Background: Cellular cues that regulate intestinal stem cell (ISC) apoptosis are unknown.
Results: Toll-like-receptor 4 (TLR4) activation on ISCs induces endoplasmic reticulum (ER) stress, leading to ISC apoptosis and necrotizing enterocolitis (NEC).
Conclusion: TLR4-induced ER stress in ISCs leads to apoptosis and NEC.
Significance: This is the first study revealing that ER stress in ISCs via immune receptors induces NEC.
Keywords: Inflammation, Innate Immunity, Necrotizing Enterocolitis, Sepsis, Toll-like Receptors (TLR)
Abstract
The cellular cues that regulate the apoptosis of intestinal stem cells (ISCs) remain incompletely understood, yet may play a role in diseases characterized by ISC loss including necrotizing enterocolitis (NEC). Toll-like receptor-4 (TLR4) was recently found to be expressed on ISCs, where its activation leads to ISC apoptosis through mechanisms that remain incompletely explained. We now hypothesize that TLR4 induces endoplasmic reticulum (ER) stress within ISCs, leading to their apoptosis in NEC pathogenesis, and that high ER stress within the premature intestine predisposes to NEC development. Using transgenic mice and cultured enteroids, we now demonstrate that TLR4 induces ER stress within Lgr5 (leucine-rich repeat-containing G-protein-coupled receptor 5)-positive ISCs, resulting in crypt apoptosis. TLR4 signaling within crypts was required, because crypt ER stress and apoptosis occurred in TLR4ΔIEC-OVER mice expressing TLR4 only within intestinal crypts and epithelium, but not TLR4ΔIEC mice lacking intestinal TLR4. TLR4-mediated ER stress and apoptosis of ISCs required PERK (protein kinase-related PKR-like ER kinase), CHOP (C/EBP homologous protein), and MyD88 (myeloid differentiation primary response gene 88), but not ATF6 (activating transcription factor 6) or XBP1 (X-box-binding protein 1). Human and mouse NEC showed high crypt ER stress and apoptosis, whereas genetic inhibition of PERK or CHOP attenuated ER stress, crypt apoptosis, and NEC severity. Strikingly, using intragastric delivery into fetal mouse intestine, prevention of ER stress reduced TLR4-mediated ISC apoptosis and mucosal disruption. These findings identify a novel link between TLR4-induced ER stress and ISC apoptosis in NEC pathogenesis and suggest that increased ER stress within the premature bowel predisposes to NEC development.
Introduction
The tightly regulated lifespan of the intestinal stem cell compartment is of critical importance for maintaining the integrity of the epithelial lining, as manifest by the fact that a variety of gastrointestinal diseases develop under conditions of increased intestinal stem cell loss (1). For instance, necrotizing enterocolitis (NEC)3 is a debilitating and often life-threatening disease of premature infants whose pathogenesis remains incompletely understood and that has been attributed in part to an acquired loss of stem cells within the premature gut (2, 3), resulting in a profound inability to heal the damaged intestinal mucosa (4–7). In seeking to understand how intestinal stem cell loss may occur in NEC, several groups, including our own, have identified that NEC occurs in the setting of exaggerated activation of the LPS receptor, namely Toll-like receptor 4 (TLR4), on intestinal epithelial cells (5, 8), because mice lacking TLR4 within the intestinal epithelium are protected from NEC development (9), whereas patients and humans with NEC show elevated intestinal TLR4 expression and signaling (10). In seeking to understand the precise mechanisms by which TLR4 activation in the gut can lead to NEC, we have recently identified that TLR4 is expressed at high levels on Lgr5-positive intestinal stem cells (11), that TLR4 regulates intestinal epithelial differentiation (9), and that exaggerated activation of TLR4 leads to apoptosis of intestinal stem cells through the up-regulation of PUMA (p53 upregulated modulator of apoptosis) (11). Importantly, however, the fundamental mechanisms by which TLR4 signaling could lead to deleterious effects on ISCs in the first place and the reasons for which premature infants may be more susceptible to NEC development than full term infants remain largely unexplored.
In seeking to address these questions, we have examined studies in other systems that have shed light on the processes by which proinflammatory signaling leads to apoptosis through the induction of endoplasmic reticulum (ER) stress. ER stress occurs after the accumulation of large amounts of unfolded proteins within the lumen of the ER (12). In response to elevated ER stress, the cell initiates the unfolded protein response, a signaling network composed of PERK, IRE1α, and ATF6, which serves to limit ER stress and maintain homeostasis (13). It is noteworthy that exaggerated ER stress has been linked to the development of colitis in recent studies (14–16) and that TLR4 signaling has been associated with ER stress induction in hematopoietic cells (17). However, a potential role for pathological ER stress in the intestinal stem cells in response to TLR4 activation in the pathogenesis of NEC—or any disease—has received little attention. Moreover, the degree to which the induction of ER stress within the intestinal stem cells induces apoptosis, the pathways involved, and the subsequent effects on intestinal inflammation, if any, remain largely unknown.
We now hypothesize that TLR4 signaling could lead to the induction of pathological ER stress within intestinal stem cells, leading to their apoptosis and to the development of NEC. We further hypothesize that higher baseline ER stress within the premature intestine could explain in part the predisposition of the premature infant to the development of NEC. In support of this hypothesis, we now report a novel link between TLR4-MyD88 signaling and PERK-CHOP activation in intestinal stem cells leading to NEC development in a process that in part requires activation of PUMA. We further show that the higher baseline ER stress of the premature mouse and human intestine places them at risk for NEC development after TLR4 becomes unexpectedly activated under the pressures of colonizing microbes outside the womb.
EXPERIMENTAL PROCEDURES
Cell, Materials, and Reagents
The small intestinal crypt derived cell line IEC-6 (American Type Culture Collection) was made deficient in specific stress genes by transduction of lentiviral particles containing gene specific shRNAs (Open Biosystems) Atf6, Xbp1, Perk, Chop, Myd88, or Trif shRNA using the four-plasmid lentiviral packaging system in permissive HEK293 cells (Invitrogen) as we previously described (11). PUMA-deficient IEC-6 cells were generated similarly, as we described (11). In all cases, control cells were treated with scrambled shRNA lentiviruses as controls as per our prior publications (11, 18). Stable integration of lentiviruses was obtained by selection using puromycin-containing media (5 μg/ml), and complete knockdown of the gene of interest was verified by RT-PCR or SDS-PAGE.
Antibodies and Other Reagents
Sources of antibodies and other reagents included: BiP (Abcam), cleaved caspase-3 (CC3) (Cell Signaling), PERK (Santa Cruz), rhodamine phalloidin and DAPI (Invitrogen), phosphorylated PERK (P-PERK) (Cell Signaling), CHOP (Cell Signaling), thapsigargin (0.5 μm for cells, 0.5 μg/kg for mice) and salubrinal (1 mg/kg) (Tocris Biosciences; Acros Organics), and Bay11 (2 μm cells; Calbiochem). LPS (Escherichia coli 0111:B4 purified by gel filtration chromatography, >99% pure; Sigma-Aldrich) was used at concentrations that we have shown to be present in mice and humans with NEC (50 μg/ml cells, 5 mg/kg for mice) (5). Treatments of LPS were of 6 h duration unless otherwise stated.
Primary intestinal cultures (enteroids) were isolated and maintained according to the methods of Sato et al. (19). SDS-PAGE and confocal microscopy were performed as in Ref. 18. Quantitative real time PCR was performed using the Bio-Rad CFX96 real time system (5) using the primers listed in Table 1.
TABLE 1.
