Background: Munc18-1 is required for membrane fusion, but the underlying mechanism is unknown.
Results: Distinct point mutations in domain 3a of Munc18-1 differentially affect the conformation of helix 12, VAMP2 binding, and membrane fusion.
Conclusion: A conformational switch in helix 12 promotes SNAREpin assembly via the VAMP2 interaction.
Significance: The Munc18-1-VAMP2 interaction may represent a general molecular mechanism of how SM proteins accelerate membrane fusion.
Keywords: Exocytosis, Membrane Fusion, Membrane Reconstitution, Membrane Trafficking, Snare Proteins, SM Protein, VAMP2/Synaptobrevin, Synaptic Vesicle
Abstract
Munc18-1, a SEC1/Munc18 protein and key regulatory protein in synaptic transmission, can either promote or inhibit SNARE complex assembly. Although the binary inhibitory interaction between Munc18-1 and closed syntaxin 1 is well described, the mechanism of how Munc18-1 stimulates membrane fusion remains elusive. Using a reconstituted assay that resolves vesicle docking, priming, clamping, and fusion during synaptic exocytosis, we show that helix 12 in domain 3a of Munc18-1 stimulates SNAREpin assembly and membrane fusion. A single point mutation (L348R) within helix 12 selectively abolishes VAMP2 binding and the stimulatory function of Munc18-1 in membrane fusion. In contrast, targeting a natural switch site (P335A) at the start of helix 12, which can result in an extended α-helical conformation, further accelerates lipid-mixing. Together with structural modeling, the data suggest that helix 12 provides a folding template for VAMP2, accelerating SNAREpin assembly and membrane fusion. Analogous SEC1/Munc18-SNARE interactions at other transport steps may provide a general mechanism to drive lipid bilayer merger. At the neuronal synapse, Munc18-1 may convert docked synaptic vesicles into a readily releasable pool.
Introduction
SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) and SEC1/Munc18 proteins are the general machinery for intracellular membrane fusion (for reviews, see Refs. 1–3). At the neuronal synapse, the v-SNARE VAMP2/synaptobrevin 2 located on synaptic vesicles pairs with its cognate t-SNARE syntaxin 1·SNAP-25 on the plasma membrane, and their SNARE motifs start forming a four-helix bundle (4–6). Zippering of the trans-SNARE complex (SNAREpin) begins at the N-terminal membrane distal halves of the SNARE motifs and sequentially progresses through the C-terminal halves and the membrane proximal linker regions (6–12). Thereby, it gradually brings the membranes in closer contact, finally culminating in the post fusion SNARE complex, which also includes interactions between the transmembrane domains of VAMP2 and syntaxin 1 (13). SNARE complex assembly drives membrane fusion and releases enough energy to overcome the repulsive forces, which prevent spontaneous lipid bilayer merger (5, 11).
SEC1/Munc18 proteins control SNARE complex assembly and are in general essential for cell survival (1, 14). In the case of neuronal exocytosis, the inactivation of the corresponding SEC1/Munc18 isoform/orthologue, Munc18-1,2 dramatically reduces transmitter secretion (15–19). Munc18-1 controls the assembly of the neuronal SNARE complex at several distinct stages. Munc18-1 binds syntaxin 1 in a closed conformation in which the regulatory N-terminal Habc domain of syntaxin 1 folds back onto the SNARE motif (20–22). In this closed conformation, Munc18-1 stabilizes syntaxin 1, escorts newly synthesized syntaxin 1 molecules from the endoplasmic reticulum to the plasma membrane, and precludes SNAP-25 and VAMP2 binding (21, 23–28). The crystal structure of the high affinity Munc18-1·syntaxin 1 binary complex shows extensive interactions of closed syntaxin 1 with a cavity formed by Munc18-1 domains 1 and 3, structurally illustrating the inability of syntaxin to bind its partner SNAREs (29). At the plasma membrane, this closed conformation can be released by Munc13-1, now favoring t-SNARE complex assembly (24, 30–33). Indeed, the initially inhibitory role of Munc18-1 can be switched to a stimulatory function accelerating membrane fusion. This stimulation requires the specific binding of Munc18-1 to VAMP2 or VAMP3 (34, 35). Molecular interactions of Munc18-1 with the membrane-proximal region of VAMP2 and the N-terminal peptide of syntaxin 1 as well as the SNARE bundle have been described, but the exact mechanism how these interactions augment membrane fusion remain to be resolved (25, 27, 28, 34–42). One possibility could be that the syntaxin 1/SNAP-25/Munc18-1/VAMP2 interactions provide a bridge between the plasma membrane and the secretory/synaptic vesicle, consistent with the vesicle docking defect observed in chromaffin cells lacking functional Munc18-1 (17, 43, 44). However, vesicle docking can be rescued in the absence of Munc18-1 by the overexpression of SNAP-25 and the stabilization of t-SNARE complexes (45). The assembled t-SNAREs in turn provide efficient binding sites for the Ca2+ sensor synaptotagmin 1 (Syt1), which is localized on synaptic vesicles and thus can bridge the two membranes (45). Indeed, cellular and in vitro assays demonstrate that Syt1 docks vesicles in a Ca2+-independent manner (45–52). However, despite the rescue of the vesicle docking phenotype, Munc18-1-deficient cells remain impaired in membrane fusion (45). Thus, Munc18-1 has a post-docking, vesicle priming function likely involving a critical step in SNARE complex assembly upstream of the Ca2+-triggered fusion process (52).
