Background: Oxygen plays a key role in organ development, including pancreatic β-cells.
Results: High oxygen conditions increase Ngn3-positive and insulin-positive cells from both mouse and human pluripotent stem cells.
Conclusion: Culturing under high oxygen conditions has a facilitative effect on pancreatic differentiation.
Significance: This new technique provides an efficient method to utilize patient-specific iPS cells for the treatment of diabetes.
Keywords: Differentiation, Embryonic Stem Cell, Hypoxia-inducible Factor, Induced Pluripotent Stem Cells, Regenerative Medicine
Abstract
Pluripotent stem cells have potential applications in regenerative medicine for diabetes. Differentiation of stem cells into insulin-producing cells has been achieved using various protocols. However, both the efficiency of the method and potency of differentiated cells are insufficient. Oxygen tension, the partial pressure of oxygen, has been shown to regulate the embryonic development of several organs, including pancreatic β-cells. In this study, we tried to establish an effective method for the differentiation of induced pluripotent stem cells (iPSCs) into insulin-producing cells by culturing under high oxygen (O2) conditions. Treatment with a high O2 condition in the early stage of differentiation increased insulin-positive cells at the terminus of differentiation. We found that a high O2 condition repressed Notch-dependent gene Hes1 expression and increased Ngn3 expression at the stage of pancreatic progenitors. This effect was caused by inhibition of hypoxia-inducible factor-1α protein level. Moreover, a high O2 condition activated Wnt signaling. Optimal stage-specific treatment with a high O2 condition resulted in a significant increase in insulin production in both mouse embryonic stem cells and human iPSCs and yielded populations containing up to 10% C-peptide-positive cells in human iPSCs. These results suggest that culturing in a high O2 condition at a specific stage is useful for the efficient generation of insulin-producing cells.
Introduction
Cell replacement therapy has become possible by utilizing artificially generated cells or organs from embryonic stem cells (ESCs),3 induced pluripotent stem cells (iPSCs), and adult stem cells. These stem cells have marked potential to develop into many different cell types in the body during early life and growth. Over the last decade, with the advent of new techniques and technologies in modern molecular biology, understanding of the underlying mechanism responsible for organ differentiation has developed rapidly. This knowledge has given rise to various new methods of manipulating stem cells in order to generate deficient organs in various diseases. To date, various differentiation methods have been developed for each cell type, including neurons, cardiomyocytes, and pancreatic endocrine cells. Many of these methods are based on mimicking the in vivo development. The development of efficient and safe methods is desired for clinical applications and studying the cause of disease.
Pluripotent stem cells are capable of spontaneous differentiation into insulin-producing cells. This is mainly carried out by preferential differentiation of stem cells into insulin-producing cells by changing the composition of the culture medium and causing the expression of dominant transcription factor genes, which are mainly involved in pancreatic development. Several groups have reported methods of generating pancreatic cell lineages from ESCs and iPSCs (1–8). These methods induce definitive endoderm differentiation in the first stage and then pancreatic specialization and maturation in the following stages, using combinations of growth factors, small molecules, and extracellular matrix. Lumelsky et al. (6) first demonstrated the successful differentiation of mouse ESCs (mESCs) to insulin-secreting structures, which was concluded to be similar to that of pancreatic islets. However, the limiting factor of this method is that the abundance of differentiated cells is relatively low. Moreover, several reports had the same issue that the differentiated cells are immature and/or not fully functional in culture. Some reports succeeded in generating functional insulin-secreting cells utilizing differentiation under implantation or co-culture with organ-matched mesenchyme (7, 8). However, such methods have a risk of teratoma or teratomatous tissue element formation in their grafts. Fifteen percent of grafts showed teratoma or a teratomatous tissue element (7). To improve this issue, establishment of safer and more efficient methods is desired.
Oxygen (O2) plays a crucial role in cellular homeostasis (9, 10). In normal tissues, the lack of oxygen contributes to cell death, whereas in stem cells, lack of O2 controls stem cell self-renewal and pluripotency by activating specific signaling pathways, such as Notch, and the expression of transcriptional factors, such as Oct4 (11, 12). Hypoxia is accompanied by the stabilization of hypoxia-inducible factors (HIFs), O2-regulated transcriptional factors that regulate an ever increasing number of genes involved in glycolytic metabolism, angiogenesis, erythropoiesis, and metastasis and mediate the adaptation of cells to decreased O2 availability (13, 14). O2 tension, the partial pressure of O2, has been shown to regulate the embryonic development of several organs, including the trachea, heart, lung, limb bud, and bone (15–19). It is also reported that O2 tension plays a key role in pancreatic development (20–23). The embryonic pancreas early in development is poorly vascularized and has a paucity of blood flow, and, at later stages, blood flow increases, and endocrine differentiation occurs at the same time (21). It has also been shown that HIF-1α protein is highly expressed in the embryonic pancreas early in development and that increasing concentrations of O2 in vitro represses HIF-1α expression and fosters the development of endocrine progenitors (22, 23). Suitable O2 concentrations should be tested for the differentiation efficiency of ESC and iPSC into pancreatic lineages. However, until now, there has been no report of such an effect on ESC and iPSC differentiation in vitro.
Here we studied the effect of increasing concentrations of O2 on the differentiation efficiency of mESC and human iPSC (hiPSC) into pancreatic lineages. A high O2 condition (60% O2) in early stages of differentiation increased the percentage of Ngn3-expressing endocrine progenitors and insulin-positive cells in both mESC and hiPSC. This effect was mediated via the inhibition of HIF-1α expression and increase of Ngn3 gene expression. Moreover, a high O2 condition was found to induce the activation of Wnt signaling. In this study, we demonstrated that culturing ESC and iPSC in a high O2 condition improved differentiation efficiency into endocrine progenitors and insulin-producing cells compared with normoxic conditions.
EXPERIMENTAL PROCEDURES
mESC and hiPSC Lines
The mESC line ING112, containing an Ins1 promoter-driven GFP reporter transgene, was established by culturing blastocysts obtained from transgenic mice homozygous for the Ins1-GFP gene (3, 24). ING112 cells were maintained on mouse embryonic fibroblast (MEF) feeders in high glucose Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 1000 units/ml leukemia inhibitory factor (Wako, Osaka, Japan), 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT), 100 μm nonessential amino acids (NEAA), 2 mm l-glutamine (l-Gln), 1 mm sodium pyruvate, 50 units/ml penicillin and 50 μg/ml streptomycin, and 100 μm β-mercaptoethanol (β-ME). The hiPSC clone 23 (C23) was previously produced by Sendai virus vector expressing OCT3/4, SOX2, KLF4, and c-MYC (25). C23 cells were maintained on mitomycin C (Nacalai Tesque, Kyoto, Japan)-treated MEF feeders in DMEM/F-12 HAM (Sigma) supplemented with 100 μm NEAA, 2 mm l-Gln, 20% knock-out serum replacement (Invitrogen), 25 units/ml penicillin and 25 μg/ml streptomycin, 100 μm β-ME, and 5 ng/ml bFGF (Peprotech, Rocky Hill, NJ).
Differentiation of mESC and hiPSC into Insulin-producing Cells
Prior to differentiation, mESCs were passaged using 0.25% trypsin and seeded at 35,000 cells/well on 4- or 24-well plates coated with 50 μg/ml poly-l-lysine (Sigma) and 2.5 μg/well laminin (Roche Applied Science). After overnight culture, the cells were cultured in high glucose (4500 mg/liter) DMEM supplemented with 10 mg/liter insulin, 5.5 mg/liter transferrin, 6.7 μg/ml sodium selenite (insulin-transferrin-selenium-G supplement, Invitrogen), 2.5 mg/ml ALBUMAX II (Invitrogen), NEAA, l-Gln, 50 units/ml penicillin and 50 μg/ml streptomycin, β-ME, 10 ng/ml activin A (R&D Systems, Minneapolis, MN), and 5 ng/ml bFGF (Peprotech) from day 1 to day 6. From day 7 to 10, the culture medium was changed to RPMI supplemented with NEAA, l-Gln, 50 units/ml penicillin, and 50 μg/ml streptomycin, β-ME, B27 supplement (Invitrogen), 50 ng/ml FGF10 (Peprotech), 250 nm KAAD-cyclopamine (Calbiochem), and 1 μm retinoic acid (Sigma). At day 11, the medium was switched to low glucose (1000 mg/liter) DMEM supplemented with insulin-transferrin-selenium-G supplement, ALBUMAX II, NEAA, l-Gln, penicillin, streptomycin, β-ME, 10 mm nicotinamide (Sigma), and 10 nm glucagon-like peptide (Sigma), and the cells were cultured until day 17. hiPSCs were passaged as small clusters using 1 unit/ml dispase (Roche Applied Science) at a 1:5 split ratio weekly and plated on mitomycin C-treated MEF feeders of 4- or 24-well plates. After a 1-week culture, the cells were cultured in differentiation medium in the same way as mouse ESCs.
Cells were cultured under high O2 concentration conditions (60% O2) for the indicated periods in a multigas incubator (APM-30DR, ASTEC, Fukuoka, Japan).
Immunostaining
Cells were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde for 30 min at room temperature (22–24 °C). Immunostaining was carried out with the standard protocol. The following primary antibodies were used: mouse anti-GFP (1:1000; Medical and Biological Laboratories, Nagoya, Japan), goat anti-Sox17 (1:200; R&D Systems), rabbit anti-Pdx1 (1:1000; Upstate Biotechnology, Inc., Lake Placid, NY), goat anti-Ngn3 (1:100; Santa Cruz Biotechnology, Inc.), goat anti-Foxa2 (1:300; Santa Cruz Biotechnology, Inc.), mouse anti-insulin (1:1000; Sigma), and rabbit anti-C-peptide (Cell Signaling Technology, Beverly, MA). Alexa488- or Alexa568-conjugated secondary antibodies were used at 1:500 dilution (Molecular Probes, Inc., Eugene, OR). Cells were counterstained with DAPI (Roche Applied Science). Images were taken with an Olympus IX81 fluorescence microscope (Olympus Optical Co. Ltd., Tokyo, Japan). Quantification was carried out using MetaMorph software (Molecular Devices). After images of marker fluorescence and DAPI fluorescence were taken in defined areas of wells in the cell culture plate, each image was thresholded to exclude background noise. The area was quantified, and the percentage of marker-positive cells was calculated by dividing the DAPI-positive area (total cell number) into the marker-positive area (supplemental Fig. S1).
