hormone-producing system of the follicle, and cells of the theca externa, together with collagen fibrils, mechanically support the structure of the follicle (8).
The development of follicles can be divided into three major phases (9). Phase 1 is characterized by a very slow growth of follicles lasting for several months. At the end of this period, numerous follicles have reached a diameter of 2–3 mm. The oocytes within these follicles do not contain significant amounts of bona fide yolk. During phase 2, some of these follicles develop further and reach a diameter of ∼6–8 mm after 60 days. Because of yolk deposition into the oocyte, these follicles acquire a yellow appearance. Finally, single follicles are selected from the pool of small yellow follicles every 25 h (synchronous with the ovulation cycle) and enter the rapid growth phase, which leads to mature follicles containing oocytes with a diameter of ∼35 mm within 7 days. The fully developed oocyte is expelled from the follicle during ovulation and enters the oviduct, where egg formation starts. This developmental scheme establishes a hierarchy of follicles present at any given time in the ovary of a mature hen. Typically, the ovary contains five to eight prominent preovulatory follicles in the rapid 7-day growth phase. These follicles are numbered from F1, the largest one that will ovulate next, to the smallest distinguishable ones, usually F5-F8.
Despite detailed knowledge at the molecular level on yolk transport and follicle growth, still very little is known about the regulatory processes selecting specific follicles to enter the rapid growth phase and leading to mature follicles. Recent attempts to identify genes that regulate the selection and recruitment of follicles to develop into mature oocytes during the bovine estrous cycle by expression profiling led to the identification of ApoER2 as a candidate for such a function (10, 11). In another study, ApoER2 has been shown to be preferentially expressed in bovine dominant follicles when compared with subordinate follicles (12). A splice variant of VLDLR, which is the major player in yolk uptake by the oocyte, is also expressed in chicken granulosa cells (13).
VLDLR and ApoER2 are also expressed in the central nervous system, where these transmembrane proteins function as Reelin receptors and are critical components of the Reelin signaling pathway (14–16). The central axis of this signaling pathway comprises the extracellular matrix protein Reelin, the two receptors ApoER2 and VLDLR, and the intracellular adapter protein disabled-1 (Dab1). Binding of Reelin to the receptors induces Src family kinase-mediated phosphorylation of Dab1, which leads, besides stimulation of other signaling pathways, to the activation of class I PI3K. This complex signaling network of Reelin via two receptors orchestrates the lamination of the cortex during embryonic brain development recently summarized in the “detach and go” (17) and the “polarity” models (18). Disruption of this pathway in mice leads to a reeler-like phenotype, characterized by severe abnormalities in the laminated structures of the brain (19–21).
Interestingly, Reelin is also expressed in the thecae of dominant bovine follicles (10), providing a potential signaling ligand for ApoER2 and VLDLR expressed in granulosa cells. Thus, we assume that a signaling pathway similar to the neuronal Reelin pathway is operating in follicles, where it is involved in the regulation of growth and development of follicular granulosa cells. Here we demonstrate that the Reelin signaling pathway functions in chicken follicles and stimulates granulosa cell proliferation of developing follicles.
EXPERIMENTAL PROCEDURES
Antibodies
The following rabbit polyclonal antisera were used. αOVR (1) and α186 (23) were applied for the detection of chicken VLDLR. chicken ApoER2 was detected with α19 (24) and α187 (23). The following antibodies were purchased from the indicated sources: rabbit polyclonal anti mouse Dab1 (400–555, Rockland; this antibody has been shown to cross-react with chicken Dab1 (25) and has already been used to evaluate Dab1 levels in chicken brain (26)); mouse monoclonal anti-Reelin, clone G10 (Millipore); rabbit polyclonal anti-p-Dab1 (Tyr-220) (Santa Cruz Biotechnology); rabbit polyclonal anti-Akt and rabbit polyclonal anti-p-Akt (Ser-473) (Cell Signaling Technology); mouse monoclonal anti-GFP, clone B-2 (Santa Cruz Biotechnology); mouse monoclonal anti-BrdU, clone 3D4 (BD Biosciences); goat monoclonal anti-mouse and anti-rabbit IgG HRP-conjugated antibodies (Jackson ImmunoResearch Laboratories); and Alexa Fluor 488-coupled goat anti-mouse and anti-rabbit IgG (H+L, Invitrogen). Rabbit polyclonal anti-chicken Dab1-E antibody (25) was a gift from Roseline Godbout (University of Alberta, Edmonton, Canada). A mouse monoclonal anti-chicken Dab1 antibody (clone 6H9-1A4) directed against a bacterially expressed recombinant fusion protein comprising amino acids 1–160 of ggDab1 containing a C-terminal HSV and His tag was produced by the Max F. Perutz Laboratories Monoclonal Antibody Facility.
Animals, BrdU Injection, and Isolation of Granulosa Sheets and Cells for Cell Culture
Sexually mature Derco brown laying hens (TETRA-SL) were purchased from Diglas Co. (Feuersbrunn, Austria) and maintained on open floor space. Animals had free access to water and feed under a daily light period of 14 h. For BrdU incorporation studies, laying hens obtained a single intraperitoneal injection of BrdU (100 mg/kg, dissolved in sterile 0.9% saline) 24 h before killing. To obtain follicles, hens were killed by decapitation. The tissue was removed immediately and kept in cold PBS. Granulosa sheets were isolated as described previously (27), with minor modifications. Briefly, large preovulatory follicles (F1-F4) were punctuated with sterile forceps. The yolk was squeezed out carefully until the granulosa sheet appeared. The isolated sheets were washed in prewarmed Hanks' balanced salt solution (PAA) to remove the adherent yolk, cut into pieces, and used for cultivation of whole sheets (F1) or processed further to obtain single cells for cultivation (F2-F4). For this purpose, granulosa sheets were digested with 1 mg/ml collagenase type IV (Sigma) in Hanks' balanced salt solution at 37 °C while stirring on a magnetic stirrer for 10 min. Then, the cells were washed with Hanks' balanced salt solution and resuspended in M199 medium with Earl's salts and GlutaMax (PAA) supplemented with 5% FCS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin (standard medium). Additionally, 50 ng/ml human pituitary FSH (Calbiochem), 25 ng/ml human recombinant activin A, and 10 ng/ml TGFα (R&D Systems) were added to the standard medium. Cells were seeded onto cell culture dishes coated with 10 μg/ml fibronectin and 15 μg/ml poly-l-ornithine and incubated at 37 °C and 5% CO2 in a humidified incubator.