The following primers were utilized in the current study for genotyping and RT-PCR analysis
| Gene | Species | Forward sequence | Reverse sequence | Amplicon size |
|---|---|---|---|---|
| bp | ||||
| ATF6 | Mouse/rat | ATTCTCAGCTGATGGCTGTCCAGT | TGGTAACTTCCAGGCGAAGCGTAA | 100 |
| ATF6KD | Rat | AGTGAGCTGCAGGTGTATTACGCT | TTCCCTGGAGTTGCAGTACAAGGT | 513 |
| CAG promoter | Mouse | CTCTGCTAACCATGTTCATGC | GCTCAATTGCTTGTCTCAGAAG | 833 |
| CHOPKD | Rat | GAGTCTAATACGTCGATCATACC | TTGATTCTTCCTCTTCGTTTCC | 300 |
| Cre | Mouse | GTTCGCAAGAACCTGATGGACA | CTAGAGCCTGTTTTGCACGTTC | 339 |
| IL-6 | Mouse/rat | GGCTAAGGACCAAGACCATCCAA | TCTGACCACAGTGAGGAATGTCCA | 138 |
| iNOS | Mouse/rat | CTGCTGGTGGTGACAAGCACATTT | ATGTCATGAGCAAAGGCGCAGAAC | 167 |
| Human | AATGAGTCCCCGCAGCCCCT | AGTCATCCCGCTGCCCCAGT | 143 | |
| PERK and PERKKD | Rat | GGCCTCGAAGCGGCAGTGAG | CTCACCAGCCAGGCAGACGC | 283 |
| PUMA | Mouse/rat | GCAGTACGAGCGGCGGAGAC | GGGCGGGTGTAGGCACCTAGT | 149 |
| PUMAKD | GGCCCAGCCTGTAAAATACT | TCAATCTGATTTTATTGAAAAGGAA | 127 | |
| RPLO | Mouse/rat/human | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC | 143 |
| Tg-TLR4 | Mouse | ACTCCACACAGGCATAGAGTGTCT | TTTGAGAGGTGGTGTAAGCCATGC | 770 |
| TLR4KO | Mouse | AGAAAATGCCAGGATGATGC | TGTCATCAGGGACTTTGCTG | 164 |
| XBP1KD | Rat | CAGACTACGTGCGCCTCTG | AGGGAGGCTGGTAAGGAACT | 233 |
| XBP1s | Mouse | GAGTCCGCAGCAGGTGC | CAAAAGGATATCAGACTCAGAATCTGAA | 80 |
| Rat | CTGAGTCCGAATCAGGTGCAG | ATCCATGGGAAGATGTTCTGG | 80 | |
| Human | GAGTCCGTGCAGCAGGTGC | CCAGAACATCTCCCCATGGATTCTGG | 80 |
Transgenic Mice Generation and Treatment
All mice experiments were approved by the Animal Care and Use Committee of the University of Pittsburgh. C57BL-6, Myd88−/−, Trif−/−, CHOP−/−, ATF6−/−, Lgr5-EGFP-IRES-creERT2, and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo reporter (mT/mG) mice were from the Jackson Laboratory. TLR4−/− mice and mice in which TLR4 was selectively deleted from the intestinal epithelium (TLR4ΔIEC) were generated in our laboratory as recently described (9). Mice lacking Xbp1 in the intestinal epithelium (XBP1−/−) were generously provided by Dr. Richard Blumberg (Harvard) and Dr. Laurie Glimcher (Cornell).
For generation of mice selectively expressing TLR4 in the intestinal epithelium (TLR4ΔIEC-OVER), we generated a Cre-dependent Tlr4 expression cassette, loxP-eGFP-STOP-loxP-Tlr4-IRES-mCherry, which was subcloned into the pTRE-Tight vector immediately downstream of the Tet-responsive promoter. In parallel, the CAG promoter-driven tTA regulatory protein expression plasmid was constructed by replacing the CMV promoter in a pTet-Off Advance vector with the CAG promoter. The TLR4 expression and CAG-tTA transgenes were co-microinjected into the oocytes from C57BL/6 mice at the transgenic facility of the University of Pittsburgh. Founder transgenic mice harboring both tTA and Tg-TLR4 transgenes were confirmed by PCR analysis of genomic DNA. This breeding strategy and confirmation of the genotype and phenotype is shown in Fig. 1. TLR4-modified mice showed no differences in overall health or viability as compared with wild-type littermates and were bred at Mendelian ratios.
FIGURE 1.
Generation of mice expressing TLR4 selectively within the intestinal epithelium. A, schematic diagram showing the generation of TLR4ΔIEC-OVER transgenic mice. The positions of coding regions of genes in the expression cassette downstream of the TRE-mini-CMV and CAG promoter are shown. B, diagrammatic representation of the breeding scheme is shown, as described under “Experimental Procedures.” C, genomic PCR from tail tip DNA confirming the genotype of the indicated strain. D, RT-PCR showing the expression of TLR4 within the small intestine or lung of the indicated strain to validate the genotype of the transgenic mice, along with RPLO as the housekeeping gene. E, expression of IL-6 by qRT-PCR in the small intestines and lungs of mice of the indicated strain showing selective induction of IL-6 within the intestine but not the lung in the TLR4−/−;IECOVER (TLR4ΔIEC-OVER) mice. Sal, saline. *, p < 0.05 versus saline; **, p < 0.05 LPS-treated TLR4−/− versus wild type; ***, LPS-treated lung versus small intestine.
For generation of Lgr5 lineage tracing reporter mice, we bred the Lgr5-EGFP-IRES-creERT2 mice (Jackson Laboratory) to a mouse expressing loxp-flanked membrane Tomato/membrane Green (mT/mG) (Jackson Laboratory). Upon treatment with tamoxifen (5 mg/kg, 24 h), this strain expresses the green fluorescent protein mG in the Lgr5 progeny. This strategy allows for lineage tracing of the Lgr5-positive cells and provides greater image clarity than the original Lgr5-eGFP reporter mice, in which the intensity of eGFP as driven by the Lgr5 promoter is often relatively weak.
To achieve in vivo knockdown of the PERK gene, mice were gavage fed twice daily (50 μl of 103–104 Pfu/ml) with purified lentiviral particles expressing Perk-ShRNA (The RNAi Consortium (TRC) shRNA clones; Thermo Scientific), which were generated using, the ViraPowerTM HiPerformTM lentiviral expression systems (Invitrogen) in permissive HEK 293 cells, according to techniques that we have utilized successfully for other genes (11). Knockdown was assessed in mucosal scrapings of the distal ileum by SDS-PAGE 4 days after the initial administration of lentivirus and was not seen in mice injected with lentivirus expressing scrambled shRNA.
Induction of Neonatal Necrotizing Enterocolitis in Wild-type and Gene-deficient Mouse Strains
NEC was induced in 5–10-day-old mice as we have described and validated in previous reports (3, 5, 11, 18, 20, 21) using formula gavage (Similac Advance infant formula (Abbott Nutrition):Esbilac (PetAg) canine milk replacer, 2:1) five times per day and hypoxia (5% O2, 95% N2) for 10 min in a hypoxic chamber (Billups-Rothenberg) twice daily for 4 days. This protocol results in the development of patchy necrosis and cytokine induction that mimics that seen in human NEC (22). Disease severity was determined on histologic sections of the terminal ileum by a pediatric pathologist blinded to the study condition according to our previously published scoring system from 0 (normal) to 3 (severe) (5).
Determination of Enterocyte Apoptosis
Enterocyte apoptosis was determined in IEC-6 cells after 6 h of treatment with LPS under concentrations that we have measured in NEC (5) (50 μg/ml) by immunostaining with antibodies to cleaved caspase-3 and performing confocal immunofluorescence analysis. In parallel, TUNEL staining was performed in paraformaldehyde (4%) fixed cells or 5-μm-thick paraffin sections of tissues according to the manufacturer's instructions (Roche Applied Science). The number of cleaved caspase-3 or TUNEL-positive cells was identified by an investigator blinded to the treatment groups using Metamorph software (Molecular Devices Corp.) and expressed as the number of cleaved caspase-3 or TUNEL-positive cells per high power field, with more than 50 fields/experiment studied and more than 100 cells/field.
Enterocyte apoptosis in vivo within the crypts was determined by measuring the number of crypt cells that are positive for cleaved caspase-3 by confocal microscopy per high power field. Over 50 fields per sample were evaluated, and each experiment was performed in triplicate.