Quantitative Real-time PCR
Total RNA was isolated from cells using a TRIzol reagent (Invitrogen) according to the manufacturer's instructions. cDNA was synthesized using a Superscript III first strand synthesis system (Invitrogen) according to the manufacturer's instructions. Quantitative real-time PCR (qPCR) analysis was performed on an ABI Prism 7300 (Applied Biosystems, Foster City, CA) using a SYBR Premix ExTaq GC (Takara, Shiga, Japan). The primer sequences for each primer set are shown in Table 1. mRNA expression data were normalized against actin expression in a corresponding sample. The data were analyzed using the relative quantification study in Sequence Detection software version 1.2 (Applied Biosystems).
TABLE 1.
Primers used in quantitative real-time PCR analysis
| Genes | Sequences (forward and reverse) | Product |
|---|---|---|
| bp | ||
| Mouse | ||
| Act | CCTCATGAAGATCCTGACCGA | 192 |
| TTGCCAATAGTGATGACCTGG | ||
| Oct4 | GAGGAAGCCGACAACAATGAGAACCTTCAG | 227 |
| TTCTGGCGCCGGTTACAGAACCATACTCGA | ||
| Sox17 | GAACAGTTGAGGGGCTACAC | 322 |
| GTTTAGGGTTTCTTAGATGC | ||
| Foxa2 | TGGTCACTGGGGACAAGGGAA | 289 |
| GCAACAACAGCAATAGAGAAC | ||
| Pdx1 | TCACTGGAGCAGGGAAGTCCT | 264 |
| TTCCGCTGTGTAAGCACCTCC | ||
| Ngn3 | ACTGCAGCAGTGGTCGAGTAC | 225 |
| AAGGGATGAGGCGCCATCCTA | ||
| Ins1 | CAGCCCTTAGTGACCAGCTA | 348 |
| ATGCTGGTGCAGCACTGATC | ||
| Vegfa | GCTACTGCCGTCCGATTGAGA | 185 |
| AGGTTTGATCCGCATGATCTGC | ||
| Hes1 | TCAACACGACACCGGACAAACC | 270 |
| GGTATTTCCCCAACACGCTCG | ||
| Human | ||
| ACT | CCTCATGAAGATCCTCACCGA | 192 |
| TTGCCAATGGTGATGACCTGG | ||
| SOX17 | GCATGACTCCGGTGTGAATCT | 103 |
| TCACACGTCAGGATAGTTGCAGT | ||
| FOXA2 | ATTGCTGGTCGTTTGTTGTG | 187 |
| TACGTGTTCATGCCGTTCAT | ||
| PDX1 | CCTTTCCCATGGATGAAGTC | 145 |
| GGAACTCCTTCTCCAGCTCTA | ||
| NGN3 | TTGCGCCGGTAGAAAGGATGAC | 249 |
| TCAGTGCCAACTCGCTCTTAGG | ||
| NEUROD1 | CCCATGGTGGGTTGTCATATATTCA | 200 |
| CCAGCATCACATCTCAAACAGCAC | ||
| MAFA | TGCAGCAGCGGCACATTC | 128 |
| CGCCAGCTTCTCGTATTTCTCCTTGT | ||
| INS | GAGGCCATCAAGCAGATCAC | 373 |
| GGCTGCGTCTAGTTGCAGTA | ||
| VEGFA | CCCTGATGAGATCGAGTACAT | 496 |
| CGGCTTGTCACATCTGCAAGT | ||
| HES1 | TCAACACGACACCGGATAAACC | 270 |
| GGTACTTCCCCAGCACACTTG |
Immunoblotting
HIF-1α levels were determined using immunoblotting. Cells were washed with PBS, scraped, and lysed in radioimmune precipitation buffer (50 mm Tris-HCl, pH 7.5, 4 mm EGTA, 150 mm NaCl, 50 mm NaF, 1 mm Na3VO4, 30 mm Na4P2O7, 10 mm EDTA, 1% Triton X-100). After sonication, the insoluble materials were removed by centrifugation at 15,000 rpm for 15 min. The supernatants were then mixed with a 5-fold amount of Laemmli sample buffer (0.38 m Tris-HCl, pH 6.8, 12% SDS, 30% β-mercaptoethanol, 10% glycerol, 0.05% bromphenol blue) and boiled for 4 min. Samples were loaded and subjected to SDS-PAGE and then transferred to a nitrocellulose membrane (Bio-Rad) and then blocked for nonspecific binding in a 5% skim milk solution. Membranes were incubated with mouse anti-HIF-1α antibody (1:1000; R&D Systems) or mouse anti-β-actin (1:1000; Chemicon, Temecula, CA) overnight at 4 °C. Then membranes were washed and incubated for 1 h with HRP-conjugated goat anti-mouse IgG antibody (1:2000; Dako, Carpinteria, CA). After washing, membranes were incubated with enhanced chemiluminescence reagent (GE Healthcare), and the immunoreactive proteins were visualized by an ImageQuant400 (GE Healthcare).
Microarray
Microarray analysis was performed on total RNA samples using a TORAY 3D-gene oligo chip (TORAY, Tokyo, Japan), according to the manufacturer's instructions. The genes induced by a high O2 environment were determined by global normalization after excluding genes of <100 intensity in the high O2 condition-treated group. Genes increased over 8-fold in the ratio of high O2 condition to normoxia are listed in Fig. 6A. Pathway analysis was performed on up-regulated genes in the high O2 condition-treated or echinomycin-treated group using GenMapp/MAPP Finder software.
FIGURE 6.
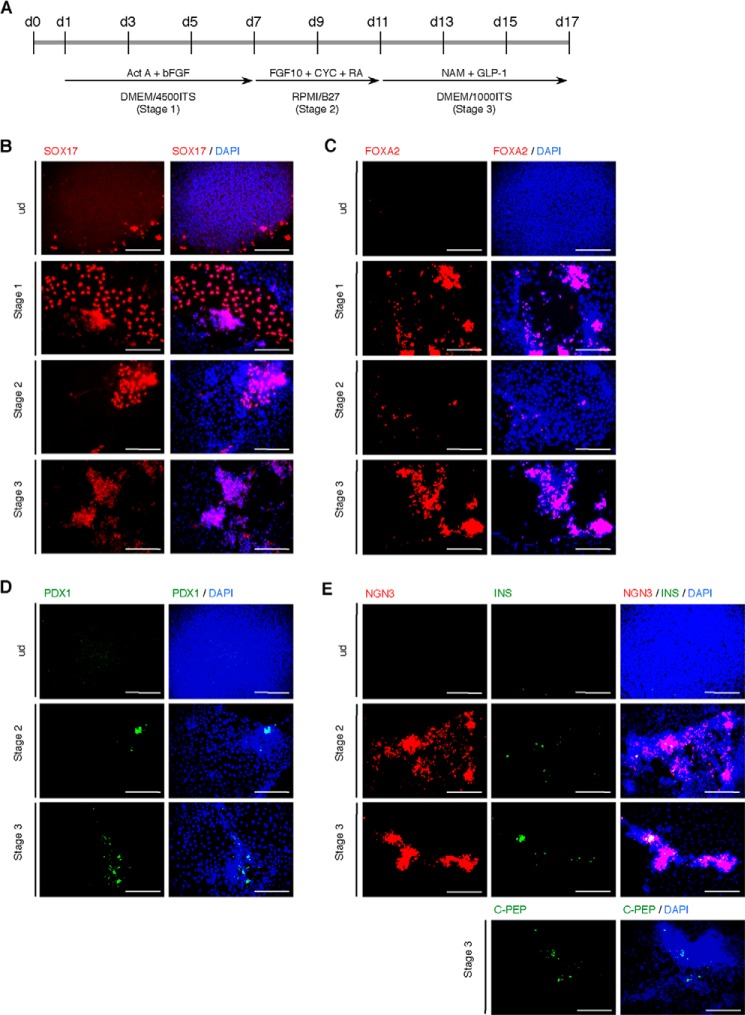
Stepwise differentiation of hiPSCs into insulin-producing cells. A, scheme of the stepwise differentiation protocol used to generate insulin-producing cells from human induced pluripotent stem C23 cells. B–E, immunofluorescence for SOX17 (B), FOXA2 (C), PDX1 (D), NGN3 (E), insulin (E), and C-peptide (E) at day 7 (stage 1), 11 (stage 2), or 17 (stage 3) of differentiation in C23 cells in normoxic condition. Scale bars, 200 μm.
Statistics
Data are shown as the mean ± S.E. Student's t test was used to identify significant differences between two conditions, and one-way analysis of variance or two-way analysis of variance followed by Tukey-Kramer's post hoc analysis was used to compare multiple conditions. p < 0.05 was considered to be significant.
