Abstract
Microbial ester kinases identified in the past 3 decades came as a surprise, as protein phosphorylation on Ser, Thr, and Tyr amino acids was thought to be unique to eukaryotes. Current analysis of available microbial genomes reveals that “eukaryote-like” protein kinases are prevalent in prokaryotes and can converge in the same signaling pathway with the classical microbial “two-component” systems. Most microbial tyrosine kinases lack the “eukaryotic” Hanks domain signature and are designated tyrosine kinases based upon their biochemical activity. These include the tyrosine kinases termed bacterial tyrosine kinases (BY-kinases), which are responsible for the majority of known bacterial tyrosine phosphorylation events. Although termed generally as bacterial tyrosine kinases, BY-kinases can be considered as one family belonging to the superfamily of prokaryotic protein-tyrosine kinases in bacteria. Other members of this superfamily include atypical “odd” tyrosine kinases with diverse mechanisms of protein phosphorylation and the “eukaryote-like” Hanks-type tyrosine kinases. Here, we discuss the distribution, phylogeny, and function of the various prokaryotic protein-tyrosine kinases, focusing on the recently discovered Mycobacterium tuberculosis PtkA and its relationship with other members of this diverse family of proteins.
Keywords: Bacterial Protein Kinases, Bacterial Signal Transduction, Signal Transduction, Signaling, Protein-tyrosine Kinase (Tyrosine Kinase), BY-kinases, Mycobacterium tuberculosis PtkA, Tyr Kinase
Introduction
Signal transduction mediated by protein phosphorylation is a complex and highly regulated means for cells to sense and respond to their environment. In eukaryotes, this process is mediated primarily by serine, threonine, and tyrosine kinases and has been intensely studied for >50 years (1). However, in prokaryotes, research progress has been slower; until their discovery in the late 1970s, protein phosphorylation was thought to be unique to eukaryotes. Although pioneering experiments identified phosphorylation on Ser and Thr residues (2, 3), it was the two-component systems consisting of histidine kinases and response regulators that were first discovered in the early 1980s (4, 5) and heavily researched in bacteria. These findings led to the initial hypothesis that Ser/Thr/Tyr phosphorylation is a eukaryotic trait, whereas His/Asp phosphorylation is restricted to prokaryotes. This understanding has since been revised on both fronts. Hundreds of homologous two-component systems have been found in eukaryotic organisms (6), and with the discovery of Ser kinase/phosphatase (7) and later Hanks-type Ser/Thr protein kinases (8), Ser/Thr protein phosphorylation is now well established in bacteria (9–11). In fact, analysis of available microbial genomes combined with recent metagenomic data from global ocean sampling revealed that “eukaryote-like” protein kinases are as prevalent in prokaryotes as their histidine kinase counterparts (12). Furthermore, we and others have shown experimentally that these two systems can converge in the same signaling pathway in both eukaryotic and prokaryotic organisms (13), broadening the complexity and integration of these signaling events.
The discovery of Ser/Thr protein kinases in bacteria left a surprising absence of identifiable Hanks-type prokaryotic protein-tyrosine kinases (PPTKs),2 despite the presence of bacterial protein-tyrosine phosphatases. Instead, proteins with homologies to various kinase and non-kinase protein families were shown to possess tyrosine kinase activity. Most notably, beginning with the discovery of Ptk in Acinetobacter johnsonii (14), an entire family of proteins with homology to MinD and ArsA ATPases have been identified to be protein-tyrosine kinases (15). With no known eukaryotic counterparts, these kinases have been termed bacterial tyrosine kinases (BY-kinases), and they are responsible for the majority of known bacterial tyrosine phosphorylation (15). Nevertheless, tyrosine kinases that do not show homology to the BY-kinases continue to be discovered in bacteria, including, among others, PtkA from Mycobacterium tuberculosis (16). As such, the BY-kinases are one family belonging to the superfamily of protein-tyrosine kinases found in bacteria, which we will refer to here as the PPTK superfamily. The other two families are the Hanks-type PPTKs and an umbrella family of “odd” PPTKs. Phylogenetic analysis of the kinase domains of known PPTKs (Table 1) demonstrated clustering into these three families (Fig. 1), supporting our classification system.
TABLE 1.
PPTKs
Provided is a list of elucidated PPTKs, known substrates, and functions categorized by protein family subtype. TAD, transmembrane activator domain; EPS, extracellular polysaccharide; TCS, two-component system.
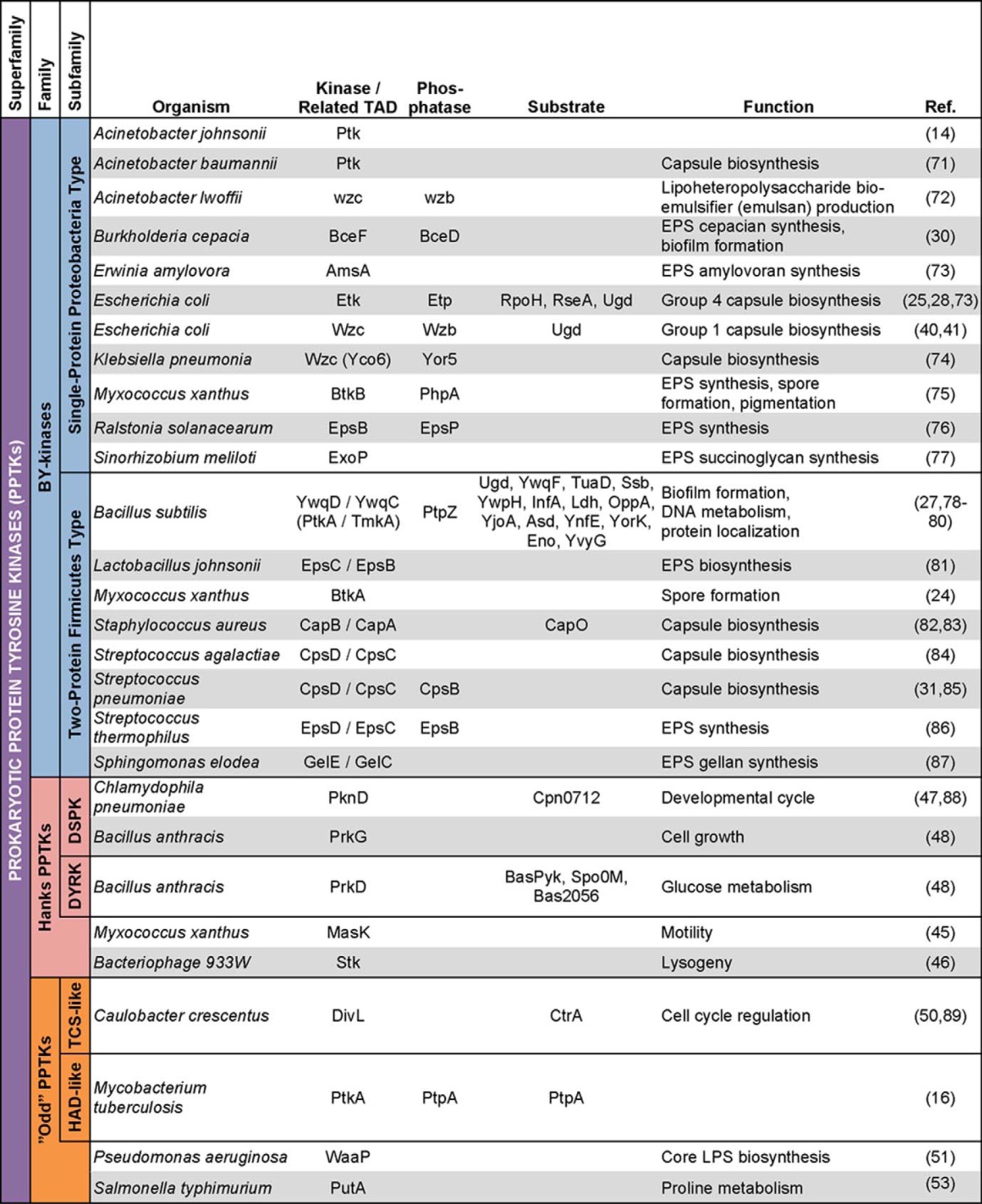
FIGURE 1.