Cultivation and Transient Transfection of Cell Lines and Preparation of Conditioned Media
NIH 3T3 fibroblasts were cultivated in DMEM high-glucose (PAA) supplemented with 10% FCS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. Transfection of the cells was performed with Lipofectamine 2000 (Invitrogen) in DMEM high-glucose (PAA) with 10% FCS according to the protocol of the manufacturer. NIH 3T3 fibroblasts were grown to 70% confluency and transfected with pEGFP-C1, pEGFP-C1 containing ggDab1-E, and pEGFP-C1 containing ggDab1-L (gifts from Roseline Godbout). After transfection, cells were kept in culture for 48 h before preparing RIPA lysates. 293 HEK cells were maintained in DMEM high-glucose (PAA) supplemented with 10% FCS, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. The medium used for 293 HEK cells stably expressing full-length mouse Reelin (a gift from Tom Curran, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA) additionally contained 0.2 mg/ml G418. Preparation of Reelin- (RCM) and mock-conditioned medium (MCM) was performed as described previously (28). Briefly, 293 HEK cells stably expressing mouse Reelin or untransfected 293 HEK cells were cultivated in OptiMEM (Invitrogen) for 48 h. The supernatant (RCM or MCM) was sterile-filtered to remove cells, aliquoted, and stored at −80 °C. Concentrated Reelin- and mock-conditioned medium was prepared as in Ref. 29. Briefly, RCM and MCM were poured into quick-seal tubes and centrifuged overnight at 40,000 rpm in an ultracentrifuge. The supernatant was removed, except for 1.5 ml, which was used for resuspension of the pellet. Concentrated RCM and MCM was sterile-filtered, aliquoted, and stored at −80 °C.
Isolation of Total RNA and RT-PCR
Isolation of total RNA was performed using TRI reagent from peqLAB. Fresh tissue was cut into small pieces and homogenized using an 18-gauge needle. All further steps were done according to the instructions of the manufacturer. 1 μg of total RNA was used for the synthesis of single-stranded cDNA using Superscript II reverse transcriptase (Invitrogen) and oligo(dT) primers. For the amplification of ggDab1-E, ggDab1-L, ggApoER2, and ggReelin, the following primers were used: ggDab1, 5′-agg cac aaa agg aca agc ag-3′ (forward) and 5′-ctg agt gac tga acc aag att ttc-3′ (reverse); ggDab1-L, 5′-tgt ttc agg ctc acg gat tg-3′ (reverse); ggApoER2 ligand-binding repeat, 5′-acc gga tga att cca gtg c-3′ (forward) and 5′-tag gga ctc ttt cct aca gc-3′ (reverse); and ggReelin, 5′-aag gca cct ctc acg aag aa-3′ (forward) and 5′-tcc agc atc aca gag tgg ag-3′ (reverse). The authenticity of the amplified PCR products was checked by DNA sequencing.
Preparation of Cell Extracts, SDS-PAGE, and Immunoblotting
Freshly isolated follicles of all developmental stages were used for the preparation of whole protein extracts. Stroma and whole follicles (swf, lwf, and syf) as well as separated thecae and granulosa sheets (F5-F1) were cut into small pieces with a scalpel, followed by homogenization with a glass potter or 18-gauge, 20-gauge, and 23-gauge needles in RIPA buffer (Cell Signaling Technology) supplemented with complete protease inhibitor mixture (Roche) and phosphatase inhibitors (2 mm Na3VO4 and 50 mm NaF). Cultivated cell lines, primary granulosa sheets, and cells were washed twice with cold PBS and lysed in RIPA buffer containing the abovementioned supplements. After vortexing for 30 s, the homogenates were incubated on ice for 15 min, followed by pulsed sonication and 15-min incubation on ice. Thereafter, the lysates were centrifuged at 21,000 × g at 4 °C for 15 min. The supernatant was shock-frozen in liquid nitrogen and stored at −80 °C until being used for protein concentration determination with the BCA protein assay kit (Pierce) and Western blot analysis. One-dimensional SDS-PAGE was followed by transfer of the proteins to a nitrocellulose membrane (Hybond C-Extra, Amersham Biosciences). Membranes were blocked with PBS-T (PBS (pH 7.4) with 0.1% Tween 20) containing 5% (w/v) nonfat dry milk (Roth) or BSA fraction V (PAA). The primary antibodies were diluted in blocking solution and incubated with the membranes overnight. The next day, the secondary HRP-conjugated antibodies were added, followed by detection with SuperSignal West Pico chemiluminescence substrate (Thermo Scientific).
Histology, Immunohistochemistry, and Immunofluorescence
Chicken follicles of different developmental stages were fixed with 4% paraformaldehyde overnight immediately after isolation. After dehydration in the Excelsior tissue processor (Thermo Scientific) and embedding in paraffin, serial sections of 5-μm thickness were prepared using a microtome (Leica). The sections were dried at 37 °C overnight, deparaffinized, and rehydrated. Slides were used for H&E staining according to standard protocols, immunohistochemical analysis, or immunofluorescence. For epitope retrieval, slides were boiled for 20 min in 10 mm citric acid (pH 6.0). Then, slides were blocked in 5% BSA fraction V (PAA) and 3% heat-inactivated goat serum (PAA) in PBS (pH 7.4). Primary antibodies were diluted in blocking solution and added to the slides for 16 h at 4 °C. For immunohistochemical analysis, the slides were incubated with a biotin-conjugated secondary antibody (Dako) followed by HRP-conjugated streptavidin (Millipore). The color reaction was performed by incubation with 3-amino-9-ethylcarbazole substrate-chromogen (Dako), and slides were mounted with an aqueous Glycergel mounting medium (Dako). The sections were analyzed with a Zeiss/Axio stereomicroscope. For immunofluorescence, slides were incubated with an anti-mouse or anti-rabbit secondary Alexa Fluor 488-coupled antibody and DAPI for nuclei counterstaining for 1 h at room temperature. After washing with PBS, the slides were mounted with fluorescent mounting medium (Dako) and analyzed with a confocal fluorescence microscope (LSM Meta, Zeiss).
Cloning and Expression of Recombinant Proteins
Preparation of mmApoER21–3,7-MBP/His, mmApoER21–3,7–8-MBP/His, mmVLDLR1–8-MBP/His, and RAP-myc/His was performed as described previously (30). For cloning of ggApoER21–8, ggApoER21–7, and ggVLDLR1–8, cDNA was prepared from total RNA from whole chicken follicles, thecae, or granulosa sheets as described above. The following primer pairs were used for the amplification of the indicated ligand-binding repeats: MBP-VLDLR1–8, 5′-ggt gca aaa gca aaa tgt gag ga-3′ (forward) and 5′-agt taa gct ttt aag gct cat cac tcc agt cct tgc-3′ (reverse); MBP-ApoER21–7, 5′-gaa acg gat ttc gct tgt gac aac gg-3′ (forward) and 5′-agc taa gct ttt aac cac att ctt tca gag gct cgt ca-3′ (reverse); and MBP-ApoER21–8, 5′-gag tgc gat aag gac cag ttc cag tgc-3′ (forward) and 5′-agc taa gct ttt aac cac att ctt tca gag gct cgt ca-3′ (reverse). PCR products were cloned into pMalc2x (New England Biolabs) via XmnI and HindIII, followed by transformation into Escherichia coli Top10 F′ cells. Positive clones were subjected to sequence analysis (LGC Genomics) and used for the expression and purification of the recombinant proteins via amylase-mediated affinity chromatography. Briefly, bacteria were grown until they reached the mid-log phase, and 1 mm isopropyl 1-thio-β-d-galactopyranoside was added to induce expression for 16 h at 30 °C. Cells were harvested, the pellet was resuspended in column buffer (20 mm Tris-HCl, 200 mm NaCl, 1 mm EDTA, 10% glycine) containing complete protease inhibitor mixture (Roche), and cells were lysed by sonication. After centrifugation, the supernatant was loaded onto an equilibrated amylase resin column that was rotated overnight at 4 °C. Elution of the fusion protein was done by adding column buffer containing 10 mm maltose. Positive fractions were identified by protein concentration determination using the method of Bradford, followed by analysis via a Coomassie-stained polyacrylamide gel.