RESULTS
High Oxygen Condition Facilitates the Differentiation of mESC into Insulin-producing Cells
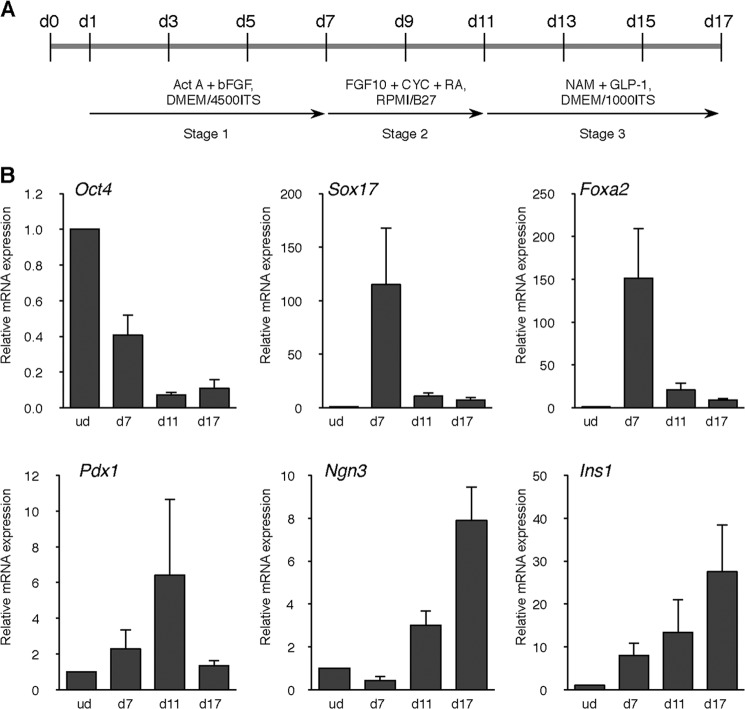
We used a modified protocol from a previous report of three-stage stepwise differentiation into insulin-producing cells (25, 26) (Fig. 1A). First, mESC ING112 cells were treated with activin A and bFGF to direct the differentiation into definitive endoderm from day 1 to day 7. By day 7, there was a steep reduction in the expression of Oct4 relating to the fact that the cells have transitioned from pluripotency to an endodermal progenitor (Fig. 1B). This is evident from the increased expression of Sox17 and Foxa2 on day 7, both of which are markers of a definitive endoderm. With the change in the composition of the medium containing B27, FGF10, KAAD-cyclopamine, and retinoic acid from day 7 onward, there was a gradual decrease in the expression of Sox17 and Foxa2. Subsequently, there was a marked increase in the expression of Pdx1 and Ngn3 on day 11, indicating the prevalence of pancreatic progenitors and endocrine progenitors in the population of the culture. With the change in the medium composition containing nicotinamide and GLP-1 on day 11, Pdx1 and Ngn3 expressions decreased, whereas the maximum level of Ins1 expression was reached.
FIGURE 1.
Stepwise differentiation of mESCs into insulin-producing cells. A, scheme of the stepwise differentiation protocol used to generate insulin-producing cells from mouse embryonic stem ING112 cells. Act A, activin A; bFGF, basic FGF; CYC, KAAD-cyclopamine; RA, retinoic acid; NAM, nicotinamide; GLP-1, glucagon-like peptide; ITS, insulin-transferrin-selenium. B, the dynamics of Oct4, Sox17, Foxa2, Pdx1, Ngn3, and Ins1 gene expression, several key markers in pancreatic differentiation, were analyzed at different stages in normoxic conditions by qPCR. n = 8 each. ud, undifferentiated cells. Error bars, S.E.
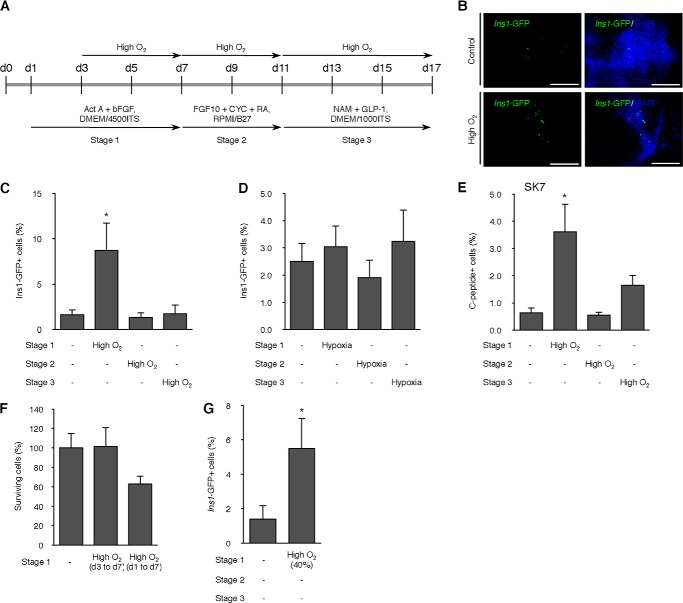
Next we examined the effects of a high O2 concentration condition on the differentiation efficiency of mESC ING112 cells. We cultured cells in a high O2 condition (60% O2) under stepwise differentiation during stage 1 (days 3–7), stage 2 (days 7–11), or stage 3 (days 11–17) (Fig. 2A). It was observed that a high O2 condition during stage 1, the early phase of differentiation, had the greatest effect on differentiation efficiency with an almost 8-fold increase (p < 0.05) in the percentage of Ins1-GFP-positive cells (Fig. 2, B and C). However, a high O2 condition during stage 2 or stage 3 had no effect on differentiation efficiency. At the beginning of these experiments, we cultured under high O2 conditions from day 1; however, earlier treatment had a deleterious effect on the percentage of surviving cells (Fig. 2F). Therefore, we used this protocol as the high O2 condition from day 3. To determine whether 60% high O2 is the best condition, we tested different levels of O2 condition. As a result, 40% O2 during stage 1 also increased the percentage of Ins1-GFP-positive cells by 4-fold (p < 0.05; Fig. 2G), but this effect was less than that of 60% O2. Instead of high O2, we used a hypoxic condition during differentiation, but there was no change compared with the normoxic condition (Fig. 2D). The high O2 condition also increased the percentage of C-peptide-positive cells in a different mESC line, SK7 (27, 28) (Fig. 2E).
FIGURE 2.
Effect of high O2 condition on differentiation efficiency of mESCs. A, scheme of the timeline of high O2 condition. B, immunofluorescence for Ins1-GFP on day 17 of differentiation in ING112 cells treated with a high O2 condition during stage 1. Scale bars, 200 μm. C, values are the percentage of Ins1-GFP-positive cells per well of the cells treated with high O2 condition (60% O2) during three different stages. *, p < 0.05 versus corresponding control. n = 8 each. D, values are the percentage of Ins1-GFP-positive cells per well of the cells treated with hypoxic condition (5% O2) during three different stages. n = 20 each. E, values are the percentage of C-peptide-positive cells per well of the cells treated with the high O2 condition (60% O2) during three different stages in mESC line SK7. *, p < 0.05 versus corresponding control. n = 6 each. F, cell viability assay on the number of viable cells after treatment with the high O2 condition (60% O2) during day 1 to day 7 or day 3 to day 7. n = 6 each. G, values are the percentage of Ins1-GFP-positive cells per well of the cells treated with the high O2 condition (40% O2) during stage 1. *, p < 0.05 versus corresponding control. n = 6 each. Error bars, S.E.
High Oxygen Condition Facilitates Differentiation into Endocrine Progenitors
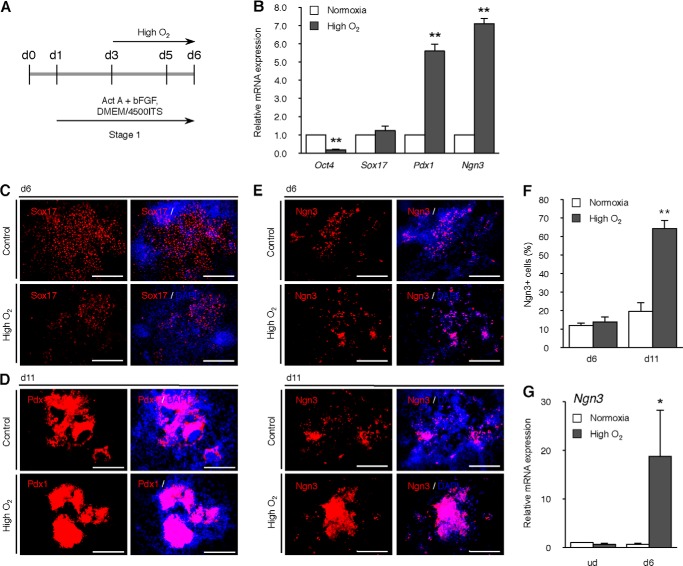
Based on the results in Fig. 2, B and C, we compared gene expression levels between the normoxia and high O2 condition groups on day 6 of stage 1 (Fig. 3A). We observed a significant decrease (p < 0.0005) in the expression of Oct4, which is indicative of the fact that the cells lost their pluripotency (Fig. 3B). Whereas there was no difference in the expression of Sox17, a marker gene of definitive endoderm, there was an almost 6-fold (p < 0.005) and 7-fold increase (p < 0.0005) in the expressions of Pdx1 and Ngn3, respectively (Fig. 3B). To determine the proportion of cells expressing each marker, immunofluorescence analysis was performed. There was no marked difference in the number of Sox17-positive cells on day 6 (Fig. 3C), and Pdx1-positive cells on day 11 (Fig. 3D), although induction of its gene expression was observed (Fig. 3B). It was confirmed that the number of Ngn3-positive cells was significantly increased on day 11 (Fig. 3E). Quantification of the percentage of Ngn3-positive cells showed an almost 3-fold (p < 0.0005) increase (Fig. 3F). These results show that the high O2 condition reduced the pluripotency of the cells and directed them markedly toward endocrine progenitors.
FIGURE 3.
Effect of high O2 condition on differentiation markers. A, scheme of the timeline of high O2 condition. B, levels of Oct4, Sox17, Pdx1, and Ngn3 genes were analyzed at day 6 of differentiation in normoxia or high O2-treated ING112 cells by qPCR. **, p < 0.01 versus corresponding control. n = 4 each. C–E, immunofluorescence for Sox17 on day 6 (C), Pdx1 on day 11 (D), and Ngn3 on day 6 or day 11 (E) of differentiation in ING112 cells treated with high O2 condition during stage 1. Scale bars, 200 μm. F, values are the percentage of Ngn3-positive cells per well at day 6 or day 11 in normoxia or high O2-treated ING112 cells. **, p < 0.01 versus corresponding control. n = 4 each. G, levels of the Ngn3 gene were analyzed on undifferentiated ING112 cells treated with high O2 condition for 3 days by qPCR. The effect of high O2 condition on differentiating cells is shown as a positive control. *, p < 0.05 versus corresponding control. n = 4 each. Error bars, S.E.
In our differentiation protocol, stage 1 contained supplements, such as activin A and bFGF, in the medium to direct toward a definitive endoderm. Next, to clarify which high O2 condition affected undifferentiated mESC or differentiating cells, we examined the effect of a high O2 condition on Ngn3 expression in undifferentiated cells. Treatment with a high O2 condition for 3 days did not affect Ngn3 expression in the undifferentiated state maintained in mESC medium compared with treatment in differentiation medium, showing that the high O2 condition affected differentiating cells (Fig. 3G).