Phylogenetic distribution of known PPTKs. Alignment of the kinase domains of known PPTKs revealed clustering into three main families of tyrosine kinases: the BY-kinases (BYK), the Hanks-type PPTKs (HYK), and the “odd” PPTKs (OYK). The BY-kinases further clustered according to their architectural subtypes: the single-protein P-type and the two-protein F-type. The Hanks-type PPTKs clustered together with the eukaryotic kinases (Src tyrosine kinase and Ser/Thr cAMP-dependent protein kinase A) and the Hanks-type Ser/Thr kinase PknB of M. tuberculosis. The DivL kinase of C. crescentus and the putative BC4770 tyrosine kinase of B. cereus clustered with the histidine kinases of E. coli (EnvZ and PhoR). P. aeruginosa WaaP is reported to contain 9 of the 11 Hanks domains (51) and clustered near the Hanks-type PPTKs. Although grouped together, the “odd” PPTKs are distantly related as expected. The following sequences were used for alignment in ClustalX2: the C-terminal catalytic domains of BY-kinases; the kinase domains of Hanks-type kinases and histidine/histidine-like kinases, identified using the ScanProsite tool (70); the catalytic domain of M. tuberculosis PtkA determined in Fig. 3; and full-length PutA and WaaP as their tyrosine kinase domains are not defined. The phylogenetic tree was displayed using Molecular Evolutionary Genetics Analysis (MEGA) 5 software.
BY-kinases
The burgeoning field of BY-kinases is highlighted in a recent set of excellent reviews (15, 17–20). To avoid redundancy, readers are encouraged to refer to these publications for broader background information. Here, we highlight our point of view based on our understanding and some recent advances.
With >6700 entries in the BY-kinase database (BYKdb) (90) as of this writing, BY-kinases represent a major class of tyrosine kinases that do not share sequence or structural homology to eukaryotic tyrosine kinases (21). Instead, the catalytic domain of BY-kinases consists of variants of the Walker A (P-loop) and Walker B motifs, which are involved in nucleotide binding and hydrolysis, respectively. BY-kinases possess an additional motif located between the Walker A and B motifs, designated Walker A′, which is also found in certain P-loop nucleotide kinases and other ATPases (15).
BY-kinases are organized into two subfamilies based on their architectural types, the Gram-negative Proteobacteria-type (P-type) and the low-GC Gram-positive Firmicutes-type (F-type), designated for their abundance in, but not restricted to, these two phyla (22). The P-type contains an N-terminal two-pass transmembrane portion with an extracellular hairpin loop domain. The intracellular portion encompasses the catalytic domain and a tyrosine-rich C-terminal domain as the site for autophosphorylation. In the F-type, the extracellular transmembrane domain and the intracellular catalytic domain are two independent proteins encoded by two separate genes. Kinase activity in this two-protein F-type requires interaction between the intracellular tail of the transmembrane activator protein and the catalytic polypeptide (23).
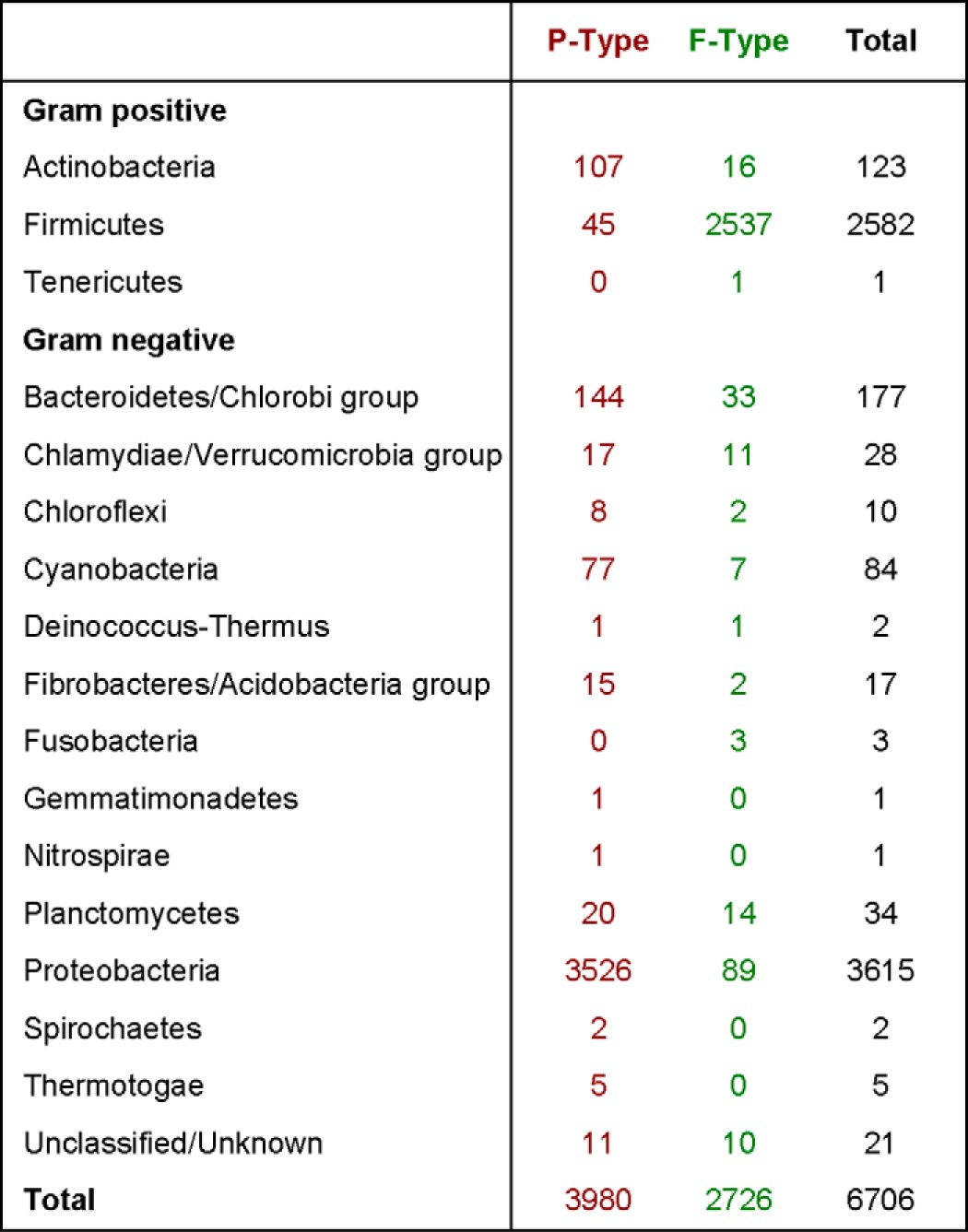
The abundance of the two subfamilies of annotated BY-kinases and their distribution among bacterial phyla is shown in Table 2. Despite their designation, both the single-protein P-type and the two-protein F-type can be found in nearly all bacterial phyla (Table 2) and, in some cases, even in the same organism (e.g. Myxococcus xanthus) (24). With the obvious exception of the Firmicutes, the predominant form of BY-kinases within each phylum is the single-protein P-type, including within the high-GC Gram-positive Actinobacteria phylum (Table 2).