Solid-phase Binding Assay
The solid-phase binding assay was performed as described previously (30). Briefly, 100 μl of TBS-C (2 mm CaCl2) containing 10 μg/ml fusion protein were incubated on a 96-well plate overnight at 4 °C. All further steps were carried out at room temperature for 1 h each, and ligands and antibodies were diluted in blocking solution (TBS-C containing 2% BSA and 0.5% Tween 20). After blocking and binding of mouse Reelin, anti-Reelin antibody (clone G10) and anti-mouse HRP-conjugated secondary antibody were used for the detection of bound Reelin. The color reaction was performed by addition of 0.1 mg/ml 3,3′,5,5′-tetramethylbenzidine in 0.1 m sodium acetate (pH 6.0) containing 10 mm H2O2. After 2 min of incubation, the color reaction was stopped by adding 0.3 m H2SO4, and the absorbance of the reaction was measured by a plate reader (Wallac Victor2 1420 multilabel counter, PerkinElmer Life Sciences) at 450 nm.
Dab1 and Akt Phosphorylation Assay
Isolated F1 granulosa sheets were cut into two pieces and cultivated overnight in standard medium supplemented with activin A and FSH. The next day, the tissue was washed once with PBS and starved in OptiMEM (Invitrogen, Life Technologies) for 4 h. The tissue was washed again with PBS before stimulation with Reelin- or mock-conditioned medium for 30 min. RIPA lysates were prepared, and phosphorylation of chicken Dab1-L and Akt was analyzed by SDS-PAGE, followed by immunoblotting. Phosphorylated Dab1 was detected with an anti p-Dab1 (Tyr-220) antibody. Total Dab1 levels were detected with an anti-chicken Dab1 antibody (6H9-1A4). An anti p-Akt (Ser-473) antibody was used to detect phosphorylated Akt. Total Akt levels were detected with an anti-Akt antibody.
EdU Incorporation Assay
Granulosa sheets (F1) and granulosa cells (F2-F4) were isolated as described above. Sheets and cells were cultivated in microslide 8-well chambers (Ibidi). For the analysis of cell proliferation, sheets and cells were incubated with 100 μm EdU for 20 h. All further steps were carried out according to the instructions of the Click-iT EdU imaging kit protocol (Invitrogen). Briefly, sheets and cells were washed and fixed for 15 min at room temperature. Then, the specimens were permeabilized, and the Click-iT reaction mixture was added. Finally, the sheets and cells were washed again and embedded in fluorescence mounting medium (Ibidi). Sheets and cells were analyzed with a confocal fluorescence microscope (LSM Meta 510, Zeiss).
Cell Proliferation Assay
Granulosa cells were seeded at a density of 5000 cells/well into a 96-well plate. Cells were kept in standard medium containing activin A and TGFα that was optionally supplemented with OptiMEM, concentrated Reelin- or mock-conditioned medium, and Reelin- or mock-conditioned medium in the presence of RAP-myc/His. Cell proliferation was determined after 1 and 4 days in culture using cell proliferation reagent WST-1 (Roche). During this incubation period, concentrated Reelin- and mock-conditioned media as well as OptiMEM were added to the cells every day to ensure continuous stimulation of the cells. RAP-myc/His was added to the cells 15 min prior to the addition of Reelin- or mock-conditioned medium. After 2 h of incubation with the reagent, the absorbance was measured with a Wallac Victor2 1420 multilabel counter (PerkinElmer Life Sciences) according to the instructions of the manufacturer.
Statistical Analysis
All values are displayed as means ± S.D. Analysis of the binding studies and statistical analysis of the proliferation assay were performed using GraphPad Prism 6. One-way analysis of variance using Tukey's multiple comparisons test or Student's t test was adopted to determine the statistical significance of the data. The significance level was set at p < 0.05.
RESULTS
Development of Chicken Follicles
To test whether the Reelin-like signaling pathway is active in chicken follicles, we dissected the ovary and tested for the spatiotemporal expression of the critical components of this pathway during development of the oocytes. Ovaries of laying hens were prepared, and follicles were selected according to specific growth phases (Fig. 1). Small white previtellogenic follicles (swf) have a diameter of about 2–4 mm and, because of the lack of vitellogenic yolk, they have a white appearance (Fig. 1A). Selected follicles from this pool enter phase 2, start to grow (large white follicles, lwf), and initiate deposition of yolk precursors (VLDL and VTG) produced in the liver (Fig. 1B). These follicles acquire a yellow appearance (small yellow follicles, syf) and reach a diameter of ∼6–8 mm. This transition period is demonstrated in Fig. 1D and ends with the beginning of the rapid growth phase, during which the follicle size increases rapidly and reaches a diameter of 35 mm prior to ovulation. In this phase, follicles are numbered individually from F6 to F1 (Fig. 1, C and D), the latter being the biggest in this hierarchy. To evaluate the presence of VLDLR, ApoER2, Dab1, and Reelin, selected follicles up to F6 were homogenized as a whole. Follicles from F5 to F1 were dissected further, and the granulosa cell layer and the theca cell layer were prepared. Cell extracts of whole follicles and separated cell layers were analyzed by Western blotting for ApoER2, VLDLR, and Dab1 and by RT-PCR for the expression of Reelin.
FIGURE 1.

Stages of chicken follicle development. A, the follicles of the first phase of development are 2–4 mm in diameter, lack bona fide yolk and the typical yellow color, and are thus named small white follicles. B, during the second developmental phase, follicles reach a diameter of 6–8 mm. The uptake of yolk precursors is initiated, and follicles start to become yellow. Follicles in this phase are called large white follicles (empty arrowheads) and small yellow follicles (black arrowheads). C, the third phase of follicle development is characterized by the uptake of large amounts of yolk precursors, accompanied by an increase in diameter from 8 to 35 mm. The biggest follicle that is the next to the ovulate is named F1. D, follicles in the transition from phase 2 to phase 3 represent the pool of follicles that will enter the final rapid growth phase and then ovulate within the next 7 days. Follicles are arranged according to size, from the smallest large white follicle (VI) to the biggest small yellow follicle (I). F6 represents the smallest follicle of the final rapid growth phase.
Expression of ApoER2 and VLDLR
As demonstrated in Fig. 2A, VLDLR is expressed throughout all stages of follicle development. As described in numerous publications, VLDLR is massively expressed by growing oocytes and mediates the transport of VLDL, VTG, and other minor yolk components from the circulation into the growing germ cell (31). Earlier studies have demonstrated that granulosa cells express a splice variant of VLDLR that differs from the variant expressed in the oocyte by the presence of the O-linked sugar domain of the receptor (VLDLR+) (13). Here (Fig. 2A), these two variants are not resolved on the gel, and the band seen in extracts derived from swf to F6 follicles represent a mixture of both forms. In granulosa cells manually separated from large follicles (F5-F1), VLDLR+ is expressed in all follicles, showing a peak between F4 and F3. Cells present in the theca layers do not express VLDLR. ApoER2, which is mainly expressed in the central nervous systems in mammals, shows strong expression throughout all stages of chicken follicle development (Fig. 2A). The expression is restricted to granulosa cells (see also Fig. 3), and the double band is produced by the presence of two different splice variants of ApoER2 containing either seven or eight ligand-binding repeats. This was evaluated by PCR analysis and sequencing of the products using mRNA derived from small yellow follicles and pooled granulosa cells from F3 and F4 follicles (Fig. 2B).