At the neuronal synapse, fast Ca2+-triggered fusion requires both the Ca2+ sensor Syt1 and complexin (Cpx) (53, 54). Complexins stabilize half-zippered SNARE complexes but block further assembly by competing with VAMP2 for the binding to the membrane proximal part of the t-SNARE (50, 55–60). Binding of Ca2+ to Syt1 perturbs the local membrane environment and releases the Cpx-mediated inhibition of SNAREpin zippering, thus triggering fusion pore opening and neurotransmitter release (50, 61–66). Biochemical studies revealed that complexin and Munc18-1 can simultaneously bind SNARE complexes, and liposome fusion assays confirmed that Munc18-1, Cpx II, Syt1, and SNAREs are sufficient to reconstitute fast Ca2+-synchronized membrane fusion (50, 52, 67). Thus, based both on cellular and reconstituted membrane fusion assays, the central issue emerges of how Munc18-1 mechanistically stimulates SNARE complex assembly, a reaction of fundamental relevance for regulated exocytosis.
To study this mechanism, we made use of reconstituted membrane fusion assays, which can resolve the inhibitory and stimulatory functions of Munc18-1 and include the Ca2+-synchronized reaction. We introduced three point mutations within domain 3a of Munc18-1 that result in a complete loss-of-function (L348R), a partial loss-of-function (M334R), and a gain-of-function (P335A) phenotype. In addition, a double mutant (P335A/L348R) demonstrated that the gain-of-function phenotype of P335A strictly depends on the VAMP2 interaction of Munc18-1. Each of these mutants retained the binding to monomeric syntaxin 1, but they differ in their inhibitory and stimulatory functions. Our data together with structural modeling suggest that the surface-exposed helix 12 in domain 3a of Munc18-1 acts as a molecular switch and template to position or fold the membrane proximal region of the VAMP2 SNARE motif and linker regions, thereby stimulating initial SNARE complex formation or/and late steps in SNAREpin zippering.
EXPERIMENTAL PROCEDURES
Munc18-1 Mutagenesis
DNA constructs were made using standard genetic manipulations. Munc18-1 cDNA subcloned into the pEG(KG) vector (a kind gift of Dr. Richard Scheller) was mutated by QuikChange site-directed mutagenesis (Stratagene) using Phusion polymerase (New England Biolabs) and the following HPLC purified mutagenesis primers (MWG Operon): M334R forward (5′-CCAGATGCTGAAGAAAAGGCCCCAGTACCAGAAGG-3′) and M334R reverse (5′-CTTCTGGTACTGGGGCCTTTTCTTCAGCATCTGGG-3′); P335A forward (5′-GATGCTGAAGAAAATGGCCCAGTACCAGAAGGAGC-3′) and P335A reverse (5′-TCCTTCTGGTACTGGGCCATTTTCTTCAGCATCTG-3′); L348R forward (5′-CAAGTATTCGACTCACCGGCACCTTGCTGAAGACTG-3′) and L348R reverse (5′-AGTCTTCAGCAAGGTGCCGGTGAGTCGAATACTTGC-3′). The integrity of the mutants was verified by DNA sequencing at GATC Biotech (sequencing primer forward (5′-CTGCTGCCTATTGAAAATG-3′) and reverse (5′-CATTTTCAATAGGCAGCAG-3′)).
Protein Purification
Recombinant mammalian His6-tagged proteins were expressed in Escherichia coli BL21(DE3) bacteria (Stratagene). Purifications of VAMP2 (amino acids (aa) 1–116), syntaxin 1 (aa 1–288), Syt1, co-expressed syntaxin 1 (aa 1–288)·SNAP-25 complexes (t-SNARE), and CpxII were performed exactly as described previously via Ni2+-nitrilotriacetic acid affinity chromatography and subsequent ion exchange chromatography (5, 50, 52). Munc18-1 was purified as follows. Recombinant rat GST-Munc18-1 encoded in the pEG(KG) vector was transformed into BL21(DE3) bacteria. Bacterial cultures in 4 liters of LB medium containing 100 μg/ml ampicillin were grown at 37 °C to an A600 of 0.8. Protein expression was induced overnight at 16 °C by the addition of 0.3 mm isopropyl-β-d-thiogalactopyranoside. The following day bacteria were collected by centrifugation and washed once with ice-cold PBS, pH 7.4. After another round of centrifugation, bacteria were resuspended in ice-cold lysis buffer (25 mm HEPES-KOH, pH 7.4, 135 mm KCl, 1 mm DTT, 10 mm methionine) in a volume of 100 ml. Protease inhibitors were added to the following concentrations: leupeptin (1.5 μg/ml), antipain (2.5 μg/ml), turkey trypsin inhibitor (25 μg/ml), benzamidine (12.5 μg/ml), Pefabloc SC (6.25 μg/ml), aprotinin (1.25 μg/ml), chymostatin (5 μg/ml), and pepstatin (2.5 μg/ml). For lysis, bacteria were passed through a Microfluidizer M110L (Microfluidics) at >10,000 p.s.i. Final concentrations of 50 units/ml Benzonase (Merck) as well as 1 mm MgCl2 were added to the bacterial lysate followed by a 10-min incubation step on ice. Subsequently, insoluble material was removed by ultracentrifugation for 1 h at 40,000 rpm at 4 °C in a 45Ti rotor (Beckman Coulter). 100 ml of the supernatant containing GST-Munc18-1 were incubated with 1 ml of glutathione (GSH)-Sepharose 4 fast flow beads (GE Healthcare) for 2 h at 4 °C on a rotating wheel. Subsequently, beads were washed in batch 6× with lysis buffer, and Munc18-1 was cleaved off the GST tag by the addition of thrombin (Calbiochem) to a final concentration of 25 units/ml for 1 h at room temperature. The Munc18-1 eluate was collected with Biospin disposable chromatography columns (Bio-Rad). 50 μl of benzamidine-Sepharose 6B beads (GE Healthcare) (washed 3× with H2O and 3× with lysis buffer) and Pefabloc SC (Roche Applied Science) (6 mm final concentration) were added overnight at 4 °C on a rotating wheel. The following day samples were centrifuged at 20,000 × g for 10 min at 4 °C, and the supernatant was loaded on a PD10 column (GE Healthcare) for buffer exchange to fusion buffer (25 mm HEPES-KOH, pH 7.4, 135 mm KCl, 1 mm DTT). Another round of centrifugation for 10 min at 55,000 rpm in a TLA 55 rotor at 4 °C (Beckman Coulter) ensured removal of aggregates. Finally, Munc18-1 aliquots were snap-frozen. The concentration of purified Munc18-1 was determined after SDS-PAGE by Coomassie Blue-staining using BSA as standard protein and ImageJ software (NIH) for quantification.