TABLE 2.
Distribution of annotated BY-kinases
The number of single-protein P-type (red) and two-protein F-type (green) BY-kinases is given for each bacterial phylum. Phyla with no known BY-kinases are not shown. Data are updated from Ref. 15.
BY-kinases are involved in many diverse functions in bacteria, such as antibiotic resistance (25), DNA replication (26) and metabolism (27), heat shock response (28), biofilm formation (29, 30), spore formation (24), and virulence (31–33). The majority of characterized BY-kinases regulate the production and export of capsular and extracellular polysaccharides (Table 1). Table 1 provides a list of known BY-kinases, their substrates, and functions.
In Escherichia coli, the cps locus, encoding the Wzc BY-kinase and its cognate tyrosine phosphatase, Wzb, is responsible for group 1 capsule biosynthesis (34, 35). Interestingly, both kinase and phosphatase activities are required for proper exopolysaccharide synthesis (36–38). Specifically, the overall level of phosphorylation of the tyrosine-rich cluster is responsible for Wzc activity (37). These findings led to a cycling hypothesis whereby Wzc alternates between phosphorylated and Wzb-mediated unphosphorylated states (20). This phenomenon highlights the elegant use of bacterial signal transduction pathways where a kinase and a phosphatase are used in concert to control synthesis of functional and structural elements. Structural dependence on phosphorylation status is also exemplified by this system. In its unphosphorylated state, Wzc forms an oligomer able to transautophosphorylate the tyrosine cluster through association of its kinase domains (39). In its phosphorylated state, Wzc dissociates into active monomers, where it is free to phosphorylate its substrates, including UDP-glucose dehydrogenase, an enzyme involved in the synthesis of polysaccharide precursors (40). Dephosphorylation of Wzc by Wzb returns Wzc to its unphosphorylated state (33, 41), completing the cycle. Initially, a low-resolution crystal structure suggested that dephosphorylated Wzc forms a tetrameric structure that spans the periplasm and interacts with the outer membrane octameric porin Wza (42). However, work by Bechet et al. showed that Wzc in its dephosphorylated state generates an octamer postulated to form a channel passing through the inner membrane (39) and interacts with the octameric outer membrane porin (42). This complex putatively forms a complete channel that is able to span both membranes. The octameric quaternary structure of Wzc agrees with the finding that the recombinant Wzc homolog in Staphylococcus aureus, CapAB fusion protein, also forms an octamer when dephosphorylated (43). This opens up a fascinating new hypothesis that Wzc-Wza may be a novel secretion system, allowing for the export of precursors for capsule biosynthesis. Further experiments are required to test this hypothesis.
The E. coli group 4 capsule (34) is synthesized by the same Wzy-dependent mechanism as the cps locus and uses the BY-kinase Etk and its cognate tyrosine phosphatase, Etp. Interestingly, Etp is tyrosine-phosphorylated, and its phosphorylation state affects capsule biosynthesis (44). Adding to the complexity of this model for capsule synthesis, neither of the E. coli BY-kinases (Etk or Wzc) are required for Etp phosphorylation, indicating there are additional unidentified PPTK(s) responsible for this phosphorylation (44).
Hanks-type Tyrosine Kinases
Although Hanks-type PPTKs are not readily identifiable in bacteria, bacteria are not completely void of these kinases. M. xanthus MasK, involved in motility, is currently the only known protein-tyrosine kinase in bacteria that bears a Hanks-type tyrosine kinase-specific active-site signature (45). Pathogenic E. coli harboring the Shiga toxin type 2-encoding bacteriophage 933W also expresses a Hanks-type PPTK, Stk. Although technically a phage protein, Stk is involved in maintaining lysogeny and bears homology to Ser/Thr protein kinases, but it instead possesses tyrosine kinase activity (46). Furthermore, the Ser/Thr kinase PknD of Chlamydophila pneumoniae was also found to autophosphorylate on Tyr residues and thus belongs to a new group of dual-specificity protein kinases (DSPKs) in bacteria (47). Two additional Hanks-type PPTKs, PrkD and PrkG, were recently identified in Bacillus anthracis, and they belong to two separate DSPK subfamilies (48). Both PrkD and PrkG autophosphorylate on Ser/Thr and Tyr residues, with PrkG transphosphorylating the artificial substrate myelin basic protein also on all three residues. Regulated predominantly by tyrosine autophosphorylation, PrkD is able to phosphorylate its endogenous substrates only on Ser and Thr (48), a mechanism typical of eukaryotic dual-specificity tyrosine phosphorylation-regulated kinases (DYRKs) (49). Therefore, PrkD represents the first known prokaryotic DYRK-like protein (48).
“Odd” Tyrosine Kinases
A handful of additional proteins, including M. tuberculosis PtkA, belonging to various protein families were also found to possess tyrosine kinase activity (Table 1). Because these kinases are few and sparse, with only one reported occurrence in each protein family, we describe these kinases here under the umbrella term “odd” PPTKs.
DivL is an essential tyrosine kinase in Caulobacter crescentus that is involved in cell cycle progression via phosphorylation of its substrate, CtrA (50). Interestingly, DivL belongs to the two-component system histidine kinase family. However, in place of the conserved histidine residue at the site of autophosphorylation, DivL is equipped with a Tyr residue that undergoes autophosphorylation (50).