In Utero Microinjection of the Fetal Intestine
Backscatter ultrasound-guided microinjection to deliver reagents directly into the fetal gut was performed as we recently described (10). At embryonic day 17.5, the uterus of a pregnant C57BL/6 mouse was exposed by laparotomy, and a fenestrated dish was placed over the uterus. A single embryo (one uterine saccule) was brought through the fenestration. The indicated reagent was injected directly into the fetal stomach, via a glass syringe, using the Vevo Imaging Station under ultrasound guidance (VisualSonics). In all cases, we injected half of the fetuses and preserved the remaining half as controls. Fetuses were delivered by cesarean section 24 h later. The total injected volume was 5 μl. In control experiments, the spontaneous fetal demise rate was under 20%, as in our prior publications (10, 22).
Human Samples
All human tissue was obtained and processed as discarded tissue via waiver of consent with approval from the University of Pittsburgh Institutional Review Board and in accordance with the University of Pittsburgh anatomical tissue procurement guidelines. Intestinal samples were obtained from human neonates undergoing resection for NEC, for unrelated indications (control), or at time of stoma closure, or from aborted fetuses and processed as we have described (10, 22).
Statistical Analysis
Statistical analysis was performed using SPSS 13.0 software. Analysis of variance was used for comparisons for experiments involving more than two experimental groups. Two-tailed Student's t test was used for comparison for experiments consisting of two experimental groups. For analysis of the severity of NEC, chi-square analysis was performed.
RESULTS
TLR4 Activation Induces ER Stress and Apoptosis in IEC-6 Enterocytes and Lgr5-positive Intestinal Stem Cells within Intestinal Crypts
We first investigated whether TLR4 activation could induce ER stress in intestinal stem cells and, if so, sought to determine the mechanisms involved. To do so, we treated the intestinal crypt-like cell line IEC-6, which we have previously shown to express TLR4 (5, 11, 23) with LPS and determined the subsequent effects on ER stress and apoptosis. As shown in Fig. 2, LPS caused an increase in ER stress in IEC-6 cells, as demonstrated by increased expression of the ER resident molecular chaperone BiP by immunoconfocal microscopy (Fig. 2A, panels i and ii) and SDS-PAGE (Fig. 2A, panel iv) and by increased expression of the ER stress-signaling molecules: spliced XBP1 (X-box binding protein 1) (XBP1s) (24), CHOP, P-PERK, and ATF6 (activating transcription factor 6) by SDS-PAGE or RT-PCR (Fig. 2A, panels iv–vi). The LPS-induced increase in ER stress was associated with an induction in apoptosis in IEC-6 cells, as measured by increased expression of CC3 by immunoconfocal microscopy (Fig. 2A, panels vii–viii and xiii) and SDS-PAGE (Fig. 2A, panel iv) and confirmed by the increased expression of TUNEL (Fig. 2A, panels x, xi, and xiv). These findings were confirmed in vivo, because LPS treatment caused an increase in ER stress within intestinal crypts of mice, as demonstrated by the increased expression of BiP within the intestinal crypts (Fig. 2B, panels i and ii) and confirmed by SDS-PAGE of mucosal lysates (Fig. 2B, panel iv) for BiP, CHOP, P-PERK, and qRT-PCR for expression of XBP1s and ATF6 (Fig. 2B, panels v and vi), and an induction in crypt apoptosis, as measured by increased expression of cleaved caspase-3 and TUNEL within the crypts (Fig. 2B, panels iv, vii, viii, x, xi, xiii, and xiv). As a positive control linking the induction of ER stress with apoptosis, the direct induction of ER stress through pretreatment with thapsigargin, which induces ER stress by inhibiting the ER Ca2+ ATPase (25), induced ER stress (Fig. 2A, panels iii–vi) and apoptosis (Fig. 2A, panels ix and xii–xiv) in IEC-6 cells and intestinal crypts (Fig. 2B, panels iii–vi, ix, and xii-xiv).
FIGURE 2.
TLR4 activation induces ER stress and apoptosis in IEC-6 enterocytes and intestinal crypts. A and B, representative confocal images of IEC-6 cells (A) or intestinal crypts in C57BL/6 mice (B) stained for BiP (panels i–iii), cleaved caspase-3 (panels vii–ix), or TUNEL (panels x-xii). Cells/mice were treated as indicated with LPS (50 μg/ml cells 1 h for P-PERK and 6 h for BIP, CHOP, and CC3; 5 mg/kg mice, 3 h for P-PERK and 6 h for BIP, CHOP, and CC3) or thapsigargin (0.5 μm cells, 0.5 mg/kg mice for time points similar to LPS treatments); SDS-PAGE showing BiP, CHOP, P-PERK, or CC3 and loading control β-actin in IEC-6 cells (A, panel iv) or wild-type mice (B, panel iv); qRT-PCR for spliced XBP1s or ATF6 in IEC-6 cells (A, panels v and vi) or mice (B, panels v and vi) under the indicated treatment. Panels xiii and xiv, quantification of cleaved caspase-3 positive or TUNEL positive IEC-6 cells or crypts/high power field. *, p < 0.05 control/saline or thapsigargin versus LPS. The results are representative of three separate experiments with three to five mice per group. The data are the means ± S.E. Scale bars, 10 μm. Arrows, apoptotic cells. Ctrl, control; Thaps, thapsigargin.
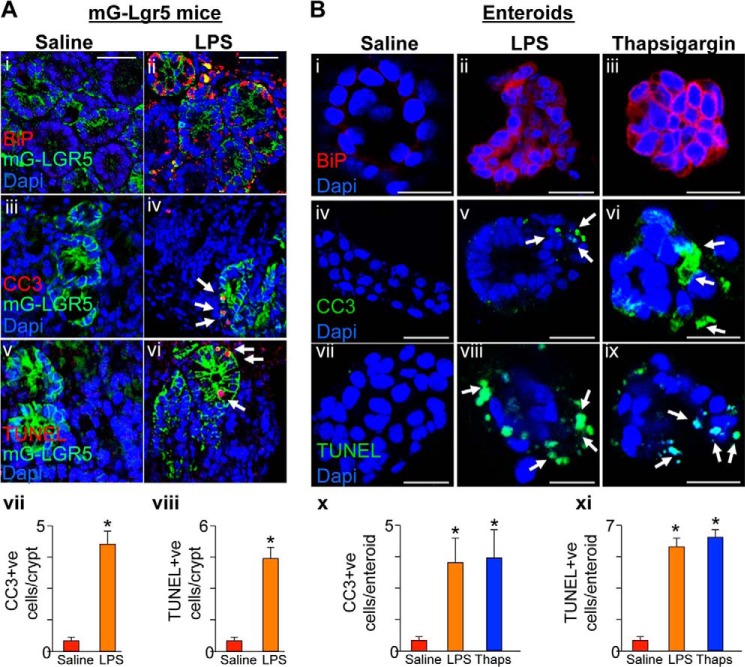
We next sought to determine whether TLR4 activation induced apoptosis via ER stress in the intestinal stem cells as opposed to other cell populations present within the intestinal crypts (26). To do so, we performed lineage tracing experiments using a novel reporter mouse line, which we generated (Lgr5-mG), which expresses the green fluorescent mG protein upon injection of tamoxifen only in the Lgr5 expressing intestinal stem cells and their progeny. All studies were performed 24 h after tamoxifen injection, ensuring that mGFP expression was confined to the intestinal stem cells and transit amplifying cells. As shown in Fig. 3A, the induction in ER stress in response to LPS as manifest by increased expression of BiP (Fig. 3A, panels i and ii) and the induction in apoptosis as measured by increased expression of CC3 and TUNEL (Fig. 3A, panels iii–viii) were both found to overlap with green mG-expressing cells present within the crypts, confirming that LPS induces ER stress and apoptosis within Lgr5 progeny. To further define the link between TLR4-induced ER stress and apoptosis of intestinal stem cells, we next harvested and maintained in culture enteroids, which represent a population of intestinal crypt cells that is enriched in Lgr5 (27) and which we have recently shown to express TLR4 (11). Treatment of enteroids with LPS increased ER stress as manifest by increased expression of BiP (Fig. 3B, panels i and ii), which was associated with increased apoptosis (Fig. 3B, panels iv, v, vii, viii, x, and xi). As a positive control, the induction of ER stress with thapsigargin increased ER stress (Fig. 3B, panel iii) and apoptosis (Fig. 3B, panels vi, ix, x, and xi) in enteroids. Taken together, these data indicate that TLR4 activation leads to the induction of ER stress in intestinal stem cells and intestinal stem cell apoptosis. We next sought to define the mechanisms involved in greater detail.