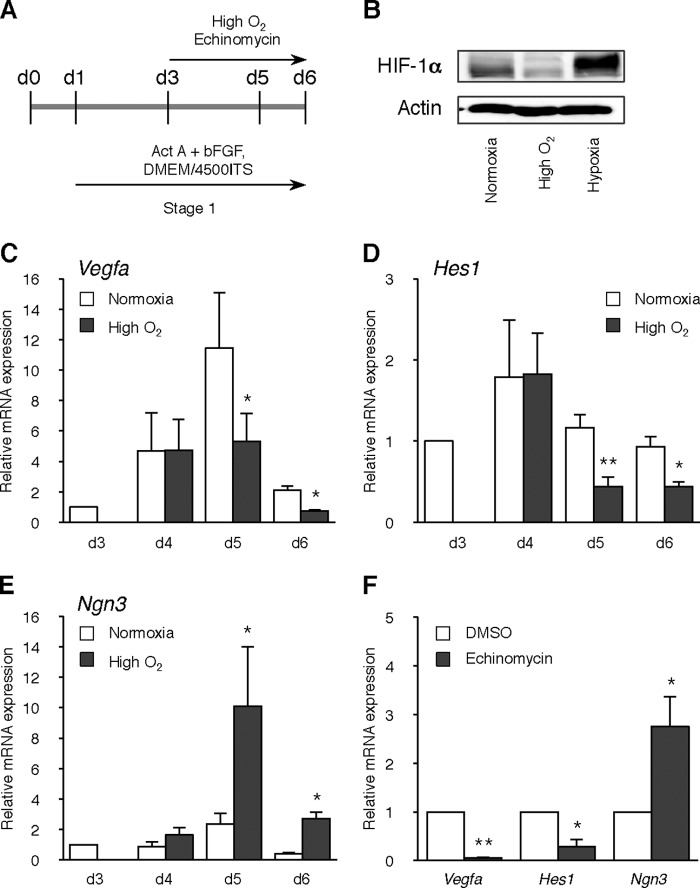
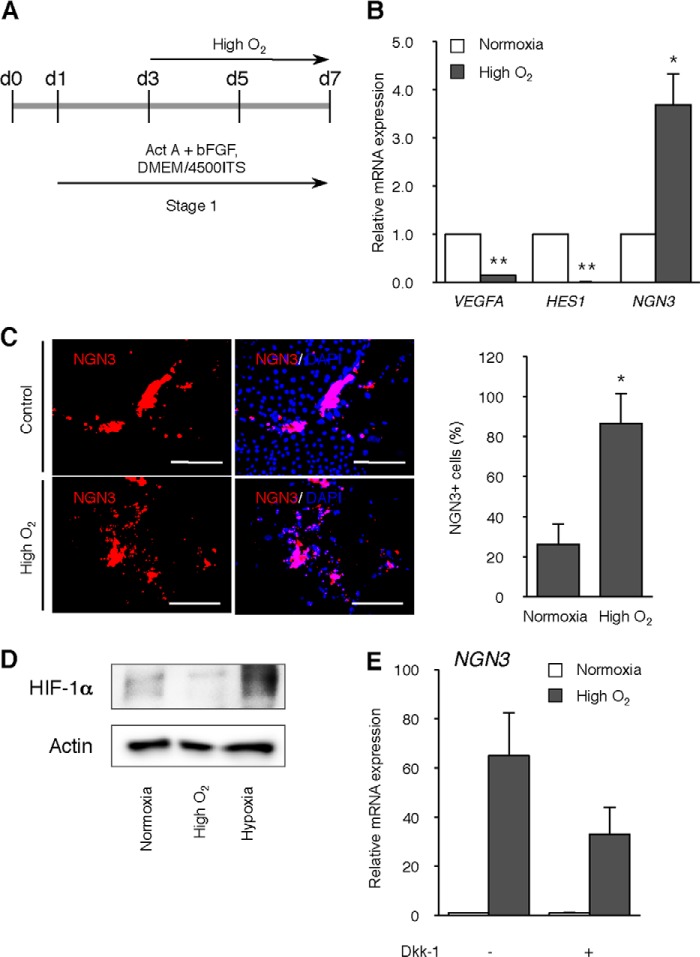
High Oxygen Condition Represses HIF-1α Protein Level and Hes1 Gene Expression
Even under normoxia, HIF-1α is reported to be expressed at a detectable level and participate in the expression of hypoxia-inducible genes in mESCs (29). Therefore, we compared HIF-1α protein level and its target gene expression between normoxic and high O2 condition groups. High O2 condition during days 3–6 of differentiation repressed HIF-1α protein level (Fig. 4, A and B). Under normoxic conditions, expression of Vegfa, a HIF-1α targeting gene, increased from day 4 with a peak level on day 5 of differentiation, showing that activation of HIF-1α occurs during differentiation, whereas the high O2 condition significantly decreased Vegfa expression on days 5 and 6 (p < 0.05, respectively; Fig. 4C). It is reported that HIF-1α activates Notch signaling in stem cells and embryonic pancreatic cells (11, 22). Hypoxia and subsequent HIF-1α expression induced expression of Hes1 (hairy and enhancer of split 1), a Notch downstream gene, and repressed Ngn3 expression, leading to the inhibition of β-cell development (22). Therefore, we compared the kinetics of Hes1 and Ngn3 expression in normoxia with those in the high O2 condition. Under differentiation, Hes1 expression was slightly increased at day 4 and gradually decreased from day 5, whereas high O2 condition significantly repressed its expression on days 5 and 6 (p < 0.01 and p < 0.05, respectively; Fig. 4D). In contrast, Ngn3 expression significantly increased on both day 5 and 6 in the high O2 condition (p < 0.05, respectively; Fig. 4E).
FIGURE 4.
Effect of high O2 condition on Notch signaling. A, scheme of the timeline of high O2 condition. B, levels of HIF-1α and β-actin (loading control) were analyzed on day 6 of differentiation in normoxia, high O2, or hypoxia (1% O2; positive control)-treated ING112 cells by immunoblotting. C–E, levels of Vegfa (C), Hes1 (D), and Ngn3 (E) gene were analyzed on days 3, 4, 5, and 6 of differentiation in normoxia or high O2-treated ING112 cells by qPCR. *, p < 0.05; **, p < 0.01 versus corresponding control. n = 8 each. F, levels of Vegfa, Hes1, and Ngn3 genes were analyzed on day 6 of differentiation in DMSO or 1 nm echinomycin-treated ING112 cells by qPCR. *, p < 0.05; **, p < 0.01 versus corresponding control. n = 3 each. Error bars, S.E.
Next we examined the effect of HIF-1α inhibition on Ngn3 expression under differentiation. Cells were treated with 1 nm echinomycin, an inhibitor of HIF-1α, from day 3 to day 6. On day 6, Ngn3 expression was significantly increased by echinomycin treatment, whereas Vegfa and Hes1 expressions were decreased (p < 0.0005, p < 0.05, and p < 0.05, respectively; Fig. 4F). These expression profiles were similar to those in the high O2 condition. Hence, it was shown that HIF-1α inhibition and subsequent repression of Notch signaling play a role in facilitated differentiation in the high O2 condition.
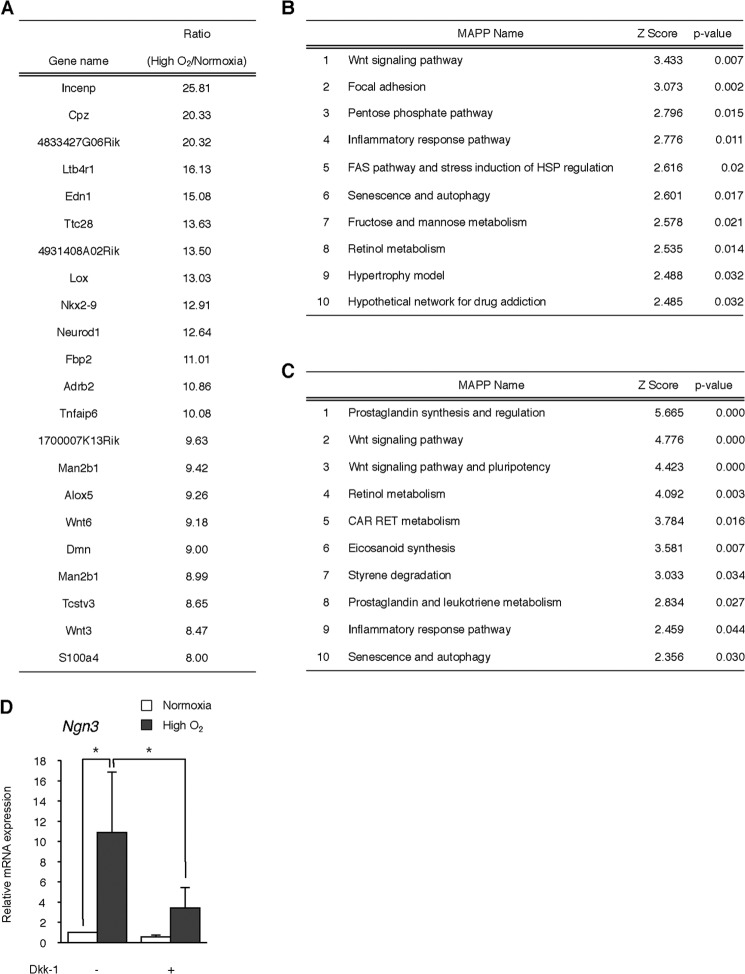
High Oxygen Condition Activates Wnt Signaling Pathway
To further clarify the effect of the high O2 condition on differentiating cells, we performed microarray analysis on normoxia or high O2-treated cells. As a result, many genes were up-regulated by the high O2 condition, and genes showing over 8-fold expression in the high O2 group compared with the normoxia group are listed in Fig. 5A. Pathway analysis using up-regulated genes in the high O2 group indicated that several genes were involved in the Wnt signaling pathway, and this pathway was ranked first (p < 0.01; Fig. 5B). Wnt3, Wnt6 (over 8-fold), Wnt5a, Wnt10a (over 4-fold; data not shown), Wnt4, Wnt7b, Wnt10b, Fzd1, Myc, and Ccnd2 (over 2-fold; data not shown) were increased in the high O2 group and detected as the Wnt signaling pathway. We also performed microarray analysis on the echinomycin-treated group. Similarly, the Wnt signaling pathway was ranked first when analyzed using up-regulated genes (p < 0.001; Fig. 5C), suggesting that HIF-1α inhibition led to the activation of Wnt signaling.