FIGURE 2.
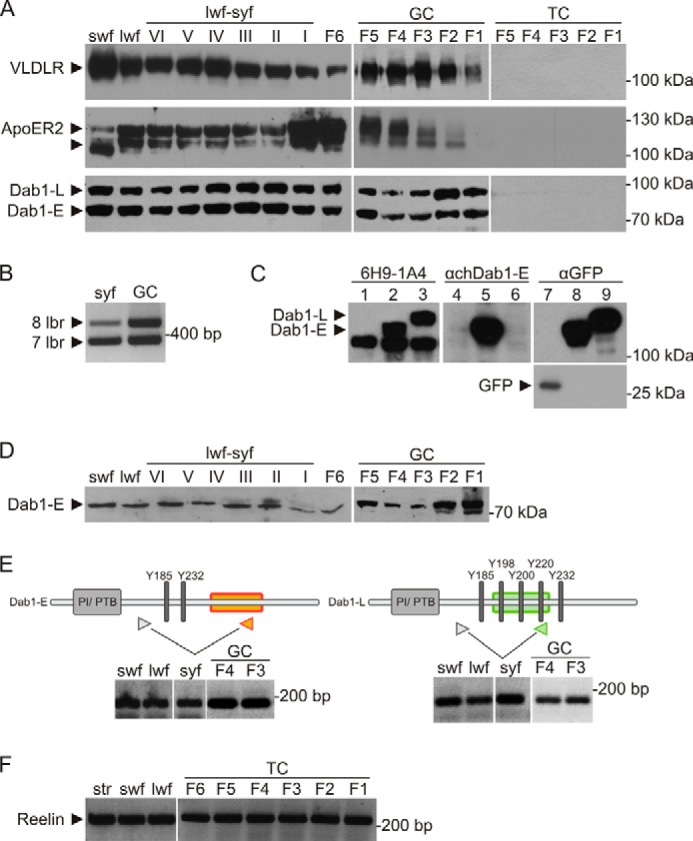
Expression of components of the Reelin signaling pathway in chicken follicles. A, RIPA lysates of whole follicles (swf, lwf, syf, and F6) and separated granulosa (GC) and theca (TC) sheets (F5-F1) were analyzed by Western blotting. A rabbit polyclonal anti-chicken VLDLR antibody (αOVR) was used to detect VLDLR (top row). A rabbit polyclonal anti-mouse ApoER2 antibody (α19) cross-reacting with chicken ApoER2 was used to detect ApoER2 (center row). A mouse monoclonal anti-chicken Dab1 antibody (6H9-1A4) was used for the detection of ggDab1-E and ggDab1-L (bottom row). B, RT-PCR with a primer pair spanning the seventh and eighth ligand-binding repeats of chicken ApoER2 was performed using cDNA from syf and granulosa cells of F3 and F4. Phase 2 and phase 3 follicles express ApoER2 transcripts coding for seven (366 bp) or eight (489 bp) ligand-binding repeats (lbr). C, specificity of a newly developed mouse monoclonal anti-chicken Dab1 antibody (6H9-1A4) was tested using RIPA lysates of NIH 3T3 fibroblasts transiently transfected with pEGFP-C1 (lanes 1, 4, and 7), pEGFP-C1 containing ggDab1-E cDNA (lanes 2, 5, and 8), or pEGFP-C1 containing ggDab1-L cDNA (lanes 3, 6, and 9). GFP-tagged ggDab1-E and ggDab1-L were detected with 6H9-1A4, with an anti-chicken Dab1-E antibody (25), and with an anti-GFP antibody (clone B-2). GFP was detected with an anti-GFP antibody (clone B-2). D, RIPA lysates of whole follicles (swf, lwf, syf, and F6) and granulosa sheets from F5 to F1 were used to detect ggDab1-E using a rabbit polyclonal anti-chicken Dab1-E antibody. E, expression of both isoforms, ggDab1-E and ggDab1-L, in the developing follicle was verified by RT-PCR using isoform-specific primer pairs, giving rise to a 158-bp PCR product (ggDab1-E) and a 128 bp PCR product (ggDab1-L). F, expression of Reelin was analyzed by RT-PCR with chicken Reelin-specific primers using cDNA of stroma (str), swf, lwf, and theca sheets (F6-F1), resulting in the amplification of a 232-bp fragment. PI/PTB, protein interaction/phosphotyrosine binding.
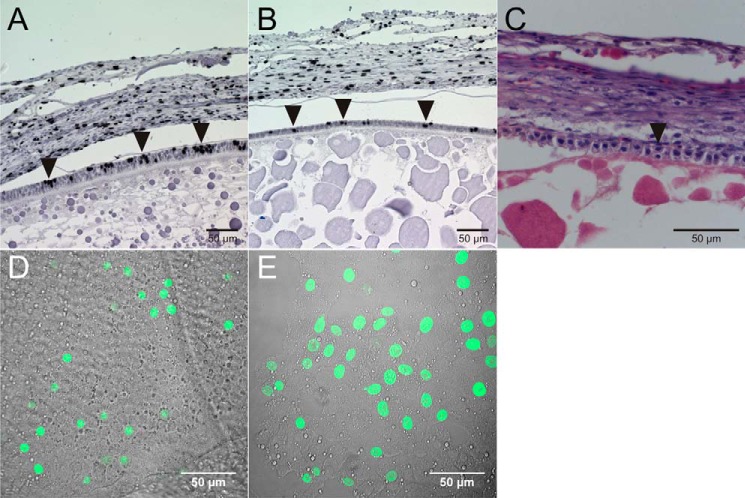
FIGURE 3.