GST-Munc18-1 Binding Assays
For the GST-Munc18-1 binding studies, usually ∼7 μg of GST-Munc18-1 or the corresponding molar amount of GST (generated by thrombin cleavage of GST-Munc18-1 to remove Munc18-1) were bound to 10 μl of GSH-Sepharose 4 fast flow beads washed 3× with lysis buffer and 3× with fusion buffer (25 mm HEPES-KOH, pH 7.4, 135 mm KCl, 1 mm DTT) containing 0.5% BSA as well as 0.5% Triton X-100 (for syntaxin 1A) or 1% n-octyl-β-d-glucopyranoside (for VAMP2). Bound amounts of GST-Munc18-1 and GST were determined after SDS-PAGE by Coomassie Blue-staining using BSA as protein standard and ImageJ as quantification software. 10 μl of beads were incubated with molar excesses of full-length VAMP2 (5×–125×) or syntaxin 1A (0.25×–4×) in fusion buffer containing 0.5% BSA as well as 0.5% Triton X-100 (for syntaxin 1A) or 1% n-octyl-β-d-glucopyranoside (for VAMP2) in a final volume of 400 μl for 1–1.5 h at 4 °C on a rotating wheel. Subsequently, beads were washed 4× with 1.4 ml of buffer. 30 μl of Laemmli buffer was added, and beads were incubated at 98 °C for 4 min. For syntaxin 1A binding, 7 μl of each reaction was analyzed by SDS-PAGE. Protein bands were stained by Coomassie Blue and quantified by ImageJ. For VAMP2 binding, 7 μl of each reaction were transferred onto a PVDF membrane via semi-dry blotting. VAMP2 was detected by immunostaining with a primary rabbit antibody directed against the VAMP2 N terminus and visualized by a fluorescently labeled secondary goat anti-rabbit antibody using the Odyssey imaging system (LI-COR Biosciences). Fluorescence intensities were quantified by ImageJ software.
Protein Reconstitution into Liposomes
SUVs and GUVs were prepared as described previously (50, 52). All lipids were from Avanti Polar Lipids with the exception of [3H]DPPC ([1,2-3H]dipalmitoyl phosphatidylcholine) and DiO (3,3′-dioctadecyloxacarbocyanine perchlorate/DiOC18(3)), which were from Invitrogen and Amersham Biosciences, respectively. The following protein/lipid mixtures were employed for the reconstitution experiments.
Syntaxin 1·SNAP-25 and syntaxin 1 liposomes 35 mol % 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 15 mol % 1,2-dioleoyl-sn-glycero-3-phosphoserine (DOPS), 20 mol % 1-hexadecanoyl-2-octadecenoyl-sn-glycero-3-phosphoethanolamine (POPE), 3 mol % liver l-α-phosphatidylinositol, 2 mol % brain l-α-phosphatidylinositol-4,5-bisphosphate, 25 mol % cholesterol (from ovine wool), and trace amounts of [3H]DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine); 5 μmol of total lipid. Syntaxin 1 and t-SNAREs were reconstituted into SUVs at a protein-to-lipid ratio of ∼1:1000. t-SNARE SUVs were used to produce t-SNARE GUVs by electro-swelling as described previously (50, 52).
VAMP2/Syt1-SUVs for the lipid-mixing assay were 30 mol % POPC, 15 mol % DOPS, 22.6 mol % POPE, 5 mol % liver l-α-phosphatidylinositol, 25 mol % cholesterol (from ovine wool), 1.6 mol % rhodamine-DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl), 0.8 mol % NBD-DPPE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-7-nitro-2,1,3-benzoxadiaziole-4-yl), and trace amounts of [3H]DPPC; 3 μmol of total lipid. SUVs were formed in the presence of Syt1 (protein to lipid ratio ∼1:850) and VAMP2 (∼1:200) using the previously described technique of dilution and dialysis followed by a Nycodenz gradient centrifugation (5).
VAMP2/Syt1-SUVs for the simultaneous content- and lipid-mixing assay: 26.5 mol % POPC, 15 mol % DOPS, 25 mol % POPE, 5 mol % liver l-α-phosphatidylinositol, 25 mol % cholesterol (from ovine wool), 3.5% DiO, and trace amounts of [3H]DPPC; 3 μmol of total lipid. The lipid mixture was dried down under a flow of N2, and the lipid film was dissolved in the presence of Syt1 (protein-to-lipid ratio ∼1:850) and VAMP2 (∼1:180) in HEPES buffer (25 mm HEPES/KOH, pH 7.4, 400 mm KCl, 1 mm DTT) containing 1.4% n-octyl-β-d-glucopyranoside. Subsequently, vesicles were formed by dilution of the solution below the critical micellar concentration. Excessive detergent was removed using a PD-10 desalting column (GE Healthcare). Sulforhodamine B (Invitrogen) was added (50 mm final), and the vesicles were snap-frozen. The extra-luminal dye was removed using a Nycodenz gradient centrifugation (5) followed by a buffer exchange (25 mm HEPES-KOH, pH 7.4, 135 mm KCl, 0.1 mm EGTA, 0,5 mm MgCl2, 1 mm DDT) using a PD MidiTrap G-25 (GE Healthcare).