FIGURE 3.
TLR4 activation induces ER stress and apoptosis in Lgr5-positive stem cells and cultured enteroids. A, representative confocal micrographs of intestinal crypts in mG-Lgr5 lineage tracing reporter mice treated with saline (panels i, iii, and v) or LPS (5 mg/kg 6 h prior; panels ii, iv, and vi) and immunostained for BiP (panels i and ii), CC3 (panels iii and iv), or TUNEL (panels v and vi). Panels vii and viii, blinded quantification of apoptosis over 50 high power fields. B, representative confocal micrographs of enteroids from the ilea of mice treated as indicated and immunostained with BiP (panels i–iii), cleaved caspase-3 (panels iv–vi), or TUNEL (panels vii–ix). Panels x and xi, blinded quantification of apoptosis over 50 high power fields. LPS treatment included enteroids (25 μg/ml) for 6 h and thapsigargin (0.5 μm) for 6 h. **, p < 0.05 saline or thapsigargin versus LPS. The results are representative of at least three separate experiments with three to five mice per group. The data are means ± S.E. Scale bars, 10 μm. Arrows, apoptotic cells.
TLR4-induced ER Stress in the Intestinal Crypts Requires the Adapter Molecule MyD88
TLR4 is known to signal through the adaptor protein MyD88 (myeloid-differentiation primary response gene 88), which leads to the induction of NFκB, and TRIF (TIR domain-containing adapter-inducing interferon-β), which induces other proinflammatory molecules, including interferon-β (28). IEC-6 cells deficient in TRIF or MyD88 were generated through lentiviral delivery of specific shRNA, resulting in the knockdown of gene expression as assessed by SDS-PAGE (Fig. 4A) but no other gene (not shown). As shown in Fig. 4B, knockdown of MyD88 in IEC-6 cells blocked the LPS-induced increase in ER stress as detected by immunostaining of cells for BiP (Fig. 4B, panel i) and SDS-PAGE analysis of IEC-6 cells lysates for expression of BiP, P-PERK, and CHOP (Fig. 4D) and expression of ATF6 by qRT-PCR (Fig. 4F) and also prevented LPS-induced apoptosis (Fig. 4, B, panels iv and vii; D; G; and H), suggesting a role for the MyD88-NFκB pathway in inducing the TLR4-mediated ER stress response (for comparison, the induction in ER stress and apoptosis in wild-type cells is shown in Fig. 2). Consistent with a role for the MyD88-NFκB signaling pathway, pretreatment of wild-type IEC-6 cells with the NFκB inhibitor Bay-11 (2 μm, 30 min prior) significantly reduced LPS-induced ER stress (Fig. 4B, panel ii) and apoptosis (Fig. 4B, panels v and viii; G; and H). Importantly, TRIF deficiency did not measurably alter the effects of LPS on ER stress (Fig. 4B, panel iii; D; and F) and apoptosis (Fig. 4B, panels vi and ix; D; G; and H) at these time points. These pathways were also relevant in vivo, because LPS caused an increase in ER stress (Fig. 4C, panels i and ii; E; and F) and apoptosis (Fig. 4C, panels iii–vi; E; G; and H) in the crypts of Trif−/− mice but not MyD88−/− mice. Taken together, these findings indicate that MyD88 is required for the TLR4-induced generation of ER stress and apoptosis within IEC-6 cells and the intestinal crypts. We next sought to determine whether the TLR4-mediated induction in crypt ER stress was required for the induction of apoptosis and to evaluate the signaling pathways involved.
FIGURE 4.
TLR4-induced ER stress in the intestinal crypts requires the adapter molecule MyD88. A, SDS-PAGE showing the knockdown of TRIF (panel i) and MyD88 (panel ii) in IEC-6 cells. Blots were stripped and reprobed for β-actin. B and C, representative confocal micrographs of IEC-6 cells (B) and crypts (C) from terminal ileum of mice deficient in MyD88 or TRIF or that were treated with Bay11 (2 μm, 30 min prior), treated with LPS (50 μg/ml for 6 h, except 1 h for P-PERK for cells, 5 mg/kg for 6 h, except 3 h for P-PERK for mice) and stained for BiP (B, panels i–iii, and C, panels i and ii), CC3 (B, panels iv–vi, and C, panels iii and iv), or TUNEL (B, panels vii–ix, and C, panels v and vi). D and E, SDS-PAGE for BiP, P-PERK, CHOP, or CC3 in IEC-6 cells (D) or mice (E) after treatment with LPS (conditions as in B and C); blots reprobed for actin. F, qRT-PCR showing the expression of ATF6 in cells (left panel) or mice (right panel) under the conditions indicated, corresponding to those in B and C. G and H, blinded evaluation of apoptosis via quantification of CC3 (G) or TUNEL (H) in LPS-treated IEC-6 cells and mice as indicated. *, p < 0.05 TRIF-deficient versus MyD88-deficient groups. The results are representative of three separate experiments with 50 high power fields and three to five mice per group. The data are means ± S.E. Scale bars, 10 μm.
TLR4-induced ER Stress Leads to Apoptosis in IEC-6 Cells and Intestinal Crypts via the PERK/CHOP Signaling Pathway
To determine whether the increase in ER stress was required for the induction in apoptosis in IEC-6 cells and intestinal crypts in response to TLR4 activation, we next treated cells or mice with LPS after selective knockdown or deletion of each of the key gene constituents of the ER stress signaling pathway (i.e., ATF6 (activating-transcription factor 6), XBP1 (X-box-binding protein 1) and PERK (protein kinase-related-PKR-like ER kinase) (14)). To knock down these ER stress genes, IEC-6 cells or wild-type mice were treated with lentivirus containing shRNA for the indicated gene or scrambled shRNA as described under “Experimental Procedures”; assessment by RT-PCR demonstrated highly efficient knockdown of the indicated ER stress gene in cells or mice treated with shRNA to the gene of interest, whereas treatment with scrambled shRNA had no effect as shown in Fig. 5 (A, panels i–iv, and B). As shown in Fig. 5C, LPS treatment of IEC-6 cells lacking ATF6 or XBP1 induced apoptosis (Fig. 5C, panels i, ii, v, vi, vii, and x) to a similar degree as in wild-type cells (see images and quantification in Fig. 2), whereas the knockdown of Perk significantly attenuated LPS-induced apoptosis (Fig. 5C, panels iii, v, viii, and x), demonstrating the importance of PERK signaling for TLR4-induced enterocyte apoptosis. To confirm this finding, we also knocked down CHOP, which functions downstream of PERK (29) from IEC-6 cells, and observed that CHOP-deficient IEC-6 cells also failed to induce apoptosis (Fig. 5C, panels iv, v, ix, and x) after LPS treatment. To evaluate which, if any, ER stress gene pathways were important in inducing LPS-mediated apoptosis in the intestinal crypts in vivo, we next obtained mice deficient in ATF6, XBP1, and CHOP and deleted PERK from the intestinal mucosa of wild-type mice through the administration of lentivirus expressing shRNA to PERK, which effectively reduced PERK expression from intestinal lysates as demonstrated by SDS-PAGE (Fig. 5B). As shown in Fig. 5D, LPS induced apoptosis in crypts in mice that were deficient in ATF6 and XBP1 at levels similar to wild-type mice (Fig. 5D, panels i, ii, v, vi, vii, and x; see controls in Fig. 2), but not in crypts from mice deficient in either PERK (Fig. 5D, panels iii, v, viii, and x) or its downstream partner CHOP (Fig. 5D, panels iv, v, ix, and x). In all cases, the administration of lentivirus expressing empty vector had no effect on PERK expression (Fig. 5B) or LPS-induced crypt apoptosis (not shown). Taken together, these findings demonstrate that the PERK/CHOP signaling pathway is important in TLR4-mediated apoptosis in IEC-6 cells and intestinal crypts.