FIGURE 5.
Microarray analysis on genes induced by high O2 condition. A, microarray analysis was performed on ING112 cells treated with high O2 condition during days 3–5 of differentiation using a TORAY 3D-gene oligo chip. The genes induced by high O2 condition are shown determined by global normalization after excluding genes of <100 intensity in the high O2 condition group. Genes increased over 8-fold in the ratio of high O2 to normoxia are listed. B and C, pathway analysis was performed on up-regulated genes in the high O2-treated (B) or echinomycin-treated (C) group using GenMapp/MAPP Finder software. D, levels of the Ngn3 gene were analyzed on day 6 of differentiation in normoxia or high O2-treated ING112 cells with or without Wnt signaling inhibitor Dkk-1 by qPCR. *, p < 0.05 versus corresponding control. n = 11 each. Error bars, S.E.
The Wnt/β-catenin pathway plays an important role in the regulation of pluripotency and pancreatic development and differentiation (1, 30–32). Hence, we examined the effect of Wnt inhibitor Dkk-1 on high O2-induced Ngn3 expression. The application of Dkk-1 led to significant repression of high O2-induced Ngn3 expression (p < 0.05; Fig. 5D), showing that activated Wnt signaling is involved in facilitated differentiation in a high O2 condition.
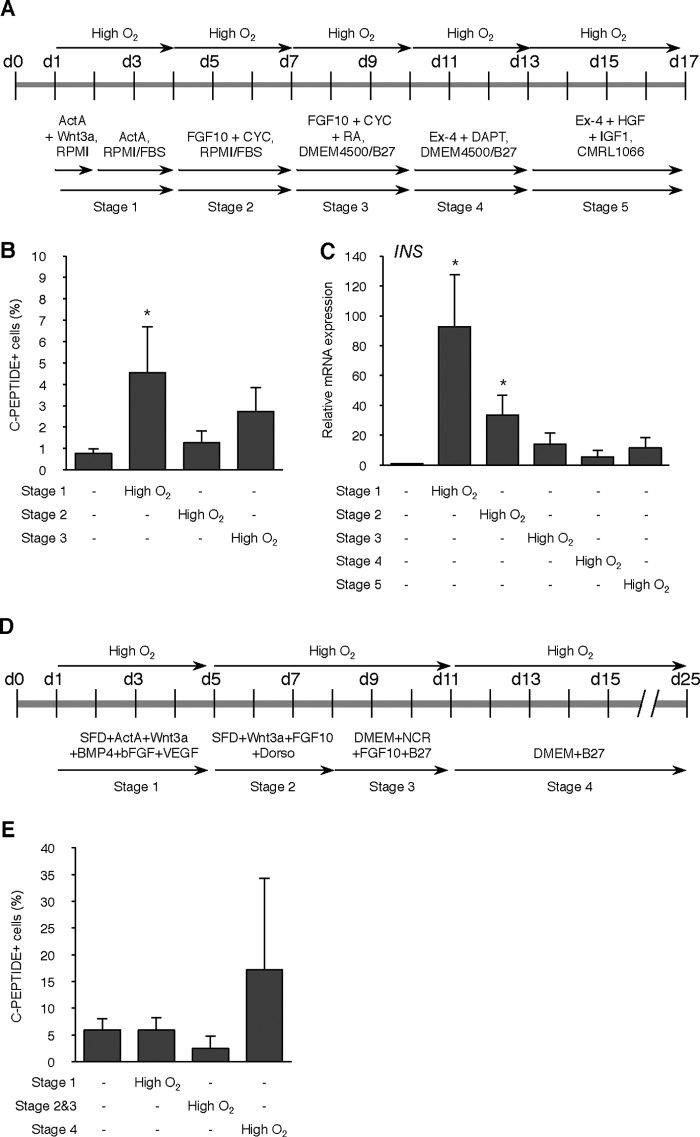
High Oxygen Condition Facilitates Differentiation of hiPSC into Insulin-producing Cells
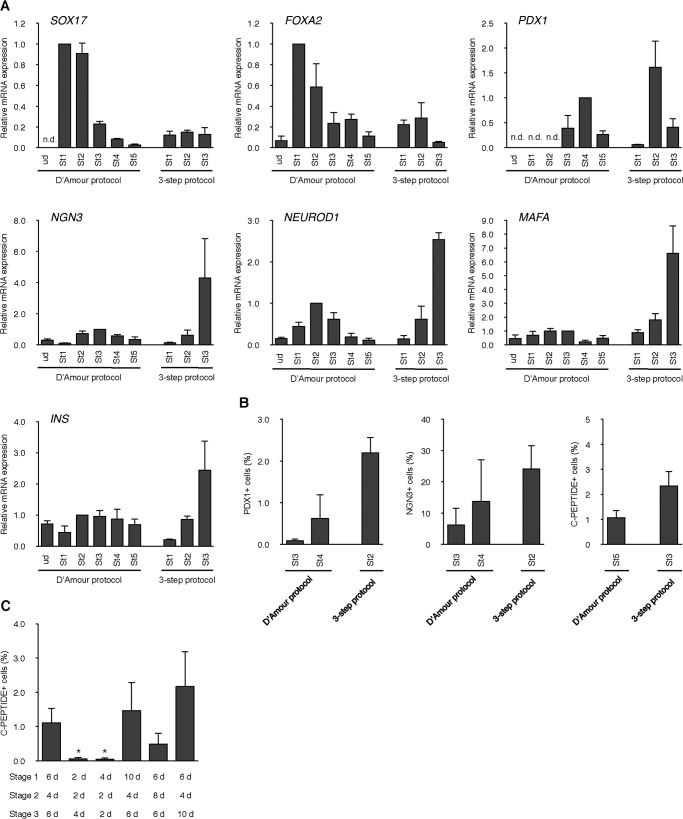
We performed pancreatic differentiation from hiPSC clone 23 (25) by our stepwise protocol (Fig. 6A) and analyzed marker expression by immunofluorescence analysis. It was confirmed that the expression of SOX17, a definitive endoderm marker, was not detected in undifferentiated cells (ud) but was markedly expressed on day 7 (stage 1) during differentiation. That signal continued to appear on day 11 (stage 2) to 17 (stage 3) (Fig. 6B). Another definitive endoderm marker, FOXA2, also began to be expressed at stage 1 and also appeared in later periods (Fig. 6C). Expression of PDX1, a marker of pancreatic progenitors, was not detected in undifferentiated and stage 1 cells (data not shown), whereas some signals were detected at stage 2 with a peak signal at stage 3 (Fig. 6D). NGN3, a marker of endocrine progenitors, was also not detected in undifferentiated and stage 1 cells (data not shown), whereas robust signals were detected at stage 2 and continued to be expressed at stage 3 (Fig. 6E). At stage 3, the termination of this differentiation protocol, several insulin- and C-peptide-positive cells were detected (Fig. 6E). Next, we examined the expression dynamics of marker genes by qPCR analysis and concurrently compared our expression dynamics with that of a previously reported protocol (32) (Fig. 7A). It was revealed that the expression dynamics of analyzed genes during differentiation by our protocol (three-step protocol) was similar to that of pancreatic β-cell development (33, 34). The D'Amour protocol consists of stage 1 to stage 5. Stage 1 guides pluripotent cells to definitive endoderm, stage 2 and 3 to pancreatic progenitors, stage 4 to endocrine progenitors, and stage 5 to hormone-expressing endocrine cells. By the D'Amour protocol, SOX17 and FOXA2 were expressed higher than in our protocol at stage 1 (Fig. 7A). In contrast, PDX1 gene expression was very high at stage 2 of our protocol and was higher than in the D'Amour protocol. Moreover, robust increases of NGN3, NEUROD1, MAFA, and INS expression were detected at the termination of our protocol (Fig. 7A). The percentages of PDX1-, NGN3-, and C-peptide-positive cells in the population of differentiated cells by our protocol were higher than those by the D'Amour protocol (Fig. 7B). Different protocols are usually used for mESC and hESC/iPSC, especially different lengths of time. Therefore, we examined the effect of altered culture times on pancreatic differentiation of hiPSC. A shorter time frame decreased the percentage of C-peptide-positive cells (p < 0.05), whereas a longer time had no effect (Fig. 7C).
FIGURE 7.
Dynamics of pancreatic differentiation marker genes. A, the dynamics of SOX17, FOXA2, PDX1, NGN3, NEUROD1, MAFA, and INS gene expression were analyzed at different stages in normoxic conditions under the D'Amour protocol or our three-step protocol by qPCR. n = 3 each. ud, undifferentiated; st, stage. B, values are the percentage of PDX1-, NGN3-, or C-peptide-positive cells/well of the cells differentiated under the D'Amour protocol or our three-step protocol. n = 3 each. C, values are the percentage of C-peptide-positive cells/well of the cells differentiated under a different time frame of our three-step protocol. *, p < 0.05 versus corresponding control. n = 4 each. d, day. Error bars, S.E.