WaaP of Pseudomonas aeruginosa and PutA of Salmonella typhimurium are not strict protein-tyrosine kinases, as other functions have been ascribed to these proteins. WaaP is described to contain 9 of the 11 conserved Hanks domains of eukaryotic kinases (51), but it is also an essential sugar kinase involved in core lipopolysaccharide biosynthesis (52). PutA has a suspiciously large array of functions. In addition to catalyzing two separate steps of proline degradation and functioning as a DNA-binding transcriptional repressor, the bifunctional PutA is described to possess Ser, Thr, and Tyr dual-specificity autophosphorylation activity (53). As no follow-up experiments on kinase activity have been performed in nearly 2 decades, it may be worthwhile to confirm PutA autophosphorylation.
The McsB protein found in Bacillus subtilis and B. anthracis was thought to use its guanidinophosphotransferase domain to autophosphorylate and phosphorylate its substrate, CtsR, on Tyr (54). However, McsB was later determined to be the first known protein-arginine kinase in bacteria (55, 56). Involved in regulating the heat shock response, McsB phosphorylates the CtsR repressor of class III heat shock response genes on Arg (54, 55). Furthermore, the annotated protein-tyrosine phosphatase that acts in opposition to McsB was also shown to be a phosphoarginine phosphatase (57).
The “odd” tyrosine kinases DivL and PtkA (described below), as well as the majority of the Hanks-type PPTKs, represent the sole tyrosine kinase found in their respective protein subfamilies (Table 1). However, the existence of one tyrosine kinase strongly suggests the existence of additional kinases within the same subfamily. For example, the annotated sensor histidine kinase BC4770 of Bacillus cereus and its orthologs in B. anthracis and Bacillus thuringiensis contain Tyr amino acids instead of His at the site of autophosphorylation (58). Therefore, these proteins likely represent additional members of the histidine kinase-like protein-tyrosine kinase subfamily to which DivL belongs. In addition, as described below, the entire operon containing the protein-tyrosine kinase PtkA in M. tuberculosis is conserved in multiple Actinomycetes species.
M. tuberculosis PtkA
Within a pathogenicity cluster in M. tuberculosis, we have identified an open reading frame that encodes a unique protein-tyrosine kinase, PtkA (16). PtkA was originally annotated as a member of the haloacid dehalogenase (HAD) superfamily based upon sequence homology. However, biochemical analysis of its enzymatic activity demonstrated that PtkA is a genuine protein-tyrosine kinase (16). PtkA is therefore the first of its kind in the HAD family, which, despite the terminology, consists of a vast number of phosphotransferases, phosphatases, ATPases, and phosphoglucomutases, in addition to dehalogenases (59).
Signature motifs of Hanks protein kinases, such as the glycine-rich domain, are not found in PtkA, nor are Walker A, A′, and B motifs typical of BY-kinases. Hence, PtkA is considered here as an odd PPTK. Interestingly, extension of the HAD domain of PtkA (hhhhDhD) bears striking resemblance to the Walker A′ motif (hhhhDXDXR), where h is a subset of hydrophobic amino acids (ILVFM) commonly found in BY-kinase conserved sequences (60). Point mutations of the three Tyr residues in PtkA revealed that Tyr-262 is the site of autophosphorylation (16). Furthermore, mutations of the first aspartate residue of the highly conserved DXD motif, which is essential for the phosphotransferase catalytic mechanism in HAD enzymes (59), abolished the tyrosine kinase activity of PtkA (16). These observations indicate that PtkA might utilize an enzymatic mechanism that is similar to that of other HAD members but has been fine-tuned for tyrosine phosphorylation. Further biochemical studies on PtkA activity are ongoing to elucidate its exact catalytic mechanism.
ptkA is located in an apparent three-gene operon upstream of ptpA, encoding the low-molecular-weight protein-tyrosine phosphatase PtpA (61, 62), and Rv2235, encoding a predicted transmembrane protein. Interestingly, with reverse analogy to the BY-kinases that are dephosphorylated by their cognate tyrosine phosphatases (41), PtkA phosphorylates the M. tuberculosis protein-tyrosine phosphatase PtpA (16). As with many of the BY-kinases involved in exopolysaccharide synthesis, PtkA and PtpA do not form classical on/off switches of signal transduction. Instead, PtpA is secreted by M. tuberculosis into the host macrophage during infection (61), and thus, the PtkA-PtpA kinase-phosphatase pair seems to be geared to a linear pathway targeting the human host protein machinery. In the host, PtpA binds to subunit H of the human vacuolar H+-ATPase pump and dephosphorylates human VPS33B (vacuolar protein sorting 33B) to inhibit phagosome acidification and block fusion with lysosomes, respectively (16, 62).
PtkA phosphorylates PtpA on Tyr-128 and Tyr-129, which are located in the D-loop of PtpA together with the catalytically critical Asp-126 residue. Further supporting PtkA phosphorylation of PtpA, the interface between PtpA and PtkA is at the P- and D-loops of PtpA (63). Although the functional role of PtpA phosphorylation by PtkA is unknown, the D-loop of PtpA switches from an “open” to a “closed” conformation upon binding to a substrate (63). Thus, PtkA phosphorylation of PtpA Tyr-128 and Tyr-129 might modulate the conformational change of the D-loop, thereby regulating the phosphatase activity of PtpA. It is also interesting to note that the human low-molecular-weight protein-tyrosine phosphatase HCPTP-A, which has 37% sequence similarity to PtpA, was found to be phosphorylated in vitro by pp60v-Src on Tyr-131 and Tyr-32 residues located in the D-loop (64). Furthermore, phosphorylation of each site results in different effects on phosphatase structure and function. Whereas Tyr-131 phosphorylation leads to a 25-fold increase in HCPTP-A activity, Tyr-132 phosphorylation serves as a signal for the recruitment and binding of the Grb2 adaptor protein. Therefore, it is tempting to speculate that PtkA phosphorylation of PtpA might similarly have dual effects by enhancing PtpA activity and signaling for the formation of a multisubunit protein complex that plays a role in M. tuberculosis pathogenesis.
Intriguingly, PtkA is predicted to localize to the mycobacterial cell wall or outer membrane (65). The last gene in the PtpA operon, Rv2235, is also predicted to possess three transmembrane helices and was previously identified in the cell wall fraction of M. tuberculosis H37Rv (66). Based on these localizations, PtkA may play a role in regulating the export of PtpA instead of, or in addition to, the maintenance of PtpA activity in the host macrophage. Further investigation is needed to fully understand the functional role of PtkA phosphorylation of PtpA.