FIGURE 5.
TLR4-induced ER stress leads to apoptosis in IEC-6 cells and intestinal crypts via the PERK-CHOP signaling pathway. A, RT-PCR showing stable knockdown of the indicated gene by mRNA expression in IEC-6 cells that had been transduced with lentivirus expressing target or scrambled (control) shRNA. B, representative SDS-PAGE showing mucosal scrapings from three separate mice that were orally fed with lentivirus expressing either empty vector of shRNA for 3 consecutive days (200 μl of 103–104 PFU/ml) and analyzed 24 h after the last lentiviral feeding. The blots are probed by SDS-PAGE for PERK, stripped, and then reprobed for actin. C and D, panels i–iv and vi–ix, representative confocal images of gene-modified IEC-6 cells (C) or crypts (D) from mice in which the indicated gene had been deleted and which were then treated with LPS (50 μg/ml for 6 h for cells, 5 mg/kg for 6 h for mice) and stained for cleaved caspase-3 or TUNEL as indicated. Arrows indicate apoptotic cells. C and D, panels v and x, blinded quantification of apoptosis in IEC-6 cells or crypts. *, p < 0.05 versus cells (C, panels v and x) or mice (D, panels v and x) deficient in ATF6 or XBP1. The results are representative of three separate experiments in triplicate with over 50 high power fields analyzed. The data are means ± S.E. Scale bars, 10 μm.
TLR4-mediated Generation of ER Stress within Intestinal Crypts Induces NEC via the PERK/CHOP Pathway
The development of NEC is associated with increased apoptosis of intestinal crypts as a consequence of exaggerated TLR4 signaling (10, 11) through mechanisms that remain incompletely understood. We next sought to define whether the induction of apoptosis in the intestinal crypts was a consequence of TLR4-induced ER stress, and if so, whether the TLR4-induced increase in ER stress within the crypts was critical to the development of NEC. As shown in Fig. 6, the induction of NEC, as achieved using a combination of formula gavage and intermittent hypoxia, resulted in the development of mucosal inflammation in wild-type mice as measured by histology (Fig. 6A, panels i and ii), by blind scoring of the histologic appearance of the terminal ileum (Fig. 6B, panel i), and by increased expression of the proinflammatory molecule iNOS in the intestinal epithelium, which has been shown to correlate with NEC severity (30, 31) (Fig. 6B, panel ii). The induction of NEC in wild-type mice was also associated with a marked increase in ER stress within the intestinal crypts, as measured by increased immunostaining for BiP (Fig. 6A, panels vii and viii) and XBP1s expression by qRT-PCR (Fig. 6C, panel i), and an increase in crypt apoptosis, as measured by expression of cleaved caspase-3 within the crypts (Fig. 6, A, panels xiii and xiv; and C, panel ii).
FIGURE 6.
TLR4-mediated generation of ER stress within intestinal crypts induces NEC development via the PERK/CHOP pathway. A, representative photomicrographs of the hematoxylin- and eosin-stained terminal ileum (panels i–vi) and confocal images of ileal crypts from newborn intestine (panels vii–xviii) from wild-type mice, mice lacking TLR4 within the intestinal epithelium (TLR4ΔIEC), mice expressing TLR4 only within the intestinal epithelium (TLR4ΔIEC-OVER), mice lacking PERK in the intestinal epithelium (PERKKD), or mice globally deficient in CHOP (CHOP−/−). The mice were either breast fed (control) or induced to develop necrotizing enterocolitis as indicated. The slides were then stained for BiP (panels vii–xii), or CC3 (panels xiii–xviii). B, NEC severity as assessed by blinded histopathology NEC severity score (panel i) or expression of iNOS by qRT-PCR in the terminal ileum (panel ii). C, panel i, qRT-PCR in ileal mucosa of XBP1s. Panel ii, blinded evaluation of cleaved caspase-3 in the intestinal crypts in three consecutive experiments over 50 high power fields with three to five mice per experiment. In all graphs: *, p < 0.05 wild-type NEC versus control; #, p < 0.05 NEC wild type versus NEC in TLR4ΔIEC mice; ##, p < 0.05 NEC in TLR4ΔIEC-OVER versus control wild type; **, p < 0.05 NEC in PERK or CHOP-deficient mice versus NEC in wild-type mice. The data are means ± S.E. Scale bars, 10 μm. Arrows, apoptotic cells. Ctrl, control.
To confirm in detail whether TLR4 signaling within the intestinal epithelium was required for induction of crypt ER stress, apoptosis, and NEC development, we next studied NEC in mice that selectively lack TLR4 (TLR4ΔIEC) or selectively express TLR4 (TLR4ΔIEC-OVER) within the intestinal epithelium (and therefore intestinal crypts). The generation of TLR4ΔIEC mice by our group was recently reported (9), and the generation of the TLR4ΔIEC-OVER mice is shown in Fig. 1, in which TLR4 is expressed only on the intestinal epithelium, on a TLR4−/− background. The results shown in Fig. 6 confirm that TLR4 signaling within the intestinal epithelium is required for the induction of ER stress and apoptosis of the crypts, because TLR4ΔIEC mice were protected from NEC development (Fig. 6, A, panel iii, and B, panels i and ii), ER stress induction in the crypts (Fig. 6, A, panel ix, and C, panel i), and crypt apoptosis (Fig. 6, A, panel xv, and C, panel ii). Strikingly, when subjected to experimental NEC, TLR4ΔIEC-OVER mice show markedly increased NEC severity (Fig. 6, A, panel iv, and B, panels i and ii), ER stress (Fig. 6, A, panel x, and C, panel i), and crypt apoptosis (Fig. 6, A, panel xvi, and C, panel ii) as compared with wild-type mice, to levels greater than that in wild-type mice. Importantly, knockdown of Perk or Chop in mice (as achieved in Fig. 5B) significantly attenuated the severity of NEC (Fig. 6, A, panels v and vi, and B, panels i and ii) and led to a reduction of ER stress (Fig. 6, A, panels xi and xii, and C, panel i) and apoptosis within the intestinal crypts (Fig. 6, A, panels xvii and xviii, and C, panel ii). Taken together, these findings demonstrate that TLR4-induced ER stress within the intestinal crypts leads to apoptosis and NEC development through PERK/CHOP signaling.
Knockdown of PERK Attenuates TLR4-induced ER Stress and Apoptosis in the TLR4ΔIEC-OVER Mice
Based upon the results shown in Fig. 6, we next sought to determine whether the knockdown of PERK in vivo could attenuate TLR4-induced ER stress and apoptosis in the intestinal crypts of the TLR4ΔIEC-OVER mice in which TLR4 is expressed exclusively in the intestinal epithelium. To do so, we delivered lentiviruses containing PERK shRNA to the gastrointestinal tracts of newborn mice as in Fig. 6 and achieved significant knockdown of PERK but not TLR4 in the intestinal mucosa, as shown in Fig. 7A. Importantly, whereas the subsequent treatment with LPS caused a significant increase in ER stress and apoptosis in the intestinal crypts of TLR4ΔIEC-OVER mice compared with saline treated controls, as manifest by increased staining for BiP and TUNEL in the crypts (Fig. 7, B, panels i, ii, iv, and v; and C, panel i) and expression of XBP1 and ATF6 by RT-PCR (Fig. 7C, panels ii and iii), the inhibition of PERK in vivo significantly attenuated the degree of ER stress and apoptosis (Fig. 7, B, panels iii and vi, and C, panels i–iii). Taken together, these findings underscore the importance of PERK in mediating the increase in ER stress and apoptosis in response to TLR4 in the crypts of TLR4ΔIEC-OVER mice.
FIGURE 7.