Lipid to protein ratios in the reconstituted liposomes were determined by measuring the lipid amounts via [3H]DPPC and quantifying the reconstituted protein after resolving them by SDS-PAGE and staining by Coomassie Blue. A BSA protein standard and ImageJ software was used for protein quantification.
SUV/GUV Lipid-mixing Assay
The lipid-mixing assay was performed as described previously (50, 52). GUVs were preincubated with 6 μm CpxII (5 min) and/or 0.9 μm Munc18-1 constructs (10 min) in fusion buffer (25 mm HEPES-KOH, pH 7.4, 135 mm KCl, 0.1 mm EGTA, 1 mm MgCl2, 1 mm DTT) at room temperature. SUVs and GUVs were mixed at room temperature in a final volume of 104 μl, and 100 μl were immediately transferred into a prewarmed 96-well plate (37 °C). NBD fluorescence (excitation 485/20 nm, emission 525/20 nm) was measured at 37 °C in a Synergy 4 plate reader (BioTek Instruments GmbH) at intervals of 10 s. After 5 min Ca2+ was added to a final concentration of 100 μm. The NBD fluorescence obtained from control incubations containing SUVs pretreated with botulinum neurotoxin D was subtracted from individual measurement sets. The fusion-dependent fluorescence was normalized to the maximal fluorescent signal obtained in the presence of 0.4% dodecylmaltoside (Fluka). Three independent fusion experiments were performed for each condition.
SUV/GUV Content-mixing Assay
The fusion assays were performed as described above. However, sulforhodamine B fluorescence (excitation 530/20 nm, emission 590/20 nm, dichroic mirror 550 nm) was measured at 37 °C in a Synergy 4 plate reader at intervals of 10 s.
Circular Dichroism
Munc18-1 peptides WT residues 324–359 (MRDLSQMLKKMPQYQKELSKYSTHLHLAEDCMKHYQ) or P335A (MRDLSQMLKKMAQYQKELSKYSTHLHLAEDCMKHYQ) were ordered from VCPBIO Lab. 5 mg of peptides were solubilized in 0.5 ml of H2O (for neutralization, 2.5 μl of 1 n NaOH were added while mixing, and pH values were checked with indicator strips) and mixed with 0.5 ml of 50 mm sodium phosphate buffer, pH 7.4. CD spectra (190–250 nm) of the peptides were collected at room temperature using a JASCO 810 polarimeter (30 μm peptide concentration in 25 mm sodium phosphate buffer, pH 7.4). A total of 10 scans were collected for each sample with a step size of 0.1 nm in a Hellma quartz cuvette with a path length of 1 mm.
Structural Alignments
The structural model for the Munc18-1/VAMP2 interaction is based on the following observations. First, within the Munc18-1 structure in complex with an isolated N-peptide of syntaxin 4 (PDB code 1PUJ), the 3a domains form a symmetric crystal contact with the respective two helices 11 and 12 forming a four-helix bundle. The position of the symmetry-related helices was subsequently used as a template to align the SNARE complex (syntaxin 1, helix 11; VAMP2, helix 12) by the program COOT (68). Second, the structural alignment was done to fit previous biochemical and biophysical data, which showed the membrane proximal region of VAMP2 (residues 75–95) in contact with a Munc18-1 region centered on helix 12 (residues 333–339) (69). The register of the alignment takes into account the cytosolic availability of VAMP2 next to its transmembrane region. Third, the model is manually validated for physically reasonable interactions and steric feasibility.
Statistical Analysis
Each experiment was repeated three times. Mean values, S.E., and 95% confidence intervals were calculated by using the Microsoft Excel 2008 software.
RESULTS
Rationale to Design Mutants Selectively Affecting the Stimulatory (Vesicle-priming) Function of Munc18-1
Previous studies already implied the functional importance of the Munc18-1 domain 3a for vesicle priming. First, a random mutagenesis approach in yeast revealed that dominant-negative Sec1 mutants cluster in domain 3a, underscoring its importance for membrane fusion (70). However, single point mutations in the orthologous mammalian Munc18-1 domain 3a showed only weak effects on regulated exocytosis (70, 71). Second, cross-linking experiments showed that residues 333–339 of Munc18-1 can interact with the C-terminal residues 87–91 of VAMP2 (Fig. 1A) (69). NMR studies extended the interacting region to residues 75–95 of VAMP2 (Fig. 1B) (69). Functional studies using a reconstituted liposome fusion assay demonstrated that mutations in the C-terminal part of VAMP2 abolish the stimulatory function of Munc18-1 (34). Third, a comparison of the Munc18-1·syntaxin 1 binary complex with the structure of Munc18-1 bound to the isolated N-terminal peptide of syntaxin 4 revealed that domain 3a in the absence of syntaxin 1 can form an extended helix (residues 295–358) (72). Remarkably, this extended helix is incompatible with the Munc18-1·syntaxin 1 binary complex. Pro-335 seems to act as a hinge point allowing residues 295–335 to adopt different conformations dependent on its interaction partners (Fig. 1C) (29, 72). In addition, a domain 3a peptide (residues 325–359) adopts a helical structure in the presence of the SNARE four-helix bundle (72). Thus, the hypothesis was forwarded that Munc18-1 domain 3a undergoes a conformational change that may allow coiled-coil interactions with SNARE complexes (72).