The PtkA-PtpA operon is conserved among numerous species of the Actinomycetes order, including the genera Rhodococcus, Corynebacterium, Gordonia, Amycolicicoccus, and Mycobacterium, albeit with limited individual protein homology (39–76% identity for PtkA) (Fig. 2). The fact that the whole operon is conserved suggests that these PtkA homologs are also protein-tyrosine kinases; however, the low-percent identity indicates that a common broader biological phenotype is associated with this operon rather than specific targets. In contrast, this operon is highly conserved (100% protein identity) uniquely within the M. tuberculosis complex (Fig. 2), which utilizes PtpA for virulence (61). In addition, individual orthologs of PtkA exist among all domains of life (Fig. 2). In total, 924 unique orthologs of PtkA were found using a FastA alignment in the KEGG Sequence Similarity Database at the time of this writing (Fig. 2). Representatives of these sequences with the highest homology to PtkA, as well as the top 15 eukaryotic orthologs, are shown as a dendrogram in supplemental Fig. S1, and they show a clear clustering of PtkA orthologs where the ptpA operon is also conserved. Interestingly, the phylogenetic distribution of the 924 orthologs revealed a subset of 220 proteins with closest homology to PtkA (supplemental Fig. S2). Sequence analysis of these 220 orthologs revealed at least five conserved domains (Fig. 3, bars), including the DXD motif (hhhDhDGTh), and two lysine residues that are required for PtkA kinase activity (hXhX(S/T)XK and KX(D/E)hhXXXh) (16). Additionally, numerous Gly and hydrophobic residues are conserved (Fig. 3, asterisks). Whereas the autophosphorylating Tyr-262 residue of PtkA is conserved only in ∼60% of the sequences, a second Tyr at position 260 is found in the majority of the remaining sequences (Fig. 3) and thus may require manual alignment. Further experiments are required to verify that these proteins are in fact tyrosine kinases and to determine whether the conserved sequences identified here are too broad or too restrictive.
FIGURE 2.
Distribution of PtkA orthologs. A simple orthology search of the KEGG Sequence Similarity Database revealed 1772 orthologs of PtkA with a minimum Smith-Waterson score of 150 using the best-best alignment FastA algorithm. Excluding orthologs found in multiple strains of the same species identified 924 unique orthologs distributed among all domains of life. The number of unique species is given after the phylum name/description, and the range of percent identity in given in parentheses. The ptpA operon, which encodes PtkA, was also conserved in 35 of the 123 Actinomycetes species (bar chart). Mtb, M. tuberculosis.
FIGURE 3.
Conserved domains of the protein-tyrosine kinase PtkA. The cluster of 220 orthologs identified in supplemental Fig. S1 was aligned with Clustal Omega and displayed using WebLogo 3. Conserved domains are highlighted with bars, and conserved residues are highlighted with asterisks.
Concluding Remarks
The prominent roles of PPTKs in bacterial physiology are highlighted by the recent discovery of 512 unique phosphotyrosine sites on 384 E. coli proteins, corresponding to up to 6% of the E. coli proteome (67). This level of tyrosine phosphorylation is far more prevalent than previously thought and exceeds even tyrosine phosphorylation events reported in mammalian cells (68). These identified phosphoproteins are involved in important cellular processes, including cell division, virulence, transport, transcription, and translation, and they are central to numerous metabolic pathways (67). Furthermore, up to almost half of the tyrosine phosphorylation events could not be accounted for by the only two known tyrosine kinases, Wzc and Etk of the BY-kinase family (67), indicating the existence of as-of-yet unidentified PPTK(s) in the E. coli genome.
The discovery of PtkA represents a new member of an atypical tyrosine kinase in bacteria and establishes a novel function of HAD proteins. Through distribution and primary sequence analysis, we propose that PtkA is a representative of a new group of “odd” PPTKs that use the HAD phosphoryl transfer mechanism for protein tyrosine phosphorylation. PtkA phosphorylation of PtpA, a key virulence factor in M. tuberculosis, marks the involvement of tyrosine kinases in microbial pathogenesis and represents an unexplored area for anti-infective drug discovery (69).
The diverse variety of PPTKs indicate that prokaryotes possess a much wider repertoire of signaling proteins compared with eukaryotes. With thousands of uncharacterized BY-kinases, novel Hanks-type DSPKs, and the recent addition to the “odd” PPTK family, it is likely that we are just beginning to scratch the surface of the prominent role of tyrosine phosphorylation in bacterial physiology.
Acknowledgments
We thank Xingji Zheng and Jeffrey Helm for useful comments.
This work was supported by Canadian Institutes of Health Research (CIHR) Operating Grant MOP-106622. This is the first article in the Thematic Minireview Series “Protein Serine/Threonine and Tyrosine Phosphorylation in Prokaryotes.”

This article contains supplemental Figs. S1 and S2.
- PPTK
- prokaryotic protein-tyrosine kinase
- BY-kinase
- bacterial tyrosine kinase
- DSPK
- dual-specificity protein kinase
- DYRK
- dual-specificity tyrosine phosphorylation-regulated kinase
- HAD
- haloacid dehalogenase.
REFERENCES
- 1. Pawson T., Scott J. D. (2005) Protein phosphorylation in signaling–50 years and counting. Trends Biochem. Sci. 30, 286–290 [DOI] [PubMed] [Google Scholar]
- 2. Wang J. Y., Koshland D. E., Jr. (1978) Evidence for protein kinase activities in the prokaryote Salmonella typhimurium. J. Biol. Chem. 253, 7605–7608 [PubMed] [Google Scholar]
- 3. Manai M., Cozzone A. J. (1979) Analysis of the protein-kinase activity of Escherichia coli cells. Biochem. Biophys. Res. Commun. 91, 819–826 [DOI] [PubMed] [Google Scholar]
- 4. Mizuno T., Wurtzel E. T., Inouye M. (1982) Osmoregulation of gene expression. II. DNA sequence of the envZ gene of the ompB operon of Escherichia coli and characterization of its gene product. J. Biol. Chem. 257, 13692–13698 [PubMed] [Google Scholar]
- 5. Tommassen J., de Geus P., Lugtenberg B., Hackett J., Reeves P. (1982) Regulation of the pho regulon of Escherichia coli K-12. Cloning of the regulatory genes phoB and phoR and identification of their gene products. J. Mol. Biol. 157, 265–274 [DOI] [PubMed] [Google Scholar]
- 6. Grebe T. W., Stock J. B. (1999) The histidine protein kinase superfamily. Adv. Microb. Physiol. 41, 139–227 [DOI] [PubMed] [Google Scholar]
- 7. LaPorte D. C., Chung T. (1985) A single gene codes for the kinase and phosphatase which regulate isocitrate dehydrogenase. J. Biol. Chem. 260, 15291–15297 [PubMed] [Google Scholar]