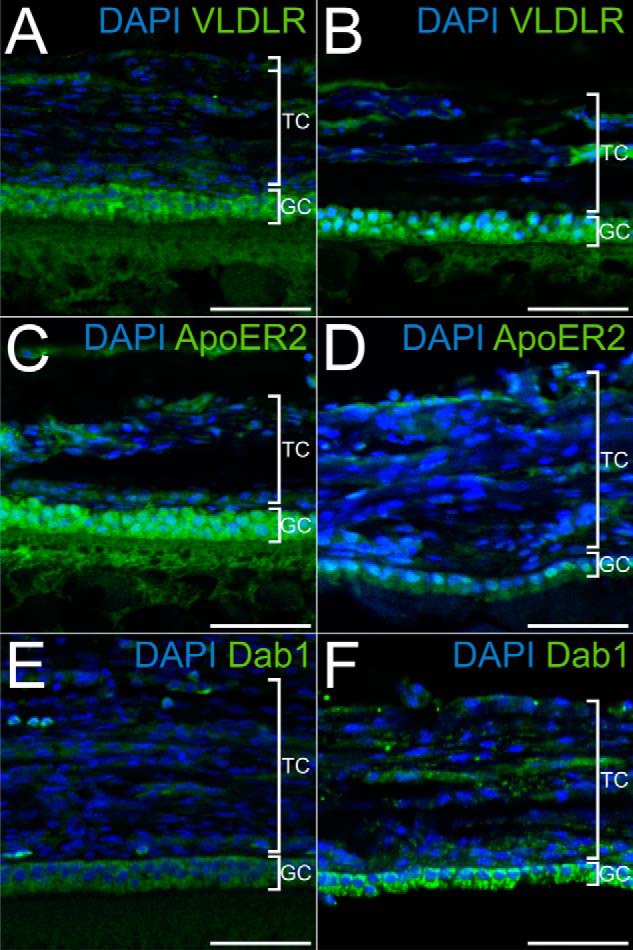
Localization of the components of the Reelin signaling pathway in the chicken follicle. A, C, and E, microtome sections of 5-μm thickness from paraffin-embedded lwf were used to localize the expression of VLDLR (A) with a polyclonal rabbit anti-mouse VLDLR antibody (α187), of ApoER2 (C) with a polyclonal rabbit anti-mouse ApoER2 antibody (α186), and of Dab1 (E) with a rabbit polyclonal anti-mouse Dab1 (D4) antibody. B, D, and F, microtome sections of 5-μm thickness from F6 follicles were used to detect VLDLR, ApoER2, and Dab1 with the same antibodies as in A, C, and E, respectively. In follicles of both developmental stages, VLDLR, ApoER2, and Dab1 are expressed in granulosa cells. TC, theca cells; GC, granulosa cells. Scale bars = 50 μm.
Expression of Dab1
To evaluate the expression of Dab1, we developed a chicken-specific monoclonal antibody against this protein because all available antibodies against mammalian Dab1 did not cross-react with the chicken homologue in Western blot analyses. This antibody (6H9-1A4) was derived from an immunization protocol using a recombinant protein containing the first 160 amino acids of chicken Dab1 (see “Experimental Procedures”). As demonstrated in Fig. 2A, this antibody recognizes two distinct bands reminiscent of the expression pattern of Dab1 in the chicken retina (32). There, two distinct isoforms of Dab1 are expressed, one termed Dab1-L that corresponds to the mammalian Dab1 responsible for transmitting the Reelin signal (19) and a shorter form termed Dab1-E that lacks critical tyrosine residues phosphorylated by activation of the Reelin pathway and contains an inclusion of 19 amino acids not present in Dab1-L (for a review, see Ref. 33). To evaluate whether the two proteins expressed in chicken granulosa cells and reacting with the new antibody indeed correspond to Dab1-E and Dab1-L, we first tested whether the antibody reacts with both isoforms. For this, we expressed both chicken variants carrying a GFP tag in 3T3 fibroblasts and performed Western blot analyses with different antibodies. As demonstrated in Fig. 2C, the monoclonal antibody (6H9-1A4) reacts with both splice variants equally well (lanes 2 and 3). The lowest band present in Dab1-E- and Dab1-L- expressing cells as well as in control cells (these cells were transfected with the empty GFP plasmid (Fig. 2C, lane 1)) most likely results from a protein expressed by 3T3 fibroblasts incidentally cross-reacting with this antibody because earlier experiments using 3T3 cells clearly showed that Dab1 is not expressed by these cells (34). To verify this result, we performed the same Western blot analysis using an antibody specific for chicken Dab1-E (33), and, as demonstrated in Fig. 2C, lanes 4-6, this antibody only reacts with the Dab1-E-fusion protein. As a control, we used an anti-GFP antibody that detects Dab1-E-GFP and Dab1-l-GFP equally well and GFP alone expressed by control cells. To further validate the presence of Dab1-E in chicken granulosa cells, we directly used the anti-chicken Dab1-E antibody on Western blot analyses evaluating protein expression of various chicken follicles and granulosa cells derived from F5-F1 follicles and verified the presence of this variant in all stages of development (Fig. 2D). Finally, we performed PCR experiments with primer pairs specific for both variants (Fig. 2E). For both pairs, the upstream primer is located in exon 6, which is present in the two alternatively spliced transcripts. The downstream primer specific for the E form is located in exon 9a, which is only present in the transcript for the E variant. The downstream primer specific for the L form is located in exon 8, which is absent from the E variant (33). Both primer pairs produced the specific product corresponding to transcripts for Dab1-E and Dab1-L (verified by sequencing) with mRNA from follicles at different stages of development and granulosa cells. Taken together, chicken granulosa cells express Dab1-E and Dab1-L throughout all stages of follicle development.
To confirm the results obtained by Western blotting we performed immunofluorescence microscopy on large white follicles (Fig. 3, A, C, and E) and large preovulatory follicles of the final growth phase (Fig. 3, B, D, and F). Expression of VLDLR (Fig. 3, A and B), ApoER2 (Fig. 3, C and D), and Dab1 (Fig. 3, E and F) is restricted to granulosa cells in both types of follicles.
Expression of Reelin
Finally, we evaluated whether Reelin is expressed in chicken follicles. To this end, we performed RT-PCR using mRNA prepared from chicken ovarian stroma and small and large white follicles and manually dissected theca cell layers from F6 to F1 follicles. Using a primer pair specific for chicken Reelin resulted in the amplification of a 232-bp fragment from mRNA that was present in all of these tissues of the chicken ovary (Fig. 2F). Sequencing of these products proved that this fragment is identical to chicken Reelin, demonstrating that Reelin is expressed in the chicken ovary, in particular in the thecae of large preovulatory follicles.
Chicken ApoER2 and VLDLR Bind Reelin
Next, we tested whether the chicken homologues of VLDLR and ApoER2 are also able to bind Reelin with high affinity like their mammalian counterparts. We produced recombinant fusion proteins made up of MBP and the respective ligand binding domains of chicken VLDLR containing eight ligand-binding repeats (ggVLDLR1–8) and of both splice variants of chicken ApoER2 expressed in follicles (see Fig. 2B) containing seven and eight ligand-binding repeats (ggApoER21–7 and ggApoER21–8) and performed ligand-binding ELISAs using mouse Reelin produced by stably transfected 293HEK cells (30). As a control, we used MBP only. As demonstrated in Fig. 4A, the binding affinities for Reelin to both variants of ggApoER2 are slightly lower (Kd values are slightly higher) than to the mouse variants (2.2 and 3.8 nm versus 0.5 and 0.8 nm) but well within the same range. For VLDLR, the affinity for Reelin is virtually the same for the receptors in both species (Fig. 4B).
FIGURE 4.