FIGURE 1.

Design of mutations to disrupt the stimulatory function of Munc18-1. A, domain architecture of Munc18-1. Amino acids (321–359), which include part of the domain 3a loop and helix 12, are shown in the single-letter code. The interaction site for the C-terminal domain (CTD) of VAMP2 is marked by a bracket (69). Mutated residues used in this study are highlighted in red. B, domain architecture of VAMP2. NMR studies showed that the membrane proximal region of VAMP2 (residues 75–95) interacts with Munc18-1 (marked by a bracket) (69). L, linker region. The botulinum neurotoxin D (BoNT/D) cleavage site is marked by a vertical line. C, Munc18-1 domain 3a can adopt two conformations. The two known crystal structures of Munc18-1 are superimposed (29, 72). The gray structure shows Munc18-1 when bound to closed syntaxin 1 (syntaxin 1 is omitted). The two anti-parallel helices (helices α11 and α12, residues 295–358) of domain 3a are connected by a bent hairpin loop (black) including an unstructured region of 21 amino acids. The golden structure shows Munc18-1 when bound to the N-peptide of syntaxin 4 (the N-peptide is omitted). The bent hairpin unfurls and extends helix 12 (magenta) with Pro-335 acting as a hinge point. Mutated residues used in this study are highlighted in cyan.
Based on these data and hypotheses, we introduced three single point mutations L348R, M334R, and P335A in domain 3a (Fig. 1, A and C), which should affect VAMP2 binding and should not disturb syntaxin 1 binding but may shift the conformational states of Munc18-1 and tested their impacts in reconstituted membrane fusion assays.
Munc18-1 Mutants Retain Binding to Monomeric Syntaxin 1
To test the ability of our mutants to bind syntaxin 1, GST-Munc18-1 constructs were immobilized on glutathione beads and incubated with increasing amounts of monomeric full-length syntaxin 1 (Fig. 2A). All mutants, Munc18-1 L348R, M334R, P335A, and P335A/L348R bound monomeric syntaxin 1 with similar efficiencies as Munc18-1 WT. In each case a 2-fold molar excess of syntaxin 1 over the distinct Munc18-1 mutants was sufficient to obtain saturable binding and a 1:1 stoichiometry. These results indicate that the Munc18-1 point mutations in domains 3a do not perturb the overall protein folding and likely retain their chaperoning function for syntaxin 1 in line with previous reports (70, 71).
FIGURE 2.
Binding of Munc18-1 constructs to syntaxin 1 and VAMP2. A, Munc18-1 mutants L348R, M334R, P335A, and P335A/L348R retain binding to monomeric syntaxin 1A. Immobilized GST-Munc18-1 constructs were incubated with the indicated amounts of syntaxin 1 for 1 h on ice, and the stoichiometry of syntaxin 1 bound to GST-Munc18-1 was determined by quantifying Coomassie Blue-stained protein bands in SDS gels by using ImageJ. All Munc18-1 mutants and the WT protein show similar binding to syntaxin 1. Unspecific binding of syntaxin 1 to immobilized GST was minimal. Error bars are 95% confidence intervals (n = 3). B, Munc18-1 mutants L348R, M334R, and P335A/L348R, but not P335A, impair binding to VAMP2. Immobilized GST-Munc18-1 constructs were incubated with an indicated molar excess of VAMP2 for 1.5 h on ice. Bound VAMP2 was visualized by Western blot analyses using an anti-VAMP2 antibody and quantified by Odyssey imaging and ImageJ software. Quantifications represent percentages of bound VAMP2 to GST-Munc18-1 mutants compared with GST-Munc18-1 WT. Introduction of L348R reduces the Munc18-1/VAMP2-interaction drastically, whereas the M334R mutant retains some ability to bind to VAMP2. P335A does not influence VAMP2 binding. Error bars are S.E. (n = 3).
Munc18-1 L348R and M334R, but Not P335A, Reduce Binding to VAMP2
Because the Munc18-1 mutants were designed to selectively perturb VAMP2 binding, the different GST-Munc18-1 constructs were immobilized and incubated with increasing amounts of full-length VAMP2. For these binding studies high concentrations and a large molar excess (up to 125-fold) of VAMP2 over Munc18-1 were employed to detect the low affinity Munc18-1-VAMP2 interaction. The binding studies revealed that Munc18-1 L348R as well as the P335A/L348R double mutants lose their ability to interact with VAMP2, whereas Munc18-1 M334R showed some residual binding activity for VAMP2 (Fig. 2B). In contrast, the P335A mutation did not significantly affect VAMP2 binding.
L348R Abolishes the Stimulatory Function of Munc18-1 in Membrane Fusion
Next we analyzed the L348R point mutation in a lipid-mixing assay using SUVs and GUVs and mimicking the docking and Ca2+-triggered fusion of synaptic vesicles at the plasma membrane (50, 52). Briefly, t-SNARE-GUVs were mixed with Syt1/VAMP2-SUVs in the absence or presence of Munc18-1 and CpxII (protein/lipid ratios are: t-SNARE ∼1:1000, Syt1 ∼1:850, VAMP2 ∼1:200). The protein/lipid ratios as well as the lipid compositions of SUVs and GUVs were largely adapted to cellular conditions (73). Preassembled t-SNARE complexes were employed to focus selectively on the stimulatory (priming) effect of Munc18-1, bypassing the inhibitory function of Munc18-1 and the requirement for Munc13-1. Briefly, Syt1-l-α-phosphatidylinositol-4,5-bisphosphate/t-SNARE interactions dock the v-SNARE SUVs to the t-SNARE GUVs, the concerted function of CpxII and Syt1 stalls the fusion reaction, and Ca2+ addition triggers fast and synchronized lipid mixing (50, 52). Lipid mixing was monitored by dilution of a FRET pair of fluorescently labeled lipids (NBD-DPPE and rhodamine-DPPE incorporated into the Syt1/VAMP2-SUVs (5, 74). Fusion of the SUVs with unlabeled t-SNARE-GUVs increases the distance between partners of the FRET pair, and the NBD fluorescence increases dramatically. Lipid-mixing was measured for 5 min in the absence of Ca2+, and subsequently Ca2+ was added (100 μm final concentration) to monitor the kinetics and the extent of Ca2+-synchronized membrane fusion for an additional 10 min.