- 8. Muñoz-Dorado J., Inouye S., Inouye M. (1991) A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell 67, 995–1006 [DOI] [PubMed] [Google Scholar]
- 9. Deutscher J., Saier M. H., Jr. (2005) Ser/Thr/Tyr protein phosphorylation in bacteria–for long time neglected, now well established. J. Mol. Microbiol. Biotechnol. 9, 125–131 [DOI] [PubMed] [Google Scholar]
- 10. Chao J., Wong D., Zheng X., Poirier V., Bach H., Hmama Z., Av-Gay Y. (2010) Protein kinase and phosphatase signaling in Mycobacterium tuberculosis physiology and pathogenesis. Biochim. Biophys. Acta 1804, 620–627 [DOI] [PubMed] [Google Scholar]
- 11. Av-Gay Y., Everett M. (2000) The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 8, 238–244 [DOI] [PubMed] [Google Scholar]
- 12. Kannan N., Taylor S. S., Zhai Y., Venter J. C., Manning G. (2007) Structural and functional diversity of the microbial kinome. PLoS Biol. 5, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chao J. D., Papavinasasundaram K. G., Zheng X., Chávez-Steenbock A., Wang X., Lee G. Q., Av-Gay Y. (2010) Convergence of Ser/Thr and two-component signaling to coordinate expression of the dormancy regulon in Mycobacterium tuberculosis. J. Biol. Chem. 285, 29239–29246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grangeasse C., Doublet P., Vaganay E., Vincent C., Deléage G., Duclos B., Cozzone A. J. (1997) Characterization of a bacterial gene encoding an autophosphorylating protein tyrosine kinase. Gene 204, 259–265 [DOI] [PubMed] [Google Scholar]
- 15. Grangeasse C., Nessler S., Mijakovic I. (2012) Bacterial tyrosine kinases: evolution, biological function and structural insights. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2640–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bach H., Wong D., Av-Gay Y. (2009) Mycobacterium tuberculosis PtkA is a novel protein tyrosine kinase whose substrate is PtpA. Biochem. J. 420, 155–160 [DOI] [PubMed] [Google Scholar]
- 17. Grangeasse C., Terreux R., Nessler S. (2010) Bacterial tyrosine-kinases: structure-function analysis and therapeutic potential. Biochim. Biophys. Acta 1804, 628–634 [DOI] [PubMed] [Google Scholar]
- 18. Whitmore S. E., Lamont R. J. (2012) Tyrosine phosphorylation and bacterial virulence. Int. J. Oral Sci. 4, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee D. C., Jia Z. (2009) Emerging structural insights into bacterial tyrosine kinases. Trends Biochem. Sci. 34, 351–357 [DOI] [PubMed] [Google Scholar]
- 20. Bechet E., Guiral S., Torres S., Mijakovic I., Cozzone A. J., Grangeasse C. (2009) Tyrosine-kinases in bacteria: from a matter of controversy to the status of key regulatory enzymes. Amino Acids 37, 499–507 [DOI] [PubMed] [Google Scholar]
- 21. Lee D. C., Zheng J., She Y. M., Jia Z. (2008) Structure of Escherichia coli tyrosine kinase Etk reveals a novel activation mechanism. EMBO J. 27, 1758–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grangeasse C., Cozzone A. J., Deutscher J., Mijakovic I. (2007) Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem. Sci. 32, 86–94 [DOI] [PubMed] [Google Scholar]
- 23. Soulat D., Jault J. M., Duclos B., Geourjon C., Cozzone A. J., Grangeasse C. (2006) Staphylococcus aureus operates protein-tyrosine phosphorylation through a specific mechanism. J. Biol. Chem. 281, 14048–14056 [DOI] [PubMed] [Google Scholar]
- 24. Kimura Y., Yamashita S., Mori Y., Kitajima Y., Takegawa K. (2011) A Myxococcus xanthus bacterial tyrosine kinase, BtkA, is required for the formation of mature spores. J. Bacteriol. 193, 5853–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lacour S., Doublet P., Obadia B., Cozzone A. J., Grangeasse C. (2006) A novel role for protein-tyrosine kinase Etk from Escherichia coli K-12 related to polymyxin resistance. Res. Microbiol. 157, 637–641 [DOI] [PubMed] [Google Scholar]
- 26. Petranovic D., Michelsen O., Zahradka K., Silva C., Petranovic M., Jensen P. R., Mijakovic I. (2007) Bacillus subtilis strain deficient for the protein-tyrosine kinase PtkA exhibits impaired DNA replication. Mol. Microbiol. 63, 1797–1805 [DOI] [PubMed] [Google Scholar]
- 27. Mijakovic I., Petranovic D., Macek B., Cepo T., Mann M., Davies J., Jensen P. R., Vujaklija D. (2006) Bacterial single-stranded DNA-binding proteins are phosphorylated on tyrosine. Nucleic Acids Res. 34, 1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klein G., Dartigalongue C., Raina S. (2003) Phosphorylation-mediated regulation of heat shock response in Escherichia coli. Mol. Microbiol. 48, 269–285 [DOI] [PubMed] [Google Scholar]
- 29. Kiley T. B., Stanley-Wall N. R. (2010) Post-translational control of Bacillus subtilis biofilm formation mediated by tyrosine phosphorylation. Mol. Microbiol. 78, 947–963 [DOI] [PubMed] [Google Scholar]
- 30. Ferreira A. S., Leitão J. H., Sousa S. A., Cosme A. M., Sá-Correia I., Moreira L. M. (2007) Functional analysis of Burkholderia cepacia genes bceD and bceF, encoding a phosphotyrosine phosphatase and a tyrosine autokinase, respectively: role in exopolysaccharide biosynthesis and biofilm formation. Appl. Environ. Microbiol. 73, 524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morona J. K., Morona R., Paton J. C. (2006) Attachment of capsular polysaccharide to the cell wall of Streptococcus pneumoniae type 2 is required for invasive disease. Proc. Natl. Acad. Sci. U.S.A. 103, 8505–8510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferreira A. S., Silva I. N., Oliveira V. H., Becker J. D., Givskov M., Ryan R. P., Fernandes F., Moreira L. M. (2013) Comparative transcriptomic analysis of the Burkholderia cepacia tyrosine kinase bceF mutant reveals a role in tolerance to stress, biofilm formation, and virulence. Appl. Environ. Microbiol. 79, 3009–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Temel D. B., Dutta K., Alphonse S., Nourikyan J., Grangeasse C., Ghose R. (2013) Regulatory interactions between a bacterial tyrosine kinase and its cognate phosphatase. J. Biol. Chem. 288, 15212–15228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Whitfield C. (2006) Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75, 39–68 [DOI] [PubMed] [Google Scholar]
- 35. Cuthbertson L., Mainprize I. L., Naismith J. H., Whitfield C. (2009) Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in Gram-negative bacteria. Microbiol. Mol. Biol. Rev. 73, 155–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wugeditsch T., Paiment A., Hocking J., Drummelsmith J., Forrester C., Whitfield C. (2001) Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J. Biol. Chem. 276, 2361–2371 [DOI] [PubMed] [Google Scholar]