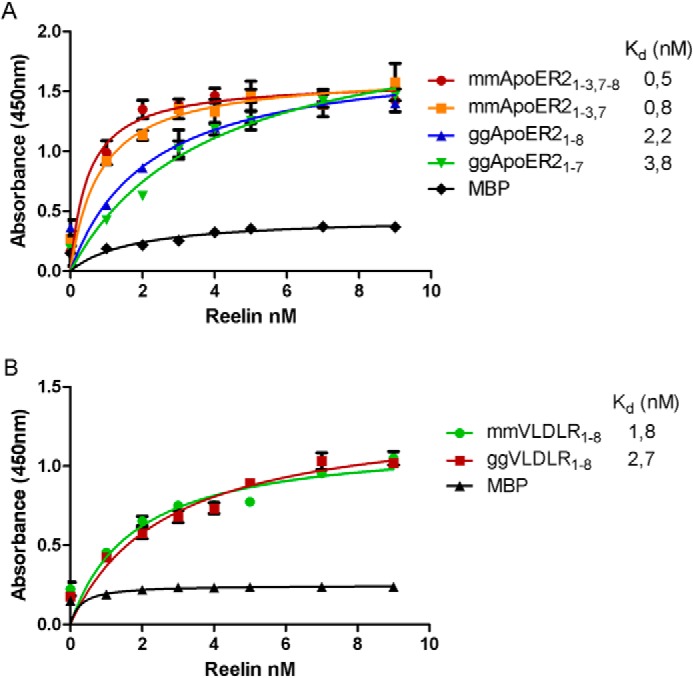
Binding of Reelin to the chicken homologues of ApoER2 and VLDLR. A, 96-well plates were coated with MBP-coupled fusion proteins containing variants of mouse and chicken ApoER2 ligand-binding domains or MBP and incubated with the indicated amounts of recombinant mouse Reelin expressed and secreted by 293 HEK cells. Bound Reelin was detected with a mouse monoclonal anti-Reelin antibody (clone G10) and the appropriate HRP-conjugated secondary antibody. The absorbance at 450 nm was measured. B, the same experiment was performed using MBP-coupled fusion proteins containing variants of mouse and chicken VLDLR or MBP. Error bars represent mean ± S.D. derived from triplicate determinations.
The Reelin Signaling Pathway Is Functional in Chicken Follicles
To evaluate whether a functional Reelin pathway exists in granulosa cells, we isolated granulosa sheets from F1 follicles and cultivated them in the presence of activin A and FSH (27). In between the basement membrane and the perivitelline membrane, the granulosa cells maintain their epitheloid characteristic in vitro. Addition of mouse Reelin to the culture medium robustly increased Dab1 phosphorylation in the cells (Fig. 5A). In the mammalian brain, phosphorylated Dab1 acts as a signaling platform, binding a variety of proteins involved in further down-stream signaling events (33). A central knot in this Reelin signaling network is the activation of PI3K (35), which leads to the phosphorylation of protein kinase B/Akt (36). As demonstrated in Fig. 5B, Reelin also activates this axis in chicken granulosa sheets, resulting in the activation of protein kinase B/Akt.
FIGURE 5.

Dab1 and Akt phosphorylation in cultivated chicken granulosa sheets. A, granulosa sheets from F1 follicles were stimulated with MCM or RCM (recombinant mouse Reelin expressed and secreted by 293 HEK cells) for 30 min, and RIPA lysates were analyzed by Western blotting. Phosphorylated Dab1 was detected with a rabbit polyclonal anti-p-Dab1 (Tyr-220) antibody, and detection of total Dab1 levels was carried out with a mouse monoclonal anti-chicken Dab1 antibody (6H9-1A4). B, the same lysates were used for the determination of Akt phosphorylation. A rabbit polyclonal anti-p-Akt (Ser-473) antibody was used to detect phosphorylated Akt. Total Akt levels were detected with a rabbit polyclonal anti-Akt antibody.
During the last growth phases (phases 2 and 3), the diameter of chicken oocytes rapidly increases from 3 to 35 mm (Fig. 1). During phase 2, where the granulosa cells still form a multicellular layer, these cells proliferate rapidly, as demonstrated in Fig. 6A. For this experiment, BrdU was injected into a laying hen. 24 h later, the ovary was removed, and follicles were examined by immunohistochemical analysis using an anti-BrdU antibody. At the beginning of phase 3, where the granulosa cell layer has transformed into a single cell layer, cell proliferation still persists, as demonstrated by BrdU incorporation (Fig. 6B) and direct visualization of dividing cells by H&E staining of the sections (Fig. 6C, arrow). To test whether Reelin signaling is stimulating granulosa cell proliferation, we cultivated granulosa sheets from F1 follicles and dispersed granulosa cells from F2 to F4 follicles (27). To prove that these cells do not lose their proliferative capacity, granulosa sheets and dispersed cells were cultivated for 24 h with a 20-h EdU pulse and analyzed by confocal microscopy. Many EdU-positive cells are present in the sheet (Fig. 6D) as well as in dispersed cells (Fig. 6E). Having demonstrated that granulosa cells still proliferate in vitro under the culture conditions used, primary dispersed granulosa cells from F2 to F4 follicles were cultivated for 4 days in the absence or presence of mouse Reelin. After 4 days, cell proliferation was increased by 36% in the presence of Reelin (Fig. 7A). The critical components of the pathway (VLDLR, ApoER2, and Dab1) were still expressed in these cells over the whole period of the experiment (4 days) (Fig. 7B). To assess that the observed effect is indeed dependent on the presence of the receptors, the experiment was repeated in the presence of increasing amounts of RAP-myc/His, a specific inhibitor of ligand recognition of members of the LDL receptor family (37) that blocks Reelin-induced Dab1 phosphorylation in primary mouse neurons (23). To prove that RAP also binds to chicken ApoER2 and VLDLR, we performed ELISA-based solid-phase binding assays as described above using recombinant RAP-myc/His as ligand. As expected, both receptors bound RAP-myc/His with high affinity (15 nm for ggApoER21–7 and ggApoER21–8 and 6 nm for ggVLDLR1–8). As demonstrated in Fig. 7C, addition of 100 μg/ml RAP-myc/His significantly reduces the proliferative effect of Reelin, whereas proliferation of granulosa cells stimulated with MCM and 100 μg/ml RAP-myc/His was not altered (Fig. 7D).
FIGURE 6.
Chicken granulosa cell proliferation in vivo and in vitro. A and B, microtome sections of small yellow follicles (A) and large preovulatory follicles (B) from laying hens injected with BrdU were used for immunohistochemical analysis with a mouse monoclonal anti-BrdU antibody and detection with the biotin/streptavidin system. Arrowheads indicate BrdU-positive and, thus, proliferating granulosa cells. C, H&E staining of a large preovulatory follicle was performed. Arrowheads indicate dividing granulosa cells. D and E, granulosa sheets from F1 follicles (D) and granulosa cells from F2 to F4 follicles (B) were cultivated in the presence of 100 μm EdU for 20 h. The Click-iT EdU imaging protocol from Invitrogen was used to visualize proliferating cells.
FIGURE 7.