As already shown in our previous study, in the absence of CpxII, Munc18-1 WT stimulates the initial lipid-mixing kinetics (time interval 10–40 s) (Fig. 3, A and B) (52). In contrast, the Munc18-1 L348R mutant lacks this stimulatory effect. The addition of CpxII reduces the initial lipid-mixing rates both in the absence and presence of Munc18, consistent with the general clamping activity of CpxII (Fig. 3, C and D). In the presence of CpxII, Munc18-1 WT showed a 6-fold stimulation (Fig. 3D). Again, the Munc18-1 L348R mutant did not stimulate the reaction in the presence of CpxII, and the fusion kinetics were similar to reactions lacking Munc18-1. All fusion reactions contained comparable amounts of CpxII and Munc18-1 constructs. To analyze if full membrane fusion occurs, we modified the SUV/GUV fusion assay and incorporated self-quenching concentrations of sulforhodamine into the lumen of the SUVs (Fig. 3, E and F). As expected from our previous cryo-EM studies, content and lipid mixing showed similar kinetics within the time resolution of the plate reader assay (Fig. 3F) (50). The L348R mutation largely abolished the stimulatory effect on content mixing.
FIGURE 3.
Munc18-1 L348R shows a loss-of-function in a SUV/GUV lipid- and content-mixing assay. VAMP2/Syt1-SUVs (2.5 nmol of lipid, 12.5 pmol of VAMP2, 3 pmol of Syt1) labeled with rhodamine-DPPE and NBD-DPPE were mixed with unlabeled t-SNARE-GUVs (14 nmol of lipid, 14 pmol of t-SNARE) in the absence or presence of either Munc18-1 WT, Munc18-1 L348R (each 90 pmol), or Cpx II (600 pmol) in a final volume of 100 μl of fusion buffer. Immediately after the addition of SUVs to GUVs, lipid-mixing was monitored by the increase of NBD fluorescence at 37 °C. After 5 min, Ca2+ was added (100 μm final concentration), and the measurement was continued for another 10 min. The results were normalized to the maximum NBD fluorescence obtained after detergent lysis of liposomes. Botulinum neurotoxin D-treated samples were subtracted as background signals. A, representative lipid-mixing experiment in the absence of CpxII. B, initial kinetics of lipid-mixing experiments in the absence of CpxII. The initial slope of the lipid-mixing kinetics was determined for the 10–40-s interval. C, representative lipid-mixing experiment in the presence of CpxII. D, initial kinetics of lipid-mixing experiments in the presence of CpxII. E, representative content-mixing assay in the presence of CpxII. VAMP2/Syt1-SUVs contained encapsulated self-quenched sulforhodamine-B. Protein and lipid concentrations were used as described above. The results were normalized to the maximum sulforhodamine-B fluorescence obtained after detergent lysis of liposomes. Signals derived from control reactions containing a 10-fold molar excess of the cytoplasmic domain of VAMP2 (VAMP-CD) over reconstituted t-SNARE were subtracted as a background signal. F, initial kinetics of content-mixing experiments in the presence of CpxII. Error bars are 95% confidence intervals (n = 3).
In summary, the mutation L348R in domain 3a selectively abolished VAMP2 binding and the stimulatory function of Munc18-1, whereas binding to monomeric syntaxin 1 was not affected.
M334R Reduced Initial Lipid-mixing Stimulation of Munc18-1 in the Absence of CpxII
Next, we introduced a point mutation, M334R, into the switch region of the Munc18-1 domain 3a, which can adopt distinct structures dependent on the presence or absence of syntaxin 1. In the absence of CpxII, this mutant still stimulated the initial lipid mixing rates (∼2-fold) but to a lesser extent than Munc18-1 WT (∼5-fold) (Fig. 4, A and B). In the presence of CpxII, which in general suppresses Ca2+-independent membrane fusion, the Munc18-1 M334R exhibited similar fusion rates as Munc18-1 WT (Fig. 4, C and D). Thus, in contrast to L348R, the M334R mutant showed a reduced but still pronounced stimulatory effect consistent with its reduced VAMP2 binding activity.
FIGURE 4.