Inhibition of PERK attenuates TLR4-induced ER stress and apoptosis in the TLR4ΔIEC-OVER mice. A, qRT-PCR showing the expression of TLR4 (panel i) or PERK (panel ii) in the small intestinal mucosa of TLR4ΔIEC-OVER mice after administration of lentiviruses containing PERK shRNA. *, p < 0.005 blue bars versus orange bars. The results are representative of three to five mice. B, representative confocal micrographs of intestinal crypts showing staining for BiP (panels i–iii) or TUNEL (panels iv–vi) in TLR4ΔIEC-OVER mice that had been treated with saline (panels i and iv) or LPS (panels ii and v) or in TLR4ΔIEC-OVER mice that had been administered by oral gavage lentiviruses expressing PERK shRNA for 3 consecutive days (200 μl of 103–104 PFU/ml) (panels iii and vi). C, panel i, blind quantification of apoptosis pertaining to groups depicted in B treated with saline or LPS as indicated. Panels ii and iii, qRT-PCR showing the expression of XBP1s (panel ii) or ATF6 (panel iii) in the intestinal mucosa of the indicated mice treated with either LPS or saline as shown. *, p < 0.05 saline versus LPS-treated TLR4ΔIEC-OVER mice; **, p < 0.05 TLR4ΔIEC-OVER mice versus TLR4ΔIEC-OVER mice + PERK shRNA. The results are representative of three separate experiments. The data are means ± S.E. Scale bars, 10 μm. Arrows, apoptotic cells. Sal, saline.
Increased Baseline ER Stress within the Fetal Bowel Predisposes to the Development of Necrotizing Enterocolitis in Mice and Humans
In the next series of studies, we sought to determine whether a higher level of ER stress could explain in part why premature infants are predisposed to the development of NEC in the first place. As shown in Fig. 8, human fetal intestine (Fig. 8A, panel i) was found to demonstrate higher ER stress within the intestinal crypts as compared with full term bowel as measured by increased immunostaining of BiP within crypts and XBP1s by RT-PCR (Fig. 8, A, panel iv versus panel vi; and D, panel i). Importantly, the degree of apoptosis was similar between fetal and full term human bowel, as measured by expression of cleaved caspase-3, (Fig. 8, A, panel vii versus panel ix; and D, panel ii), consistent with their normal histology and reflective of the absence of exaggerated TLR4 signaling when NEC is not present. By contrast, the presence of NEC in premature human infants (Fig. 8A, panel ii) was associated with increased ER stress within the crypts (Fig. 8, A, panel v, and D, panel i) and increased apoptosis (Fig. 8, A, panel viii, and D, panel ii) within the intestinal crypts. Given that we have shown that premature infant bowel displays high levels of TLR4 compared with term bowel (10, 18), these findings are consistent with the notion that exaggerated TLR4 signaling on the background of already increased ER stress within the premature gut can lead to crypt apoptosis and NEC. To determine therefore whether the increase in ER stress was required for the TLR4-induced apoptosis within the intestinal crypts of the premature gut, we next investigated a pregnant mouse model in which LPS was delivered directly into the lumen of the fetal bowel using ultrasound-guided microinjection. As shown in Fig. 8B, the fetal mouse intestine shows high ER stress at baseline compared with term mouse (Figs. 2B, panels i–vi; and 8, B, panel iv, and D, panel i) resembling the human situation (Fig. 8, A, panels iv and vi, and D, panel i). The delivery of LPS into the fetal bowel caused a significant increase in mucosal disruption (Fig. 8B, panel ii) and intestinal crypt ER stress (Fig. 8, B, panel v; C; and D, panel i), as well as apoptosis within the intestinal crypts (Fig. 8, B, panel viii, and D, panel ii), consistent with the human situation in which NEC develops in the premature gut. Strikingly, the co-delivery of the agent salubrinal, which was identified as a small molecule capable of protecting cells from ER stress-induced apoptosis (32), directly into the fetal bowel 24 h prior to harvest markedly reduced LPS-induced apoptosis (Fig. 8, B, panel ix; C; and D, panel ii) within the intestinal crypts. Taken together, these findings demonstrate that high baseline levels of ER stress present within the fetal bowel predispose to the development of crypt apoptosis upon TLR4 activation leading to the development of NEC and may provide a novel mechanism by which prematurity predisposes to the development of this devastating disease.
FIGURE 8.
Increased baseline ER stress within the fetal bowel predisposes to the development of necrotizing enterocolitis in mice and humans. A and B, representative photomicrographs of human (A) and mouse (B) terminal ileum (panels i–iii) and confocal images of ileal crypts (panels iv–ix) from human fetal intestine, a premature infant with NEC, full term infant, or E17 fetal mouse intestine injected intraintestinally with saline, LPS, or LPS + salubrinal as indicated and then stained for BiP (panels iv–vi) or cleaved CC3 (panels vii–ix). C, Western blot of P-PERK and CHOP in in utero injected fetal intestine. LPS intragastric dose was 5 μl of 1 mg/ml stock, and salubrinal intragastric dose was 1 mg/ml. The total injected volume was 5 μl. D, XBP1s expression by qRT-PCR in the ileal mucosa (panel i) blinded evaluation of apoptosis in the ileum of mouse and human tissue (panel ii). *, p < 0.05 versus human full term control; **, p < 0.05 mouse fetal saline versus mouse term control; ***, p < 0.05 mouse fetal LPS versus mouse saline and mouse LPS + salubrinal; #, p < 0.05 human NEC versus term control or fetal; ##, p < 0.05 mouse fetal LPS-injected versus saline or LPS + salubrinal injected fetal mice. The results are representative of three separate experiments with at least one mother per group and the injection of at least four pups. The data are means ± S.E. Scale bars, 10 μm. Arrows, apoptotic cells. Ctrl, control; Sal, saline; Salub, salubrinal.
PUMA Is Required for the Induction in Apoptosis in Response to TLR4-induced ER Stress in the Intestinal Crypts
In the final series of studies, we sought to interrogate in greater detail potential pathways linking the induction of ER stress after TLR4 signaling with the induction of apoptosis in the intestinal stem cells. To do so, we focused on the role of PUMA, which we and others have shown to be important in the pathways that govern apoptosis in the intestinal stem cells in response to exogenous stimuli (11, 33, 34), although we note that a role for PUMA in ER, stress-mediated apoptosis has not been determined. As shown in Fig. 9A, although LPS causes an induction of PUMA in wild-type IEC-6 cells (Fig. 9A, panel i) and mice (Fig. 9A, panel ii), the inhibition of PERK in either IEC-6 cells or the intestinal mucosa of mice significantly reduced LPS-induced PUMA expression (Fig. 9A, panels i and ii), suggesting that PERK is required for the induction of PUMA in response to LPS within the intestinal epithelium (evidence for PUMA knockdown in IEC-6 cells is shown in Fig. 9B). Importantly, compared with wild-type IEC-6 cells or mice, the inhibition of PUMA in either IEC-6 cells or the intestinal mucosa significantly reduced LPS-induced ER stress (Fig. 9, C, panels i and ii versus panels iv and v; D, panels i and ii; E, panels i and ii; and F) and apoptosis (Fig. 9, C, panels vii and viii versus panels x and xi; D, panels ii–iv; E, panel iii; and F), suggesting a role for PUMA and other proapoptotic pathways in the induction of ER stress after TLR4 activation. Consistent with this possibility, pretreatment of IEC-6 cells with the pan-caspase inhibitor Z-VAD reduces ER stress (Fig. 9, C, panel iii, and E, panels i and ii) and apoptosis (Fig. 9, C, panel ix, and E, panel iii) after TLR4 activation. In control experiments, pretreatment of IEC-6 cells with thapsigargin increased ER stress and apoptosis (Fig. 9, C, panels vi and xii, and E), confirming that the ER stress response pathway is otherwise intact in the PUMA-deficient cells. Taken together, these findings identify a novel pathway explaining the link between TLR4-induced ER stress and apoptosis through the induction of PUMA and, along with the results above, provide novel insights into mechanisms regulating intestinal stem cell death in necrotizing enterocolitis.
FIGURE 9.