- 37. Paiment A., Hocking J., Whitfield C. (2002) Impact of phosphorylation of specific residues in the tyrosine autokinase, Wzc, on its activity in assembly of group 1 capsules in Escherichia coli. J. Bacteriol. 184, 6437–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Obadia B., Lacour S., Doublet P., Baubichon-Cortay H., Cozzone A. J., Grangeasse C. (2007) Influence of tyrosine-kinase Wzc activity on colanic acid production in Escherichia coli K12 cells. J. Mol. Biol. 367, 42–53 [DOI] [PubMed] [Google Scholar]
- 39. Bechet E., Gruszczyk J., Terreux R., Gueguen-Chaignon V., Vigouroux A., Obadia B., Cozzone A. J., Nessler S., Grangeasse C. (2010) Identification of structural and molecular determinants of the tyrosine-kinase Wzc and implications in capsular polysaccharide export. Mol. Microbiol. 77, 1315–1325 [DOI] [PubMed] [Google Scholar]
- 40. Grangeasse C., Obadia B., Mijakovic I., Deutscher J., Cozzone A. J., Doublet P. (2003) Autophosphorylation of the Escherichia coli protein kinase Wzc regulates tyrosine phosphorylation of Ugd, a UDP-glucose dehydrogenase. J. Biol. Chem. 278, 39323–39329 [DOI] [PubMed] [Google Scholar]
- 41. Vincent C., Doublet P., Grangeasse C., Vaganay E., Cozzone A. J., Duclos B. (1999) Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J. Bacteriol. 181, 3472–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collins R. F., Beis K., Dong C., Botting C. H., McDonnell C., Ford R. C., Clarke B. R., Whitfield C., Naismith J. H. (2007) The 3D structure of a periplasm-spanning platform required for assembly of group 1 capsular polysaccharides in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 104, 2390–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olivares-Illana V., Meyer P., Bechet E., Gueguen-Chaignon V., Soulat D., Lazereg-Riquier S., Mijakovic I., Deutscher J., Cozzone A. J., Laprévote O., Morera S., Grangeasse C., Nessler S. (2008) Structural basis for the regulation mechanism of the tyrosine kinase CapB from Staphylococcus aureus. PLoS Biol. 6, e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nadler C., Koby S., Peleg A., Johnson A. C., Suddala K. C., Sathiyamoorthy K., Smith B. E., Saper M. A., Rosenshine I. (2012) Cycling of Etk and Etp phosphorylation states is involved in formation of group 4 capsule by Escherichia coli. PLoS ONE 7, e37984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomasson B., Link J., Stassinopoulos A. G., Burke N., Plamann L., Hartzell P. L. (2002) MglA, a small GTPase, interacts with a tyrosine kinase to control type IV pili-mediated motility and development of Myxococcus xanthus. Mol. Microbiol. 46, 1399–1413 [DOI] [PubMed] [Google Scholar]
- 46. Tyler J. S., Friedman D. I. (2004) Characterization of a eukaryotic-like tyrosine protein kinase expressed by the Shiga toxin-encoding bacteriophage 933W. J. Bacteriol. 186, 3472–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnson D. L., Mahony J. B. (2007) Chlamydophila pneumoniae PknD exhibits dual amino acid specificity and phosphorylates Cpn0712, a putative type III secretion YscD homolog. J. Bacteriol. 189, 7549–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arora G., Sajid A., Arulanandh M. D., Singhal A., Mattoo A. R., Pomerantsev A. P., Leppla S. H., Maiti S., Singh Y. (2012) Unveiling the novel dual specificity protein kinases in Bacillus anthracis: identification of the first prokaryotic dual specificity tyrosine phosphorylation-regulated kinase (DYRK)-like kinase. J. Biol. Chem. 287, 26749–26763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aranda S., Laguna A., de la Luna S. (2011) DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J. 25, 449–462 [DOI] [PubMed] [Google Scholar]
- 50. Wu J., Ohta N., Zhao J. L., Newton A. (1999) A novel bacterial tyrosine kinase essential for cell division and differentiation. Proc. Natl. Acad. Sci. U.S.A. 96, 13068–13073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao X., Lam J. S. (2002) WaaP of Pseudomonas aeruginosa is a novel eukaryotic type protein-tyrosine kinase as well as a sugar kinase essential for the biosynthesis of core lipopolysaccharide. J. Biol. Chem. 277, 4722–4730 [DOI] [PubMed] [Google Scholar]
- 52. Walsh A. G., Matewish M. J., Burrows L. L., Monteiro M. A., Perry M. B., Lam J. S. (2000) Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Mol. Microbiol. 35, 718–727 [DOI] [PubMed] [Google Scholar]
- 53. Ostrovsky P. C., Maloy S. (1995) Protein phosphorylation on serine, threonine, and tyrosine residues modulates membrane-protein interactions and transcriptional regulation in Salmonella typhimurium. Genes Dev. 9, 2034–2041 [DOI] [PubMed] [Google Scholar]
- 54. Kirstein J., Zühlke D., Gerth U., Turgay K., Hecker M. (2005) A tyrosine kinase and its activator control the activity of the CtsR heat shock repressor in B. subtilis. EMBO J. 24, 3435–3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fuhrmann J., Schmidt A., Spiess S., Lehner A., Turgay K., Mechtler K., Charpentier E., Clausen T. (2009) McsB is a protein arginine kinase that phosphorylates and inhibits the heat-shock regulator CtsR. Science 324, 1323–1327 [DOI] [PubMed] [Google Scholar]
- 56. Jung K., Jung H. (2009) A new mechanism of phosphoregulation in signal transduction pathways. Sci. Signal. 2, pe71. [DOI] [PubMed] [Google Scholar]
- 57. Fuhrmann J., Mierzwa B., Trentini D. B., Spiess S., Lehner A., Charpentier E., Clausen T. (2013) Structural basis for recognizing phosphoarginine and evolving residue-specific protein phosphatases in Gram-positive bacteria. Cell Rep. 3, 1832–1839 [DOI] [PubMed] [Google Scholar]
- 58. Anderson I., Sorokin A., Kapatral V., Reznik G., Bhattacharya A., Mikhailova N., Burd H., Joukov V., Kaznadzey D., Walunas T., Markd'Souza, Larsen N., Pusch G., Liolios K., Grechkin Y., Lapidus A., Goltsman E., Chu L., Fonstein M., Ehrlich S. D., Overbeek R., Kyrpides N., Ivanova N. (2005) Comparative genome analysis of Bacillus cereus group genomes with Bacillus subtilis. FEMS Microbiol. Lett. 250, 175–184 [DOI] [PubMed] [Google Scholar]
- 59. Burroughs A. M., Allen K. N., Dunaway-Mariano D., Aravind L. (2006) Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J. Mol. Biol. 361, 1003–1034 [DOI] [PubMed] [Google Scholar]
- 60. Jadeau F., Bechet E., Cozzone A. J., Deléage G., Grangeasse C., Combet C. (2008) Identification of the idiosyncratic bacterial protein tyrosine kinase (BY-kinase) family signature. Bioinformatics 24, 2427–2430 [DOI] [PubMed] [Google Scholar]
- 61. Bach H., Papavinasasundaram K. G., Wong D., Hmama Z., Av-Gay Y. (2008) Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe 3, 316–322 [DOI] [PubMed] [Google Scholar]