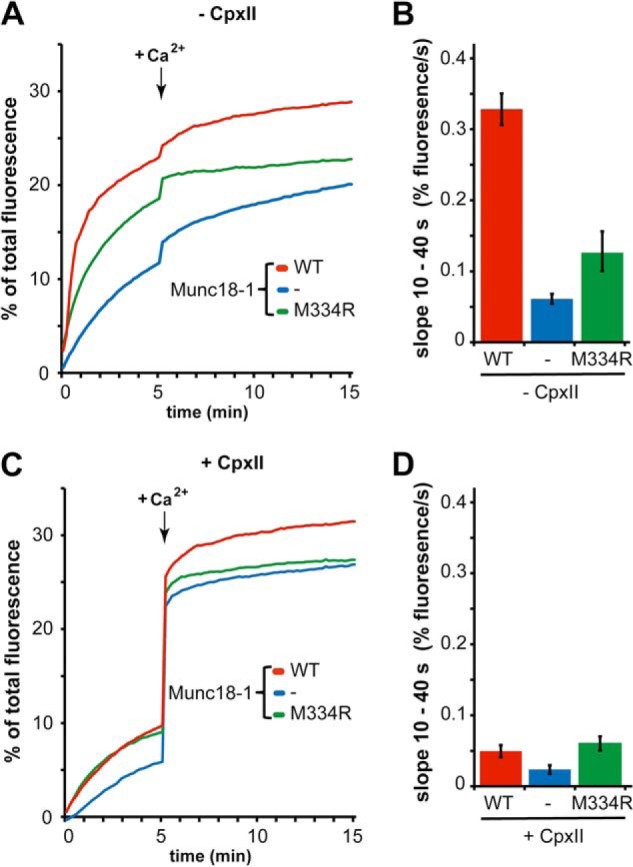
Munc18-1 M334R reduces the initial lipid-mixing rates in a SUV/GUV lipid-mixing assay in the absence of CpxII. VAMP2/Syt1-SUVs were mixed with t-SNARE-GUVs in the absence or presence of either Munc18-1 WT, Munc18-1 M334R, or CpxII, and lipid-mixing was monitored as described in the legend to Fig. 3. A, representative lipid-mixing experiment in the absence of CpxII. B, initial kinetics of lipid-mixing experiments in the absence of CpxII. The initial slope of the lipid-mixing kinetics was determined for the 10–40-s interval. C, representative lipid-mixing experiment in the presence of CpxII. D, initial kinetics of lipid-mixing experiments in the presence of CpxII. Error bars are 95% confidence intervals (n = 3).
Munc18-1 P335A Showed a Gain of Function in Stimulating v-/t-SNARE Assembly as Shown by Lipid Mixing
As demonstrated by the crystal structure analyses, Munc18-1 domain 3a can adopt a bent hairpin and an extended helical hairpin, suggesting that Pro-335 might function as a hinge/switch point (Fig. 1C). We now directly tested if the conversion of the helix breaking amino acid proline into an alanine favors the transition to the extended helical hairpin conformation as predicted by in silico analyses (Fig. 5A) (75–77). We analyzed the secondary structure of Munc18-1 domain 3a peptides including residues 324–359, containing either a proline or an alanine in position 335. Circular dichroism (CD) spectroscopy of the domain 3a peptide WT revealed a characteristic random-coil structure as has been reported previously (Fig. 5B) (72). The minimum in the CD spectrum was centered around 200 nm, which is typical for unstructured peptides. However, the introduction of an alanine at position Pro-335 changed the spectrum. The minima were now centered around 223 as well as 205 nm, and a maximum at 190 nm appeared, which are the characteristic features of an α-helical structure. Thus, the replacement of a proline by an alanine dramatically increased α-helicity, consistent with corresponding changes observed by Pro/Ala substitutions in unrelated peptides (78–80).
FIGURE 5.

P335A induces an α-helical conformation in helix 12. A, in silico analysis of a Munc18-1 domain 3a peptide (residues 324–359) indicates that P335A enhances the formation of an α-helical structure. Depending on the prediction algorithm used (PSIPRED, JNET, Prof), a flexible helical conformation was suggested for the peptide WT. For the peptide P335A a strict α-helical conformation was adopted for all prediction algorithms used. B, CD spectra of Munc18-1 domain 3a peptides (residues 324–359) WT, or P335A. 30 μm peptides were analyzed in 25 mm sodium phosphate buffer at 25 °C. The Munc18-1 peptide WT shows a typical random coil spectrum with a minimum at 200 nm, whereas the peptide P335A shows a characteristic α-helical spectrum with minima centered around 205 and 223 nm respectively as well as a maximum at about 190 nm. Representative CD spectra are shown.
Given the distinct conformational change upon introduction of the P335A exchange, we wondered whether this mutant shows any effect in the reconstituted SUV/GUV fusion assay. Remarkably, Munc18-1 P335A further increased the initial lipid-mixing rates in the presence of CpxII by >3-fold (Fig. 6). In the absence of CpxII, the initial lipid-mixing stimulation by the P335A mutant was too fast to be resolved in the plate reader assay. To make sure that this pronounced stimulation depends on the specific interaction of Munc18-1 with VAMP2, we made use of the L348R mutation, which abolished VAMP2 binding (Fig. 2B). Indeed, the double mutant P335A/L348R lacked the stimulatory effect of Munc18-1, demonstrating that the stimulation by P335A is specific. The P335A gain-of-function mutant still bound monomeric syntaxin 1, indicating that the alanine mutation only increases the propensity to form an extended helical hairpin but may nevertheless adopt the bent hairpin conformation, compatible with the binding of closed syntaxin 1 (Fig. 2A).
FIGURE 6.

Munc18-1 P335A shows a gain of function in a SUV/GUV lipid-mixing assay. VAMP2/Syt1-SUVs were mixed with t-SNARE-GUVs together with CpxII in the absence or presence of either Munc18-1 WT, Munc18-1 P335A, or Munc18-1 P335A/L348R, and lipid-mixing was monitored. A, representative lipid-mixing experiment in the presence of CpxII. B, initial kinetics of lipid-mixing experiments in the absence of CpxII. Error bars are 95% confidence intervals (n = 3).