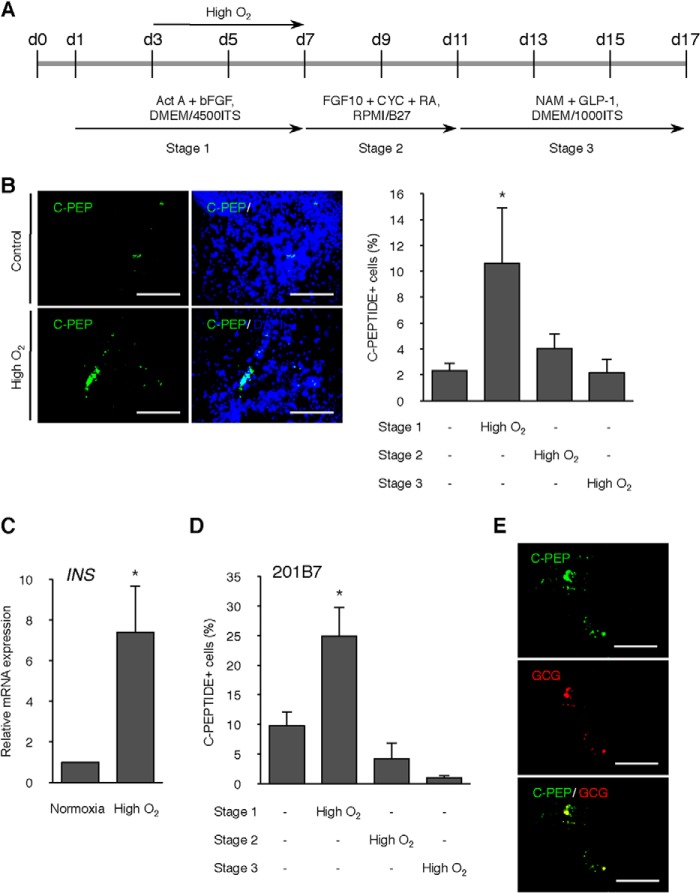
Next we investigated whether the high O2 condition has an effect on the pancreatic differentiation of hiPSC (Fig. 8A). The effect of the high O2 condition during differentiation showed a similar result to that of mESC. Immunofluorescence analysis revealed a significant increase of the percentage of C-peptide-positive cells by the high O2 condition during stage 1 (p < 0.05; Fig. 8B). It was confirmed that there was a significant increase in INS gene expression (p < 0.05; Fig. 8C). These effects were also observed in another hiPSC line, 201B7 (35) (p < 0.05; Fig. 8D). We performed immunostaining for glucagon to determine whether differentiated cells are monohormonal or polyhormonal by our protocol. A few glucagon-positive cells were observed in the differentiated cells treated with the high O2 condition (Fig. 8E). Moreover, co-expression with C-peptide appeared in a few cells, suggesting that some cells were polyhormonal for insulin and glucagon.
FIGURE 8.
Effect of high O2 condition on differentiation efficiency of hiPSCs. A, scheme of the timeline of high O2 condition. B, immunofluorescence for C-peptide on day 17 of differentiation in C23 cells treated with high O2 condition during stage 1. Scale bars, 200 μm. Right graph, values are the percentage of C-peptide-positive cells/well of the cells treated with high O2 condition during three different stages. *, p < 0.05 versus corresponding control. n = 11 each. C, level of the INS gene was analyzed at day 17 of differentiation in normoxia or high O2-treated C23 cells by qPCR. *, p < 0.05 versus corresponding control. n = 4 each. D, values are the percentage of C-peptide-positive cells/well of the cells treated with high O2 condition during three different stages in hiPSC line 201B7. *, p < 0.05 versus corresponding control. n = 8 each. E, immunofluorescence for C-peptide and glucagon on day 17 of differentiation in C23 cells treated with high O2 condition during stage 1. Scale bars, 200 μm. Error bars, S.E.
High Oxygen Condition Facilitates Differentiation of hiPSC into Endocrine Progenitors
Corresponding with our results on mESC, we observed that NGN3 expression was significantly increased on day 7 by the high O2 condition during stage 1, whereas VEGFA and HES1 expression were significantly decreased (p < 0.0000005 and p < 0.000005, respectively; Fig. 9, A and B). Immunofluorescence analysis confirmed that the percentage of NGN3-positive cells was increased by this treatment (Fig. 9C). To further determine whether HIF-1α inhibition and Wnt signaling activation are involved in the case of hiPSC, we performed Western blot analysis of HIF-1α and microarray analysis of high O2-treated hiPSCs. The high O2 condition repressed the HIF-1α protein level (Fig. 9D). By microarray analysis, many genes were found to be up-regulated by the high O2 condition, as listed in supplemental Fig. S2A. Some of these genes were determined as the Wnt receptor signaling pathway in the GO biological process (supplemental Fig. S2B), suggesting that Wnt signaling was also activated in high O2-treated hiPSCs. In addition, Dkk-1 treatment weakened high O2-induced NGN3 expression (Fig. 9E). These results in hiPSC are similar to those observed in mouse ESC, thereby indicating that both human and mouse pluripotent cells follow a similar pathway in a high O2 condition.
FIGURE 9.
Effect of high O2 condition on NGN3 gene expression of hiPSCs. A, scheme of the timeline of high O2 condition. B, levels of VEGFA, HES1, and NGN3 genes were analyzed at day 7 of differentiation in normoxia or high O2-treated C23 cells by qPCR. *, p < 0.05; **, p < 0.01 versus corresponding control. n = 4 each. C, immunofluorescence for NGN3 on day 11 of differentiation in C23 cells treated with high O2 condition during stage 1. Scale bars, 200 μm. Right graph, values are the percentage of NGN3-positive cells/well of the cells treated with high O2 condition during stage 1. *, p < 0.05 versus corresponding control. n = 3 each. D, levels of HIF-1α and β-actin (loading control) were analyzed on day 6 of differentiation in normoxia, high O2, or hypoxia (1% O2; positive control)-treated C23 cells by immunoblotting. E, levels of the NGN3 gene were analyzed on day 6 of differentiation in normoxia or high O2-treated C23 cells with or without Wnt signaling inhibitor Dkk-1 by qPCR. n = 4 each. Error bars, S.E.
We tested whether the high O2 condition had an effect even in the D'Amour protocol and Nostro protocol (1) (Fig. 10A). Using this protocol, we also saw a significant increase in the percentage of C-peptide-positive cells by the high O2 condition from day 1 to day 4 (Stage 1; p < 0.05) and also in the INS expression by high O2 condition from day 1 to day 4 (Stage 1; p < 0.05) and from day 4 to day 7 (Stage 2; p < 0.05) (Fig. 10, B and C). However, in the Nostro protocol, we did not observe any facilitative effect of the high O2 condition on the percentage of C-peptide-positive cells (Fig. 10, D and E).
FIGURE 10.
Effect of high O2 condition on differentiation efficiency of hiPSCs in D'Amour and Nostro protocol. A, scheme of the D'Amour differentiation protocol and the timeline of high O2 condition. Ex-4, exendin-4; HGF, hepatocyte growth factor; IGF1, insulin-like growth factor 1. B, values are the percentage of C-peptide-positive cells/well of the cells treated with high O2 condition during three different stages. *, p < 0.05 versus corresponding control. n = 6 each. C, levels of INS gene were analyzed on day 17 of differentiation in cells treated with high O2 condition during five different stages by qPCR. *, p < 0.05 versus corresponding control. n = 7 each. D, scheme of the Nostro differentiation protocol and the timeline of high O2 condition. E, values are the percentage of C-peptide-positive cells/well of the cells treated with high O2 condition during three different stages. n = 3 each. Error bars, S.E.
DISCUSSION
Insulin-secreting pancreatic β-cells are essential regulators of the mammalian metabolism. The absence of functional β-cells leads to hyperglycemia and diabetes, making patients dependent on exogenously supplied insulin. Recent insights into β-cell development, combined with the discovery of pluripotent stem cells, have led to an unprecedented opportunity to generate new β-cells for transplantation therapy and drug screening (36, 37). It is important to mimic the in vivo developmental stages of pancreatic organogenesis in which cells are transitioned through mesendoderm, definitive endoderm, foregut endoderm, pancreatic progenitor, and the endocrine progenitor stage, until mature β-cells are obtained from pluripotent stem cells (38). Oxygen tension, the partial pressure of oxygen, has been shown to regulate the stem cell function and embryonic development of several organs, including the pancreas (9, 10, 15–23). In the present study, we demonstrated that a high O2 condition during the in vitro differentiation of ESC and iPSC has a facilitative effect on generating pancreatic progenitors and insulin-producing cells.
In our stepwise differentiation protocol, the cells transitioned through definitive endoderm, pancreatic progenitor, endocrine progenitor, and insulin-producing cells, as revealed by qPCR analysis. Induction of Pdx1 and Ngn3 gene expressions appeared on day 7 (stage 1), showing that a slight transition to pancreatic progenitors and endocrine progenitors had already started during stage 1 (Fig. 1B). With this protocol, treatment with a high O2 condition during stage 1 (toward definitive endoderm) increased differentiation efficiency into endocrine progenitors and subsequent insulin-producing cells. This was demonstrated by a significant increase of Ngn3-positive cells and Ngn3 gene expression (Fig. 3, B, E, and F). Ngn3 is a basic helix-loop-helix transcription factor expressed in cell progenitors that is necessary to initiate the endocrine differentiation program in pancreatic development (39, 40), and its gene expression is inversely regulated by HIF-1α (22). Down-regulation of Notch signaling will yield cells that express Ngn3 (41). Ngn3 gene expression and pancreatic endocrine development are tightly regulated by Hes1, which is an inhibitory bHLH factor activated by Notch signaling and binds to the proximal promoter of Ngn3 and specifically blocks promoter activity (39, 42). It has been shown that HIF-1α activates Notch-responsive promoters and increases the expression of Notch direct downstream genes, including Hes1 (11). During differentiation, HIF-1α signaling is moderately activated even in normoxic conditions, revealed by HIF-1α protein expression and an increase of its target gene Vegfa, whereas a high O2 condition markedly repressed both expressions (Fig. 4, B and C). Supporting our results, it is reported that HIF-1α signaling of cultured stem cells is activated during spontaneous differentiation even in normoxic conditions, showing a time-dependent increase of Vegfa (43). A high O2 condition might increase cellular O2 concentration and lead to inhibition of HIF-1α signaling. The high O2 condition significantly repressed Hes1 gene expression on days 5 and 6 (Fig. 4D). Consistent with previous reports, inhibition of HIF-1α signaling might lead to repression of Hes1 expression and subsequent induction of Ngn3 expression in a high O2 condition. Furthermore, the HIF-1α inhibitor echinomycin had an effect similar to that of the high O2 condition (Fig. 4F). These data indicate that inhibition of HIF-1α signaling is involved in the facilitative effect of the high O2 condition on pancreatic differentiation. The high O2 condition had no effect on Sox17 gene expression and the number of immunoreactive cells (Fig. 3, B and C), suggesting that its treatment might affect the transition from definitive endoderm to pancreatic progenitor or endocrine progenitor. This is supported by the finding that its treatment did not increase Ngn3 expression in the undifferentiated state (Fig. 3G). The high O2 condition significantly decreased Oct4 gene expression (Fig. 3B). Oct4 gene expression is directly regulated by HIF-2α, also a hypoxia-dependent factor (12); therefore, it is considered that the high O2 condition might repress Oct4 gene expression via HIF-2α inhibition.