- 62. Wong D., Bach H., Sun J., Hmama Z., Av-Gay Y. (2011) Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc. Natl. Acad. Sci. U.S.A. 108, 19371–19376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stehle T., Sreeramulu S., Löhr F., Richter C., Saxena K., Jonker H. R., Schwalbe H. (2012) The apo-structure of the low molecular weight protein-tyrosine phosphatase A (MptpA) from Mycobacterium tuberculosis allows for better target-specific drug development. J. Biol. Chem. 287, 34569–34582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tailor P., Gilman J., Williams S., Couture C., Mustelin T. (1997) Regulation of the low molecular weight phosphotyrosine phosphatase by phosphorylation at tyrosines 131 and 132. J. Biol. Chem. 272, 5371–5374 [DOI] [PubMed] [Google Scholar]
- 65. Song H., Sandie R., Wang Y., Andrade-Navarro M. A., Niederweis M. (2008) Identification of outer membrane proteins of Mycobacterium tuberculosis. Tuberculosis 88, 526–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mawuenyega K. G., Forst C. V., Dobos K. M., Belisle J. T., Chen J., Bradbury E. M., Bradbury A. R., Chen X. (2005) Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol. Biol. Cell 16, 396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hansen A. M., Chaerkady R., Sharma J., Díaz-Mejía J. J., Tyagi N., Renuse S., Jacob H. K., Pinto S. M., Sahasrabuddhe N. A., Kim M. S., Delanghe B., Srinivasan N., Emili A., Kaper J. B., Pandey A. (2013) The Escherichia coli phosphotyrosine proteome relates to core pathways and virulence. PLoS Pathog. 9, e1003403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Olsen J. V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. (2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 69. Wong D., Chao J. D., Av-Gay Y. (2013) Mycobacterium tuberculosis-secreted phosphatases: from pathogenesis to targets for TB drug development. Trends Microbiol. 21, 100–109 [DOI] [PubMed] [Google Scholar]
- 70. de Castro E., Sigrist C. J., Gattiker A., Bulliard V., Langendijk-Genevaux P. S., Gasteiger E., Bairoch A., Hulo N. (2006) ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34, W362–W365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Russo T. A., Luke N. R., Beanan J. M., Olson R., Sauberan S. L., MacDonald U., Schultz L. W., Umland T. C., Campagnari A. A. (2010) The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect. Immun. 78, 3993–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nakar D., Gutnick D. L. (2003) Involvement of a protein tyrosine kinase in production of the polymeric bioemulsifier emulsan from the oil-degrading strain Acinetobacter lwoffii RAG-1. J. Bacteriol. 185, 1001–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ilan O., Bloch Y., Frankel G., Ullrich H., Geider K., Rosenshine I. (1999) Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 18, 3241–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Preneta R., Jarraud S., Vincent C., Doublet P., Duclos B., Etienne J., Cozzone A. J. (2002) Isolation and characterization of a protein-tyrosine kinase and a phosphotyrosine-protein phosphatase from Klebsiella pneumoniae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 131, 103–112 [DOI] [PubMed] [Google Scholar]
- 75. Kimura Y., Kato T., Mori Y. (2012) Function analysis of a bacterial tyrosine kinase, BtkB, in Myxococcus xanthus. FEMS Microbiol. Lett. 336, 45–51 [DOI] [PubMed] [Google Scholar]
- 76. Milling A., Babujee L., Allen C. (2011) Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS ONE 6, e15853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Niemeyer D., Becker A. (2001) The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J. Bacteriol. 183, 5163–5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mijakovic I., Poncet S., Boël G., Mazé A., Gillet S., Jamet E., Decottignies P., Grangeasse C., Doublet P., Le Maréchal P., Deutscher J. (2003) Transmembrane modulator-dependent bacterial tyrosine kinase activates UDP-glucose dehydrogenases. EMBO J. 22, 4709–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jers C., Pedersen M. M., Paspaliari D. K., Schütz W., Johnsson C., Soufi B., Macek B., Jensen P. R., Mijakovic I. (2010) Bacillus subtilis BY-kinase PtkA controls enzyme activity and localization of its protein substrates. Mol. Microbiol. 77, 287–299 [DOI] [PubMed] [Google Scholar]
- 80. Macek B., Mijakovic I., Olsen J. V., Gnad F., Kumar C., Jensen P. R., Mann M. (2007) The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6, 697–707 [DOI] [PubMed] [Google Scholar]
- 81. Horn N., Wegmann U., Dertli E., Mulholland F., Collins S. R., Waldron K. W., Bongaerts R. J., Mayer M. J., Narbad A. (2013) Spontaneous mutation reveals influence of exopolysaccharide on Lactobacillus johnsonii surface characteristics. PLoS ONE 8, e59957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Soulat D., Grangeasse C., Vaganay E., Cozzone A. J., Duclos B. (2007) UDP-acetyl-mannosamine dehydrogenase is an endogenous protein substrate of Staphylococcus aureus protein-tyrosine kinase activity. J. Mol. Microbiol. Biotechnol. 13, 45–54 [DOI] [PubMed] [Google Scholar]
- 83. Lomas-Lopez R., Paracuellos P., Riberty M., Cozzone A. J., Duclos B. (2007) Several enzymes of the central metabolism are phosphorylated in Staphylococcus aureus. FEMS Microbiol. Lett. 272, 35–42 [DOI] [PubMed] [Google Scholar]
- 84. Rubens C. E., Heggen L. M., Haft R. F., Wessels M. R. (1993) Identification of cpsD, a gene essential for type III capsule expression in group B streptococci. Mol. Microbiol. 8, 843–855 [DOI] [PubMed] [Google Scholar]
- 85. Bender M. H., Yother J. (2001) CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276, 47966–47974 [DOI] [PubMed] [Google Scholar]
- 86. Minic Z., Marie C., Delorme C., Faurie J. M., Mercier G., Ehrlich D., Renault P. (2007) Control of EpsE, the phosphoglycosyltransferase initiating exopolysaccharide synthesis in Streptococcus thermophilus, by EpsD tyrosine kinase. J. Bacteriol. 189, 1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moreira L. M., Hoffmann K., Albano H., Becker A., Niehaus K., Sá-Correia I. (2004) The gellan gum biosynthetic genes gelC and gelE encode two separate polypeptides homologous to the activator and the kinase domains of tyrosine autokinases. J. Mol. Microbiol. Biotechnol 8, 43–57 [DOI] [PubMed] [Google Scholar]
- 88. Johnson D. L., Stone C. B., Bulir D. C., Coombes B. K., Mahony J. B. (2009) A novel inhibitor of Chlamydophila pneumoniae protein kinase D (PknD) inhibits phosphorylation of CdsD and suppresses bacterial replication. BMC Microbiol. 9, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Reisinger S. J., Huntwork S., Viollier P. H., Ryan K. R. (2007) DivL performs critical cell cycle functions in Caulobacter crescentus independent of kinase activity. J. Bacteriol. 189, 8308–8320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jadeau F., Grangeasse C., Shi L., Mijakovic I., Deleage G., Combet C. (2012) BYKdb: the Bacterial protein Tyrosine Kinase database. Nucleic Acids Research 40, D321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]