PUMA is required for the induction in apoptosis in response to TLR4-induced ER stress in the intestinal crypts. A, qRT-PCR for PUMA in wild-type or PERK-deficient IEC-6 cells (panel i) or mice (panel ii) after treatment with saline (control) or LPS for 6 h as indicated. *, p < 0.05 versus control wild-type cells or mice; **, p < 0.01 versus LPS-treated wild-type cells or mice versus PERK-deficient cells or mice. The data are means ± S.E. The results are representative of three separate experiments. B, qRT-PCR for PUMA in wild-type or PUMA-deficient IEC-6 cells showing the extent of PUMA knockdown. *, p < 0.05 wild-type versus PUMAKD cells. C, representative confocal micrographs of wild-type or PUMA-deficient IEC-6 cells that were either untreated or treated with LPS, LPS + Z-VAD, or thapsigargin as indicated, then stained for BiP (panels i–vi) or TUNEL (panels vii–xii) as indicated. Arrows, apoptotic cells. D, representative confocal micrographs showing the crypts of PUMA−/− mice that were injected with saline or LPS (5 mg/kg for 6 h). Tissues were stained for BiP (panels i and ii) or TUNEL (panels iii and iv). Scale bars, 10 μm. E and F, qRT-PCR or quantification of apoptosis in wild-type or PUMA-deficient IEC-6 cells (E) or PUMA−/− mice (F) under the conditions indicated. *, p < 0.05 LPS versus control wild-type IEC-6 cells; #, p < 0.05 LPS versus LPS + Z-VAD IEC-6 cells; **, p < 0.05 thapsigargin versus other groups in PUMAKD cells. The results are representative of three separate experiments with over five mice per group or over 50 high power field per group as indicated. The data are means ± S.E. Ctrl, control; Thaps, thapsigargin.
DISCUSSION
We now present multiple independent lines of evidence to support our conclusion that TLR4 signaling induces ER stress in intestinal stem cells, which leads to their apoptosis and initiates the development of NEC. These include: 1) TLR4 activation induces ER stress and apoptosis of crypt like IEC-6 cells, enteroids, and intestinal crypts; 2) deletion of the ER stress genes PERK or CHOP results in a loss of apoptosis in response to TLR4 signaling; 3) TLR4 activation by LPS injection and overexpression of TLR4 within the intestinal crypts lead to a marked increase in ER stress and apoptosis of ISCs; 4) lineage tracing experiments demonstrate that TLR4 activation induces ER stress and apoptosis in Lgr5+ intestinal stem cells; 5) strategies that reduce ER stress by silencing of PERK or CHOP attenuate the degree of enterocyte apoptosis and prevent the development of NEC; and 6) ER stress-induced crypt apoptosis occurs in part through a novel pathway in which the induction of PERK by TLR4 leads to the induction of PUMA, which is required for the subsequent induction of apoptosis, thereby mechanistically linking TLR4 signaling with ER stress-induced cell death. Taken together, these findings identify a novel pathway linking TLR4 signaling in the intestinal stem cells with the generation of ER stress and the induction of apoptosis, in the pathogenesis of this devastating disease.
The current findings extend our knowledge regarding the role of ER stress in the development of intestinal inflammation, which until now has been thought to represent a protective strategy that exists in highly secretory cells of the intestinal epithelium including paneth and goblet cells (14). We also now identify a critical role for TLR4-induced ER stress within the intestinal stem cells, which has not been examined previously. Previous studies in this area have demonstrated that the highly active protein production apparatus of these secretory cells within the intestinal epithelium requires a very efficient unfolded protein response, the failure of which has been shown to lead to mucosal injury and thus increased susceptibility to enteritis or colitis (15, 16). These prior studies, however, have not examined the role of ER stress in the initial induction of the mucosal injury, have been confined to adult tissue, and have not examined intestinal inflammatory diseases in which TLR4 activation has been shown to play a major role such as NEC. By contrast, the current study indicates that in contrast to the full term bowel, the premature intestine exists in a state of heightened ER stress at baseline, such that the subsequent activation of TLR4 provides a crucial “second hit,” which results in irreparable activation apoptosis pathways in the ISCs. It is tempting now to speculate that the higher level of ER stress that exists within the intestinal mucosa of the premature host evolved as a consequence of in utero development as a means to protect the developing fetus from the consequences of the high mRNA and protein production that characterizes the premature state and that was never “expected” to encounter subsequent TLR4 activation by microbes, given the evolutionarily unlikely event of premature birth. Indeed, the very role for TLR4 in the gut and within the intestinal stem cells remains incompletely explained, although we have determined that this receptor plays a largely developmental role in the gut distinct from its role in pattern recognition, because the genetic inhibition of Tlr4 in mice leads to an abnormally differentiated intestinal epithelium, characterized by increased goblet cell differentiation (9). We now speculate that the exposure of the premature intestine, whose baseline ER stress levels are high, to the harsh microbial climate of the postnatal world, leads to TLR4 activation, apoptosis, and subsequently the development of NEC.
It is noteworthy that the PERK-CHOP branch of the ER stress cascade is required for this effect, because mice lacking an effective PERK-CHOP signaling apparatus are protected from disease development in vivo. Studies of CHOP deletion in mice have revealed that CHOP is required for ER stress-mediated cell death in response to several disease states including diabetes (35, 36) and atherosclerosis (37), and the current findings extend the scope of CHOP-mediated disease induction to the newborn population. Further studies are needed to determine whether humans with activating mutations in PERK-CHOP are at greater risk of NEC development than premature cohorts that do not develop this disease, much as mutations in the ER stress gene XBP1 have been linked to colitis (16). It is also important to note that we have now identified that PUMA serves as a novel and important mechanistic link between the induction of ER stress in response to TLR4 through PERK and the subsequent induction of apoptosis, which is the signature role of PUMA within cells. By tying together these two previously unlinked processes, we hope to gain further insights into the steps by which cell death occurs in response to ER stress induction, so as to design processes to reverse these events from propagating.
We readily acknowledge that although we have now shown that Lgr5-positive ISCs show increased ER stress, we cannot exclude the possibility that other ISC or crypt-associated cell populations including Bmi1 at the +4 level may be similarly TLR4 expressing and responsive to ER stress (38). Additional studies on this less frequent cell population are required to determine in greater detail the precise contribution of each of the various ISC populations to the mucosal healing response in the premature bowel and how ER stress affects these in response to TLR4 activation. Although the current work represents the first observation that TLR4 can impair ISCs through the induction of ER stress leading to the development of NEC, the current work is consistent with and extends the findings of Heijmans et al. (39), who describe that the unfolded protein response plays an important role in the differentiation of ISCs, that inhibition of PERK signaling leads to ISC accumulation, and that induction of ER stress results in the loss of ISCs in vivo, which in their system could be explained by an increase in differentiation as opposed to apoptosis. Importantly, the link between TLR4 signaling in the intestinal stem cell compartment and intestinal stem cell apoptosis was strengthened in the current work by overexpression of TLR4 in the intestinal epithelium using the villin promoter, which targets the crypts and leads to increased apoptosis of ISCs and injury. These findings imply that although physiological ER stress signaling plays a role in maintenance of normal ISCs under homeostatic conditions, as demonstrated by Heijmans et al. (39), pathological ER stress as it occurs in the ISCs upon TLR4 signaling is deleterious, leading to apoptosis and the development of disease.
In summary, we have now described a novel pathway linking TLR4 activation with the induction of ER stress in the intestinal stem cells to the development of NEC and how increased baseline ER stress of the premature gut may confer a particular susceptibility of the premature host to NEC development. Such findings, we believe, not only offer new insights into the molecular requirements that lead to NEC development but also identify novel therapeutic approaches for this devastating disease.
This work was supported, in whole or in part, by National Institutes of Health Grants 1R01GM078238 and 1R01DK083752 (to D. J. H.), P50GM053789 (to D. J. H. and T. R. B.), R01DK088199 (to R. B.), and R01-DK083541 (to G. K. G.). This work was also supported by funds from the Hartwell Foundation (to D. J. H.) and Harvard Digestive Diseases Center Grant DK0034854 (to R. B.).
- NEC
- necrotizing enterocolitis
- ISC
- intestinal stem cell
- TLR
- Toll-like receptor
- ER
- endoplasmic reticulum
- CC3
- cleaved caspase-3
- P-PERK
- phosphorylated PERK
- qRT-PCR
- quantitative RT-PCR
- Z-VAD
- benzyloxycarbonyl.