Effect of Reelin on proliferation of chicken granulosa cells. A, granulosa cells from F2 to F4 follicles were cultivated under standard conditions or in the presence of concentrated MCM or RCM (recombinant mouse Reelin expressed and secreted by 293 HEK cells and concentrated by ultracentrifugation) for 4 days. Proliferation of cells was determined by application of the cell proliferation reagent WST-1 from Roche and measurement of the absorbance at 450 and 600 nm. Values represent means ± S.D. n.s., not significant; ***, p = 0.0002; ****, p < 0.0001; one-way analysis of variance, Tukey's multiple comparisons test, GraphPad Prism). B, granulosa cells from F2 to F4 follicles were cultivated under standard conditions for 4 days (d) and tested for the expression of VLDLR (top panel), ApoER2 (center panel), and Dab1 (bottom panel) by Western blot analysis using the same antibodies as described in Fig. 2A. C, granulosa cells from F2 to F4 follicles were cultivated in the presence of RCM (recombinant mouse Reelin expressed and secreted by 293 HEK cells and concentrated by ultracentrifugation) and different concentrations of RAP-myc/His for 4 days. Proliferation of cells was determined as described above. Values represent means ± S.D. **, p = 0.0016; ***, p = 0.0001; ****, p < 0.0001; one-way analysis of variance, Tukey's multiple comparisons test). D, granulosa cells from F2 to F4 follicles were cultivated in the presence of MCM and RAP-myc/His for 4 days. Proliferation of the cells was determined as described above. Values represent means ± S.D. (Student's t test, GraphPad Prism).
DISCUSSION
The Reelin signaling pathway is known to operate in only a few cell types present in the nervous system. During embryonic brain development, Reelin orchestrates neuronal migration to form laminated structures of the cortex, hippocampus, cerebellum, and olfactory bulb. In addition, the Reelin pathway also seems to be involved in the development of the mammalian retina (38). Disruption of the pathway leads to a reduction in the number of rod bipolar cells. Interestingly, in the chicken retina, a splice variant of Dab1 is also expressed that functions independently of the Reelin signaling pathway (25).
Here we report, for the first time, that a functional Reelin pathway is operating in another organ, i.e. the chicken ovary. Oocytes grow within follicles composed of the oocyte proper (the egg yolk), surrounded by layers of specialized cells and acellular matrices. Close to the oocyte, separated by the zona pellucida, reside the granulosa cells, which are separated from the theca cells by an unusual basement membrane (39). Granulosa cells express the Reelin receptors ApoER2 and a splice variant of VLDLR. In contrast to the oocyte, which expresses massive amounts of VLDLR-, the granulosa cell-specific form contains an O-linked sugar domain (VLDLR+) that is absent from the oocyte-specific variant. This is an interesting situation because two adjacent cell systems express two different variants from the same gene that have completely different functions. VLDLR- transports yolk precursors (VLDL and VTG) via receptor-mediated endocytosis. VLDLR+ binds Reelin and transmits a signal. The fact that VLDL and VTG have to bypass the granulosa cells that express VLDLR+ without being taken up by these cells is surprising and awaits further evaluation. In addition to the Reelin receptors, granulosa cells also express Dab1, a prerequisite to be responsive to Reelin (40). Reelin, which is also present in the follicle, is expressed in the theca layer, setting the stage for a functional Reelin signaling axis between theca and granulosa cells. As demonstrated here, this pathway is indeed operating in this organ, triggering phosphorylation of Dab1 and subsequent activation of PI3K/Akt in granulosa cells, stimulating proliferation of these cells. This proproliferative signal might be especially important during the last growth phase of the follicle. Before the transition from phase 2 to 3, granulosa cells grow within a multicellular layer and remain in an undifferentiated state until selected follicles enter the last growth phase, establishing the preovulatory hierarchy. At the transition to the last growth phase, granulosa cells differentiate and express high levels of follicle-stimulating hormone receptor (6). In addition, the multicellular layer becomes a monolayer with epithelial-like properties (22). During this phase, the follicle diameter increases from 8 to 35 mm, and the surface area of the oocyte that has to be covered by granulosa cells expands by a factor of 20. Thus, despite their differentiation, granulosa cells have to keep their proliferative capacity because there is no pool of proliferating precursor cells available from which granulosa cells can be recruited. As demonstrated in Fig. 6, B and C, granulosa cells divide within the monolayer. In such a setting, Reelin is produced in the theca cell layer in direct vicinity of the granulosa cells, expressing all components required for transmitting the Reelin signal and stimulating cell proliferation to ensure tight coverage and support of the rapidly growing oocyte.
Acknowledgments
We thank Harald Rumpler and Philipp Tondl for technical assistance.
This work was supported by Austrian Science Fund (FWF) Grant P 24767-B21 (to J. N.).
- VLDL
- very low density lipoprotein
- VTG
- vitellogenin
- VLDLR
- very low density lipoprotein receptor
- RIPA
- radioimmune precipitation assay
- MBP
- maltose binding protein
- RCM
- Reelin-conditioned medium
- MCM
- mock-conditioned medium
- gg
- Gallus gallus
- swf
- small white follicle
- lwf
- large white follicle
- syf
- small yellow follicle
- EdU
- 5-ethynyl-2′-deoxyuridine
- RAP
- receptor-associated protein.
REFERENCES
- 1. Bujo H., Hermann M., Kaderli M. O., Jacobsen L., Sugawara S., Nimpf J., Yamamoto T., Schneider W. J. (1994) Chicken oocyte growth is mediated by an eight ligand binding repeat member of the LDL receptor family. EMBO J. 13, 5165–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider W. J., Nimpf J. (2003) LDL receptor relatives at the crossroad of endocytosis and signaling. Cell. Mol. Life Sci. 60, 892–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takahashi S., Kawarabayasi Y., Nakai T., Sakai J., Yamamoto T. (1992) Rabbit very low density lipoprotein receptor. A low density lipoprotein receptor-like protein with distinct ligand specificity. Proc. Natl. Acad. Sci. U.S.A. 89, 9252–9256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perry M. M., Gilbert A. B., Evans A. J. (1978) Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J. Anat. 125, 481–497 [PMC free article] [PubMed] [Google Scholar]
- 5. Huang E. S., Kao K. J., Nalbandov A. V. (1979) Synthesis of sex steroids by cellular components of chicken follicles. Biol. Reprod. 20, 454–461 [DOI] [PubMed] [Google Scholar]
- 6. Kim D., Ocón-Grove O., Johnson A. L. (2013) Bone morphogenetic protein 4 supports the initial differentiation of hen (Gallus gallus) granulosa cells. Biol. Reprod. 88, 161. [DOI] [PubMed] [Google Scholar]
- 7. Hermann M., Lindstedt K. A., Foisner R., Mörwald S., Mahon M. G., Wandl R., Schneider W. J., Nimpf J. (1998) Apolipoprotein A-I production by chicken granulosa cells. FASEB J. 12, 897–903 [DOI] [PubMed] [Google Scholar]