By microarray analysis, we found that the Wnt signaling pathway is activated in high O2 condition-treated cells (Fig. 5B). Wnt inhibitor Dkk-1 partially repressed high O2 condition-induced Ngn3 expression (Fig. 5D). It is reported that hypoxia inhibits Wnt signaling via HIF-1α competing with T-cell factor-4 (TCF-4) for direct binding to β-catenin (44). The high O2 condition might inhibit HIF-1α signaling, and compensatory Wnt signaling was activated. This is supported by the finding that genes induced by the HIF-1α inhibitor echinomycin are also involved in the Wnt signaling pathway (Fig. 5C). The canonical Wnt cascade has emerged as a critical regulator of self-renewal and pluripotency in stem cells (30, 45–49). In contrast, it is also reported that Wnt/β-catenin signaling promotes the differentiation, not self-renewal, of embryonic stem cells (1, 32, 50, 51). Nostro et al. (1) showed that Wnt signaling induces a posterior endoderm fate, the primed stage from definitive endoderm, and at optimal concentrations enhances the development of pancreatic lineage cells. In this report, Wnt signaling did not affect the levels of PDX1 but did increase INS expression in hiPSC with Wnt3a treatment at the stage of definitive endoderm to pancreatic endoderm. This report is consistent with our findings that a high O2 condition activates Wnt signaling and facilitates differentiation from definitive endoderm into pancreatic fate. In the developing embryo, a key step in the generation of endoderm-derived cell types is patterning the appropriate region of the gut tube along the anterior-posterior axis. Studies using different model systems have shown that in gastrulation, Wnt signaling is restricted to the posterior region of the embryo and, together with FGF signaling, is responsible for the induction of a posterior phenotype (52, 53). Wnt signaling activated by a high O2 condition might function to promote the development of a posterior phenotype in mESC and hiPSC cultures.
The high O2 condition also facilitates pancreatic differentiation of hiPSC. Our stepwise differentiation protocol generated insulin-producing cells larger than the D'Amour protocol (32). However, in the case of SOX17 and FOXA2, a robust increase was observed in the D'Amour protocol (Fig. 7A), indicating that the induction of definitive endoderm was more efficient than our three-step protocol. This might have been due to the difference of the activin A concentration (D'Amour protocol, 100 ng/ml; our protocol, 10 ng/ml), because induction of definitive endoderm by activin A is reported to increase in a dose-dependent fashion (54–56). However, at stage 2 of our protocol, there was a marked increase in the expression of PDX1, greater than at stage 4 of the D'Amour protocol. Furthermore, NGN3, NEUROD1, MAFA, and INS gene expressions were higher in our protocol, indicating that the induction of pancreatic fate from definitive endoderm in our protocol was more efficient than with the D'Amour protocol. Using this protocol, hiPSCs more efficiently differentiated into endocrine progenitors and insulin-producing cells in the high O2 condition during stage 1, similar to mESCs. The effect of the high O2 condition was also observed in the D'Amour protocol but not in the Nostro protocol (1) (Fig. 10, A–E). Nostro et al. included VEGF during stage 1, probably to support endothelial development for pancreatic differentiation of hESC (1, 57). The high O2 condition repressed VEGF expression in our study (Fig. 9B), and a similar effect is expected to occur in the Nostro protocol. This effect may compete with the addition of VEGF. Therefore, the high O2 condition seems to have no facilitative effect in the Nostro protocol.
Insulin-producing cells obtained in our study did not secrete insulin by high glucose stimulation (data not shown), and some cells were polyhormonal because co-expression of insulin and glucagon occurred. During normal human embryogenesis, β-cells are not generated until ∼10 weeks after endoderm specification (58), whereas in hiPSC differentiation cultures, this typically occurs in 2–3 weeks (32). It is possible that pancreatic differentiation in human ESC/iPSC culture may be accelerated by rapid changing of the transcriptional network and/or epigenetic modifications by changing supplements (growth factor and inhibitors, etc). For proper β-cells, it may be necessary to change the extracellular environment and signal more precisely to mimic normal human embryogenesis.
In a previous study, Shah et al. (21) stated that the early mammalian embryo is located within the uterus, with a non-existent or immature cardiovascular system and blood supply, but, despite this hypoxic environment, the embryo is still able to undergo rapid growth and organogenesis. Furthermore, they showed that the number of Ngn3-positive cells was not altered by hypoxia treatment in pancreatic explants, whereas the number of insulin-positive cells was decreased by hypoxia, implying that high oxygen may only be required at later stages during pancreatic differentiation, namely endocrine progenitor to β-cell. However, in our study, the number of Ngn3-positive cells differentiated from mESCs and hiPSCs was increased by the high O2 condition (Figs. 3 (E and F) and 9C). There are some differences between the culture environments of dissociated mESCs and pancreatic explants. HIF-1α levels seem to be different between mESC culture and pancreatic explants because of spatial and temporal patterns of cell-cell interactions. Moreover, the external signals were different because our study used chemically defined medium, whereas pancreatic explants were maintained in serum-containing medium. These differences seemed to have caused the discrepancy.
A previous study modulated the O2 environment for pancreatic differentiation (59). Cheng et al. used a 5% O2 environment for maintaining and differentiating human endodermal progenitor cells into β-cells. However, they did not mention the reason for using a hypoxic environment and did not compare the effect on differentiation cultured under hypoxia with normoxia. In our study, a hypoxic condition (5% O2) during differentiation had no facilitative effect on the number of Ins1-GFP-positive cells of mESCs. This discrepancy seems to have been particularly caused by the cell density during differentiation. In their studies, endodermal progenitor cells were plated in 12-well dishes at 3–4 × 105 cells/well as dissociated at the start of differentiation, whereas in our study, hiPSCs were grown for 7 days as a colony before the start of differentiation. In the colony state, cells appeared to promote a hypoxic phenotype because HIF-1α was expressed at a detectable level even in normoxia (Fig. 9D). Therefore, in our case, the high O2 condition rather than the hypoxic condition facilitated pancreatic differentiation.
In conclusion, the present study showed that a high O2 condition during differentiation has facilitative effects on generating insulin-producing cells from mESCs and hiPSCs. This effect was due to the inhibition of Notch signaling and activation of Wnt signaling during definitive endoderm to pancreatic fate. We also found that HIF-1α inhibition during differentiation accelerated the generation of pancreatic lineages. These observations would provide an efficient method of utilizing patient-specific iPS cells for the treatment of diabetes.
Acknowledgment
We thank A. Maeda for experimental support.
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Japan Society for the Promotion of Science through its “Funding Program for Next Generation World-leading Researchers,” by the Uehara Memorial Foundation, and by the Takeda Science Foundation.

This article contains supplemental Figs. 1 and 2.
- ESC
- embryonic stem cell
- mESC
- mouse embryonic stem cell
- iPSC
- induced pluripotent stem cell
- hiPSC
- human induced pluripotent stem cell
- HIF
- hypoxia-inducible factor
- MEF
- mouse embryo fibroblast
- NEAA
- nonessential amino acid(s)
- β-ME
- β-mercaptoethanol
- bFGF
- basic fibroblast growth factor
- qPCR
- quantitative PCR.