REFERENCES
- 1. Simons B. D., Clevers H. (2011) Stem cell self-renewal in intestinal crypt. Exp. Cell Res. 317, 2719–2724 [DOI] [PubMed] [Google Scholar]
- 2. Chen C. L., Yu X., James I. O., Zhang H. Y., Yang J., Radulescu A., Zhou Y., Besner G. E. (2012) Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab. Invest. 92, 331–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sodhi C. P., Shi X. H., Richardson W. M., Grant Z. S., Shapiro R. A., Prindle T., Jr., Branca M., Russo A., Gribar S. C., Ma C., Hackam D. J. (2010) Toll-like receptor-4 inhibits enterocyte proliferation via impaired β-catenin signaling in necrotizing enterocolitis. Gastroenterology 138, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feng J., Besner G. E. (2007) Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J. Pediatr. Surg. 42, 214–220 [DOI] [PubMed] [Google Scholar]
- 5. Leaphart C. L., Cavallo J., Gribar S. C., Cetin S., Li J., Branca M. F., Dubowski T. D., Sodhi C. P., Hackam D. J. (2007) A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J. Immunol. 179, 4808–4820 [DOI] [PubMed] [Google Scholar]
- 6. Neal M. D., Sodhi C. P., Dyer M., Craig B. T., Good M., Jia H., Yazji I., Afrazi A., Richardson W. M., Beer-Stolz D., Ma C., Prindle T., Grant Z., Branca M. F., Ozolek J., Hackam D. J. (2013) A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J. Immunol. 190, 3541–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richter J. M., Schanbacher B. L., Huang H., Xue J., Bauer J. A., Giannone P. J. (2012) LPS-binding protein enables intestinal epithelial restitution despite LPS exposure. J. Pediatr. Gastroenterol. Nutr. 54, 639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jilling T., Simon D., Lu J., Meng F. J., Li D., Schy R., Thomson R. B., Soliman A., Arditi M., Caplan M. S. (2006) The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J. Immunol. 177, 3273–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sodhi C. P., Neal M. D., Siggers R., Sho S., Ma C., Branca M. F., Prindle T., Jr., Russo A. M., Afrazi A., Good M., Brower-Sinning R., Firek B., Morowitz M. J., Ozolek J. A., Gittes G. K., Billiar T. R., Hackam D. J. (2012) Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Good M., Siggers R. H., Sodhi C. P., Afrazi A., Alkhudari F., Egan C. E., Neal M. D., Yazji I., Jia H., Lin J., Branca M. F., Ma C., Prindle T., Grant Z., Shah S., Slagle D., 2nd, Paredes J., Ozolek J., Gittes G. K., Hackam D. J. (2012) Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proc. Natl. Acad. Sci. U.S.A. 109, 11330–11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neal M. D., Sodhi C. P., Jia H., Dyer M., Egan C. E., Yazji I., Good M., Afrazi A., Marino R., Slagle D., Ma C., Branca M. F., Prindle T., Jr., Grant Z., Ozolek J., Hackam D. J. (2012) Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J. Biol. Chem. 287, 37296–37308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 13. Tabas I., Ron D. (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13, 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaser A., Adolph T. E., Blumberg R. S. (2013) The unfolded protein response and gastrointestinal disease. Semin. Immunopathol. 35, 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao S. S., Zimmermann E. M., Chuang B. M., Song B., Nwokoye A., Wilkinson J. E., Eaton K. A., Kaufman R. J. (2013) The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology 144, 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaser A., Lee A. H., Franke A., Glickman J. N., Zeissig S., Tilg H., Nieuwenhuis E. E., Higgins D. E., Schreiber S., Glimcher L. H., Blumberg R. S. (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woo C. W., Cui D., Arellano J., Dorweiler B., Harding H., Fitzgerald K. A., Ron D., Tabas I. (2009) Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by Toll-like receptor signalling. Nat. Cell Biol. 11, 1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afrazi A., Sodhi C. P., Good M., Jia H., Siggers R., Yazji I., Ma C., Neal M. D., Prindle T., Grant Z. S., Branca M. F., Ozolek J., Chang E. B., Hackam D. J. (2012) Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J. Immunol. 188, 4543–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sato T., Vries R. G., Snippert H. J., van de Wetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., Clevers H. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 20. Leaphart C. L., Qureshi F., Cetin S., Li J., Dubowski T., Batey C., Beer-Stolz D., Guo F., Murray S. A., Hackam D. J. (2007) Interferon-γ inhibits intestinal restitution by preventing gap junction communication between enterocytes. Gastroenterology 132, 2395–2411 [DOI] [PubMed] [Google Scholar]
- 21. Richardson W. M., Sodhi C. P., Russo A., Siggers R. H., Afrazi A., Gribar S. C., Neal M. D., Dai S., Prindle T., Jr., Branca M., Ma C., Ozolek J., Hackam D. J. (2010) Nucleotide-binding oligomerization domain-2 inhibits Toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology 139, 904–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gribar S. C., Sodhi C. P., Richardson W. M., Anand R. J., Gittes G. K., Branca M. F., Jakub A., Shi X. H., Shah S., Ozolek J. A., Hackam D. J. (2009) Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J. Immunol. 182, 636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neal M. D., Leaphart C., Levy R., Prince J., Billiar T. R., Watkins S., Li J., Cetin S., Ford H., Schreiber A., Hackam D. J. (2006) Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J. Immunol. 176, 3070–3079 [DOI] [PubMed] [Google Scholar]
- 24. Calfon M., Zeng H., Urano F., Till J. H., Hubbard S. R., Harding H. P., Clark S. G., Ron D. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 [DOI] [PubMed] [Google Scholar]
- 25. Kaufman R. J. (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 [DOI] [PubMed] [Google Scholar]
- 26. Clevers H. (2013) The intestinal crypt, a prototype stem cell compartment. Cell 154, 274–284 [DOI] [PubMed] [Google Scholar]
- 27. Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., Clevers H. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- 28. Medzhitov R. (2001) Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 [DOI] [PubMed] [Google Scholar]
- 29. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ford H., Watkins S., Reblock K., Rowe M. (1997) The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J. Pediatr. Surg. 32, 275–282 [DOI] [PubMed] [Google Scholar]
- 31. Cetin S., Leaphart C. L., Li J., Ischenko I., Hayman M., Upperman J., Zamora R., Watkins S., Ford H. R., Wang J., Hackam D. J. (2007) Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G1347–G1358 [DOI] [PubMed] [Google Scholar]
- 32. Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., Yuan J. (2005) A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 33. Hikisz P., Kiliańska Z. M. (2012) PUMA, a critical mediator of cell death: one decade on from its discovery. Cell Mol. Biol. Lett. 17, 646–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qiu W., Carson-Walter E. B., Liu H., Epperly M., Greenberger J. S., Zambetti G. P., Zhang L., Yu J. (2008) PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell 2, 576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oyadomari S., Koizumi A., Takeda K., Gotoh T., Akira S., Araki E., Mori M. (2002) Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song B., Scheuner D., Ron D., Pennathur S., Kaufman R. J. (2008) Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J. Clin. Invest. 118, 3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thorp E., Li G., Seimon T. A., Kuriakose G., Ron D., Tabas I. (2009) Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 9, 474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King S. L., Dekaney C. M. (2013) Small intestinal stem cells. Curr. Opin. Gastroenterol. 29, 140–145 [DOI] [PubMed] [Google Scholar]
- 39. Heijmans J., van Lidth de Jeude J. F., Koo B. K., Rosekrans S. L., Wielenga M. C., van de Wetering M., Ferrante M., Lee A. S., Onderwater J. J., Paton J. C., Paton A. W., Mommaas A. M., Kodach L. L., Hardwick J. C., Hommes D. W., Clevers H., Muncan V., van den Brink G. R. (2013) ER stress causes rapid loss of intestinal epithelial stemness through activation of the unfolded protein response. Cell Reports 3, 1128–1139 [DOI] [PubMed] [Google Scholar]