- 8. Recheis B., Osanger A., Haubenwallner S., Schneider W. J., Nimpf J. (2000) Chicken coagulation factor XIIIA is produced by the theca externa and stabilizes the ovarian follicular wall. J. Biol. Chem. 275, 35320–35327 [DOI] [PubMed] [Google Scholar]
- 9. Johnson A. L. (1990) Steroidogenesis and action of steroids in the hen ovary. Critical Reviews in Poultry Biology 2, 319–346 [Google Scholar]
- 10. Fayad T., Lefebvre R., Nimpf J., Silversides D. W., Lussier J. G. (2007) Low-density lipoprotein receptor-related protein 8 (LRP8) is upregulated in granulosa cells of bovine dominant follicle. Molecular characterization and spatio-temporal expression studies. Biol. Reprod. 76, 466–475 [DOI] [PubMed] [Google Scholar]
- 11. Fayad T., Lévesque V., Sirois J., Silversides D. W., Lussier J. G. (2004) Gene expression profiling of differentially expressed genes in granulosa cells of bovine dominant follicles using suppression subtractive hybridization. Biol. Reprod. 70, 523–533 [DOI] [PubMed] [Google Scholar]
- 12. Sisco B., Hagemann L. J., Shelling A. N., Pfeffer P. L. (2003) Isolation of genes differentially expressed in dominant and subordinate bovine follicles. Endocrinology 144, 3904–3913 [DOI] [PubMed] [Google Scholar]
- 13. Bujo H., Lindstedt K. A., Hermann M., Dalmau L. M., Nimpf J., Schneider W. J. (1995) Chicken oocytes and somatic cells express different splice variants of a multifunctional receptor. J. Biol. Chem. 270, 23546–23551 [DOI] [PubMed] [Google Scholar]
- 14. Frotscher M. (2010) Role for Reelin in stabilizing cortical architecture. Trends Neurosci. 33, 407–414 [DOI] [PubMed] [Google Scholar]
- 15. Rice D. S., Curran T. (2001) Role of the reelin signaling pathway in central nervous development. Annu. Rev. Neurosci. 24, 1005–1039 [DOI] [PubMed] [Google Scholar]
- 16. Tissir F., Goffinet A. M. (2003) Reelin and brain development. Nat. Rev. Neurosci. 4, 496–505 [DOI] [PubMed] [Google Scholar]
- 17. Cooper J. A. (2008) A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 31, 113–119 [DOI] [PubMed] [Google Scholar]
- 18. Jossin Y. (2011) Polarization of migrating cortical neurons by Rap1 and N-cadherin. Revisiting the model for the Reelin signaling pathway. Small GTPases 2, 322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Howell B. W., Gertler F. B., Cooper J. A. (1997) Mouse disabled (mDab1). A Src binding protein implicated in neuronal development. EMBO J. 16, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sheldon M., Rice D. S., D'Arcangelo G., Yoneshima H., Nakajima K., Mikoshiba K., Howell B. W., Cooper J. A., Goldowitz D., Curran T. (1997) Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice (see comments). Nature 389, 730–733 [DOI] [PubMed] [Google Scholar]
- 21. Trommsdorff M., Gotthardt M., Hiesberger T., Shelton J., Stockinger W., Nimpf J., Hammer R. E., Richardson J. A., Herz J. (1999) Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97, 689–701 [DOI] [PubMed] [Google Scholar]
- 22. Schuster M. K., Schmierer B., Shkumatava A., Kuchler K. (2004) Activin A and follicle-stimulating hormone control tight junctions in avian granulosa cells by regulating occludin expression. Biol. Reprod. 70, 1493–1499 [DOI] [PubMed] [Google Scholar]
- 23. Strasser V., Fasching D., Hauser C., Mayer H., Bock H. H., Hiesberger T., Herz J., Weeber E. J., Sweatt J. D., Pramatarova A., Howell B., Schneider W. J., Nimpf J. (2004) Receptor clustering is involved in Reelin signaling. Mol. Cell. Biol. 24, 1378–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Novak S., Hiesberger T., Schneider W. J., Nimpf J. (1996) A new low density lipoprotein receptor homologue with 8 ligand binding repeats in brain of chicken and mouse. J. Biol. Chem. 271, 11732–11736 [DOI] [PubMed] [Google Scholar]
- 25. Gao Z., Monckton E. A., Glubrecht D. D., Logan C., Godbout R. (2010) The early isoform of disabled-1 functions independently of Reelin-mediated tyrosine phosphorylation in chick retina. Mol. Cell. Biol. 30, 4339–4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kisliouk T., Meiri N. (2013) MiR-138 promotes the migration of cultured chicken embryonic hypothalamic cells by targeting reelin. Neuroscience 238, 114–124 [DOI] [PubMed] [Google Scholar]
- 27. Schmierer B., Schuster M. K., Shkumatava A., Kuchler K. (2003) Activin and follicle-stimulating hormone signaling are required for long-term culture of functionally differentiated primary granulosa cells from the chicken ovary. Biol. Reprod. 68, 620–627 [DOI] [PubMed] [Google Scholar]
- 28. Brandes C., Kahr L., Stockinger W., Hiesberger T., Schneider W. J., Nimpf J. (2001) Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not α 2-macroglobulin. J. Biol. Chem. 276, 22160–22169 [DOI] [PubMed] [Google Scholar]
- 29. Blake S. M., Strasser V., Andrade N., Duit S., Hofbauer R., Schneider W. J., Nimpf J. (2008) Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 27, 3069–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koch S., Strasser V., Hauser C., Fasching D., Brandes C., Bajari T. M., Schneider W. J., Nimpf J. (2002) A secreted soluble form of ApoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling. EMBO J. 21, 5996–6004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nimpf J., Schneider W. J. (1998) The VLDL receptor. An LDL receptor relative with eight ligand binding repeats, LR8. Atherosclerosis 141, 191–202 [DOI] [PubMed] [Google Scholar]
- 32. Katyal S., Godbout R. (2004) Alternative splicing modulates Disabled-1 (Dab1) function in the developing chick retina. EMBO J. 23, 1878–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Z., Godbout R. (2013) Reelin-Disabled-1 signaling in neuronal migration. Splicing takes the stage. Cell. Mol. Life Sci. 70, 2319–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mayer H., Duit S., Hauser C., Schneider W. J., Nimpf J. (2006) Reconstitution of the Reelin signaling pathway in fibroblasts demonstrates that Dab1 phosphorylation is independent of receptor localization in lipid rafts. Mol. Cell. Biol. 26, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bock H. H., Jossin Y., Liu P., Förster E., May P., Goffinet A. M., Herz J. (2003) PI3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 278, 38772–38779 [DOI] [PubMed] [Google Scholar]
- 36. Beffert U., Morfini G., Bock H. H., Reyna H., Brady S. T., Herz J. (2002) Reelin-mediated signaling locally regulates PKB/Akt and GSK-3β. J. Biol. Chem. 277, 49958–49964 [DOI] [PubMed] [Google Scholar]
- 37. Willnow T. E. (1998) Receptor-associated protein (RAP). A specialized chaperone for endocytic receptors. Biol. Chem. 379, 1025–1031 [PubMed] [Google Scholar]
- 38. Rice D. S., Nusinowitz S., Azimi A. M., Martínez A., Soriano E., Curran T. (2001) The reelin pathway modulates the structure and function of retinal synaptic circuitry. Neuron 31, 929–941 [DOI] [PubMed] [Google Scholar]
- 39. Hummel S., Christian S., Osanger A., Heid H., Nimpf J., Schneider W. J. (2007) Identification of a novel chondroitin-sulfated collagen in the membrane separating theca and granulosa cells in chicken ovarian follicles. The granulosa-theca cell interface is not a bona fide basement membrane. J. Biol. Chem. 282, 8011–8018 [DOI] [PubMed] [Google Scholar]
- 40. Howell B. W., Herrick T. M., Hildebrand J. D., Zhang Y., Cooper J. A. (2000) Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr. Biol. 10, 877–885 [DOI] [PubMed] [Google Scholar]