REFERENCES
- 1. Nostro M. C., Sarangi F., Ogawa S., Holtzinger A., Corneo B., Li X., Micallef S. J., Park I. H., Basford C., Wheeler M. B., Daley G. Q., Elefanty A. G., Stanley E. G., Keller G. (2011) Stage-specific signaling through TGFβ family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138, 861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kunisada Y., Tsubooka-Yamazoe N., Shoji M., Hosoya M. (2012) Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 8, 274–284 [DOI] [PubMed] [Google Scholar]
- 3. Higuchi Y., Shiraki N., Yamane K., Qin Z., Mochitate K., Araki K., Senokuchi T., Yamagata K., Hara M., Kume K., Kume S. (2010) Synthesized basement membranes direct the differentiation of mouse embryonic stem cells into pancreatic lineages. J. Cell Sci. 123, 2733–2742 [DOI] [PubMed] [Google Scholar]
- 4. Maehr R., Chen S., Snitow M., Ludwig T., Yagasaki L., Goland R., Leibel R. L., Melton D. A. (2009) Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 106, 15768–15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang D., Jiang W., Liu M., Sui X., Yin X., Chen S., Shi Y., Deng H. (2009) Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 19, 429–438 [DOI] [PubMed] [Google Scholar]
- 6. Lumelsky N., Blondel O., Laeng P., Velasco I., Ravin R., McKay R. (2001) Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292, 1389–1394 [DOI] [PubMed] [Google Scholar]
- 7. Kroon E., Martinson L. A., Kadoya K., Bang A. G., Kelly O. G., Eliazer S., Young H., Richardson M., Smart N. G., Cunningham J., Agulnick A. D., D'Amour K. A., Carpenter M. K., Baetge E. E. (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 [DOI] [PubMed] [Google Scholar]
- 8. Sneddon J. B., Borowiak M., Melton D. A. (2012) Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature 491, 765–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keith B., Simon M. C. (2007) Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mohyeldin A., Garzón-Muvdi T., Quiñones-Hinojosa A. (2010) Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150–161 [DOI] [PubMed] [Google Scholar]
- 11. Gustafsson M. V., Zheng X., Pereira T., Gradin K., Jin S., Lundkvist J., Ruas J. L., Poellinger L., Lendahl U., Bondesson M. (2005) Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev. Cell 9, 617–628 [DOI] [PubMed] [Google Scholar]
- 12. Covello K. L., Kehler J., Yu H., Gordan J. D., Arsham A. M., Hu C. J., Labosky P. A., Simon M. C., Keith B. (2006) HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 20, 557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Semenza G. L. (1999) Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15, 551–578 [DOI] [PubMed] [Google Scholar]
- 14. Chan D. A., Giaccia A. J. (2007) Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 26, 333–339 [DOI] [PubMed] [Google Scholar]
- 15. Jarecki J., Johnson E., Krasnow M. A. (1999) Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99, 211–220 [DOI] [PubMed] [Google Scholar]
- 16. Provot S., Zinyk D., Gunes Y., Kathri R., Le Q., Kronenberg H. M., Johnson R. S., Longaker M. T., Giaccia A. J., Schipani E. (2007) Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J. Cell Biol. 177, 451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schipani E., Ryan H. E., Didrickson S., Kobayashi T., Knight M., Johnson R. S. (2001) Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 15, 2865–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon M. C., Keith B. (2008) The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 9, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tian H., Hammer R. E., Matsumoto A. M., Russell D. W., McKnight S. L. (1998) The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12, 3320–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heinis M., Soggia A., Bechetoille C., Simon M. T., Peyssonnaux C., Rustin P., Scharfmann R., Duvillié B. (2012) HIF1α and pancreatic β-cell development. FASEB J. 26, 2734–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shah S. R., Esni F., Jakub A., Paredes J., Lath N., Malek M., Potoka D. A., Prasadan K., Mastroberardino P. G., Shiota C., Guo P., Miller K. A., Hackam D. J., Burns R. C., Tulachan S. S., Gittes G. K. (2011) Embryonic mouse blood flow and oxygen correlate with early pancreatic differentiation. Dev. Biol. 349, 342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinis M., Simon M. T., Ilc K., Mazure N. M., Pouysségur J., Scharfmann R., Duvillié B. (2010) Oxygen tension regulates pancreatic β-cell differentiation through hypoxia-inducible factor 1α. Diabetes 59, 662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fraker C. A., Alvarez S., Papadopoulos P., Giraldo J., Gu W., Ricordi C., Inverardi L., Domínguez-Bendala J. (2007) Enhanced oxygenation promotes β-cell differentiation in vitro. Stem Cells 25, 3155–3164 [DOI] [PubMed] [Google Scholar]
- 24. Hara M., Wang X., Kawamura T., Bindokas V. P., Dizon R. F., Alcoser S. Y., Magnuson M. A., Bell G. I. (2003) Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 284, E177–E183 [DOI] [PubMed] [Google Scholar]
- 25. Kaitsuka T., Noguchi H., Shiraki N., Kubo T., Wei F. Y., Hakim F., Kume S., Tomizawa K. (2014) Generation of functional insulin-producing cells from mouse ES cells through 804G cell-derived extracellular matrix and protein transduction of transcription factors. Stem Cells Transl. Med. 3, 114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakano D., Shiraki N., Kikawa K., Yamazoe T., Kataoka M., Umeda K., Araki K., Mao D., Matsumoto S., Nakagata N., Andersson O., Stainier D., Endo F., Kume K., Uesugi M., Kume S. (2014) VMAT2 identified as a regulator of late-stage β-cell differentiation. Nat. Chem. Biol. 10, 141–148 [DOI] [PubMed] [Google Scholar]
- 27. Gu G., Wells J. M., Dombkowski D., Preffer F., Aronow B., Melton D. A. (2004) Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131, 165–179 [DOI] [PubMed] [Google Scholar]
- 28. Shiraki N., Yoshida T., Araki K., Umezawa A., Higuchi Y., Goto H., Kume K., Kume S. (2008) Guided differentiation of embryonic stem cells into Pdx1-expressing regional-specific definitive endoderm. Stem Cells 26, 874–885 [DOI] [PubMed] [Google Scholar]
- 29. Hu C. J., Iyer S., Sataur A., Covello K. L., Chodosh L. A., Simon M. C. (2006) Differential regulation of the transcriptional activities of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in stem cells. Mol. Cell Biol. 26, 3514–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reya T., Clevers H. (2005) Wnt signalling in stem cells and cancer. Nature 434, 843–850 [DOI] [PubMed] [Google Scholar]
- 31. Yamaguchi T. P. (2001) Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 11, R713–R724 [DOI] [PubMed] [Google Scholar]
- 32. D'Amour K. A., Bang A. G., Eliazer S., Kelly O. G., Agulnick A. D., Smart N. G., Moorman M. A., Kroon E., Carpenter M. K., Baetge E. E. (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 [DOI] [PubMed] [Google Scholar]
- 33. Oliver-Krasinski J. M., Stoffers D. A. (2008) On the origin of the beta cell. Genes Dev. 22, 1998–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murtaugh L. C. (2007) Pancreas and beta-cell development: from the actual to the possible. Development 134, 427–438 [DOI] [PubMed] [Google Scholar]
- 35. Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 [DOI] [PubMed] [Google Scholar]
- 36. Pagliuca F. W., Melton D. A. (2013) How to make a functional β-cell. Development 140, 2472–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baetge E. E. (2008) Production of beta-cells from human embryonic stem cells. Diabetes Obes. Metab. 10, 186–194 [DOI] [PubMed] [Google Scholar]
- 38. Champeris Tsaniras S., Jones P. M. (2010) Generating pancreatic beta-cells from embryonic stem cells by manipulating signaling pathways. J. Endocrinol. 206, 13–26 [DOI] [PubMed] [Google Scholar]
- 39. Gradwohl G., Dierich A., LeMeur M., Guillemot F. (2000) Neurogenenin 3 is required for the development of the four endocrine cell lineages of the pancrease. Proc. Natl. Acad. Sci. U.S.A. 97, 1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu G., Dubauskaite J., Melton D. A. (2002) Direct evidence for the pancreatic lineage: Ngn3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 [DOI] [PubMed] [Google Scholar]
- 41. Apelqvist A., Li H., Sommer L., Beatus P., Anderson D. J., Honjo T., Hrabe de Angelis M., Lendahl U., Edlund H. (1999) Notch signalling controls pancreatic cell differentiation. Nature 400, 877–881 [DOI] [PubMed] [Google Scholar]
- 42. Lee J. C., Smith S. B., Watada H., Lin J., Scheel D., Wang J., Mirmira R. G., German M. S. (2001) Regulation of the pancreatic pro-endocrine gene neurogenin3. Diabetes 50, 928–936 [DOI] [PubMed] [Google Scholar]
- 43. Lee S. W., Jeong H. K., Lee J. Y., Yang J., Lee E. J., Kim S. Y., Youn S. W., Lee J., Kim W. J., Kim K. W., Lim J. M., Park J. W., Park Y. B., Kim H. S. (2012) Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol. Med. 4, 924–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaidi A., Williams A. C., Paraskeva C. (2007) Interaction between β-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 9, 210–217 [DOI] [PubMed] [Google Scholar]
- 45. Sato N., Meijer L., Skaltsounis L., Greengard P., Brivanlou A. H. (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat. Med. 10, 55–63 [DOI] [PubMed] [Google Scholar]
- 46. ten Berge D., Kurek D., Blauwkamp T., Koole W., Maas A., Eroglu E., Siu R. K., Nusse R. (2011) Embryonic stem cells require Wnt proteins to prevent differentiation to epiblast stem cells. Nat. Cell Biol. 13, 1070–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sokol S. Y. (2011) Maintaining embryonic stem cell pluripotency with Wnt signaling. Development 138, 4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yi F., Pereira L., Hoffman J. A., Shy B. R., Yuen C. M., Liu D. R., Merrill B. J. (2011) Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat. Cell Biol. 13, 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyabayashi T., Teo J. L., Yamamoto M., McMillan M., Nguyen C., Kahn M. (2007) Wnt/β-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc. Natl. Acad. Sci. U.S.A. 104, 5668–5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davidson K. C., Adams A. M., Goodson J. M., McDonald C. E., Potter J. C., Berndt J. D., Biechele T. L., Taylor R. J., Moon R. T. (2012) Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. U.S.A. 109, 4485–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nostro M. C., Cheng X., Keller G. M., Gadue P. (2008) Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell 2, 60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Keenan I. D., Sharrard R. M., Isaacs H. V. (2006) FGF signal transduction and the regulation of Cdx gene expression. Dev. Biol. 299, 478–488 [DOI] [PubMed] [Google Scholar]
- 53. McLin V. A., Rankin S. A., Zorn A. M. (2007) Repression of Wnt/β-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development 134, 2207–2217 [DOI] [PubMed] [Google Scholar]
- 54. Kubo A., Shinozaki K., Shannon J. M., Kouskoff V., Kennedy M., Woo S., Fehling H. J., Keller G. (2004) Development of definitive endoderm from embryonic stem cells in culture. Development 131, 1651–1662 [DOI] [PubMed] [Google Scholar]
- 55. Gadue P., Huber T. L., Paddison P. J., Keller G. M. (2006) Wnt and TGF-β signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 103, 16806–16811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sulzbacher S., Schroeder I. S., Truong T. T., Wobus A. M. (2009) Activin A-induced differentiation of embryonic stem cells into endoderm and pancreatic progenitors: the influence of differentiation factors and culture conditions. Stem Cell Rev. 5, 159–173 [DOI] [PubMed] [Google Scholar]
- 57. Gouon-Evans V., Boussemart L., Gadue P., Nierhoff D., Koehler C. I., Kubo A., Shafritz D. A., Keller G. (2006) BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat. Biotechnol. 24, 1402–1411 [DOI] [PubMed] [Google Scholar]
- 58. Spence J. R., Wells J. M. (2007) Translational embryology: using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Dev. Dyn. 236, 3218–3227 [DOI] [PubMed] [Google Scholar]
- 59. Cheng X., Ying L., Lu L., Galvão A. M., Mills J. A., Lin H. C., Kotton D. N., Shen S. S., Nostro M. C., Choi J. K., Weiss M. J., French D. L., Gadue P. (2012) Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 10, 371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]