The Inhibitory Function of Munc18-1 Is Unimpaired by L348R and M334R but Is Turned into a Constitutive Stimulatory Function by P335A
The previous experiments showed that all Munc18-1 mutants retained syntaxin 1 binding, and we have used preassembled t-SNARE complexes to analyze their effects on v-/t-SNARE complex assembly. However, these experiments do not provide information about the inhibitory function of Munc18-1, blocking t-SNARE assembly. Actually, our data that P335A adopts a helical conformation indicates that this mutant may eliminate the inhibitory function of Munc18-1 WT. Thus, we made use of a reconstituted lipid-mixing assay, which resolves both the stimulatory as well as the inhibitory function (35). In this assay, instead of preassembled t-SNAREs, monomeric syntaxin 1 was reconstituted into SUVs, and SNAP-25 was added as a soluble component. To generate the inhibitory syntaxin 1·Munc18-1 binary complex, syntaxin 1 liposomes were preincubated with Munc18-1, resulting in a block of the subsequent t-SNARE and v-/t-SNARE complex formation detected by a loss of lipid mixing. However, when the Munc18-1-inhibited syntaxin 1 SUVs were simultaneously preincubated for a prolonged period of time in the presence of SNAP-25 and VAMP2-SUVs under conditions that block membrane fusion (at low temperature) but allow an interaction of Munc18-1 with VAMP2-SUVs, the inhibition can be converted into a stimulation upon increasing the temperature (Fig. 7A). As a note, in the presence of Syt1, which binds the t-SNAREs, vesicles dock efficiently, as shown in the SUV/GUV fusion assay, and the stimulatory effect of Munc18-1 was observed in the absence of any low temperature preincubation.
FIGURE 7.
Munc18-1 L348R and M334R retain their inhibitory function in a SUV/SUV lipid-mixing assay, whereas Munc18-1 P335A shows a stimulatory function. A, incubation schemes to resolve the dual function of Munc18-1. To determine the inhibitory function of Munc18-1, syntaxin 1-SUVs (100 nmol of lipid, 100 pmol of syntaxin 1) were preincubated at room temperature with 100 pmol Munc18-1 for 30 min followed by the addition of 300 pmol soluble SNAP-25 for 1 h on ice. Subsequently, VAMP2-SUVs (7.2 nmol of lipid, 36 pmol of VAMP2) were added (in a final volume of 80 μl of fusion buffer), and lipid-mixing was immediately monitored (denoted as 0 h VAMP2-SUVs in the figure) for 120 min at 37 °C. To measure the stimulatory function of Munc18-1, syntaxin 1-SUVs were preincubated at room temperature for 30 min with 100 pmol of the indicated Munc18-1 constructs. Then, 300 pmol of soluble SNAP-25 and VAMP2-SUVs (7.2 nmol of lipid, 36 pmol of VAMP2) were added simultaneously and incubated for 1 h on ice (denoted as 1 h VAMP2 in the figure). Subsequently, lipid mixing was monitored for 120 min at 37 °C. B–D, representative experiments for Munc18-1 L348R, M334R, and P335A. Lipid mixing in the absence of SNAP-25 was subtracted as a background signal. E–G, initial kinetics of lipid-mixing experiments for Munc18-1 L348R, M334R, and P335A. The initial slope of the lipid-mixing kinetics was determined for the 5–10-min interval. Error bars are 95% confidence intervals (n = 3).
Briefly, syntaxin 1-SUVs (protein/lipid ratio ∼1:1000) were preincubated at room temperature for 30 min with Munc18-1 to stabilize syntaxin 1 in its inhibitory closed conformation. The subsequent addition of SNAP-25 does not result in the formation of a t-SNARE complex because SNAP-25 alone is not sufficient to release the Munc18-1 inhibition in the absence of Munc13 (24). Therefore, upon the addition of VAMP2-SUVs (protein/lipid ratio ∼1:200) and instant warming up of the reaction to 37 °C to start lipid mixing, Munc18-1 showed a clear inhibitory effect (Fig. 7, B and E).
In this assay the Munc18-1 L348R mutant behaved similarly to Munc18-1 WT and inhibited lipid-mixing. However, upon preincubation of all components at low temperature, L348R did not stimulate lipid mixing, in contrast to Munc18-1 WT. This result was consistent with the SUV/GUV fusion assay (compare Figs. 7, B and E, with 3, A–D). Concerning Munc18-1 M334R, the stimulatory function of this mutant was not significantly impaired but showed a reduction in the initial fusion kinetics, again in accordance with the SUV/GUV fusion assay (compare Figs. 7, C and F, with 4, A–D). As expected, the inhibitory role of M334R is comparable with that of Munc18-1 WT.
In contrast, Munc18-1 P335A did not show any inhibitory effect; instead it profoundly stimulated the initial fusion kinetics (Fig. 7, D and G). Remarkably, this stimulation did not require a preincubation of the liposomes at low temperature.
DISCUSSION
Our data suggest that domain 3a of Munc18-1, in particular helix 12, functions as a central hub controlling SNARE complex assembly at several levels. First, extending helix 12 at its N terminus (P335A) apparently favors an open, instead of a closed syntaxin 1 conformation, allowing efficient SNAP-25 binding. Such a bimodal switch is consistent with previously published structural studies, demonstrating that this region can adopt two alternative conformations (29, 72). Second, in addition to favoring an open syntaxin 1 conformation, the extended helix 12 seems to activate VAMP2 for SNAREpin assembly and membrane fusion. Third, based on L348R mutation and structural modeling, the exposed surface of helix 12 could function as folding template for VAMP2 binding stimulating the initiation of SNARE complex formation or/and SNARE complex zippering (see below). Fourth, Munc18-1 stimulates the Ca2+-independent reaction but also increases the final fusion signal, likely by rising the number of functional v-SNAREs or t-SNAREs. Such a role of regulatory factors and also of Munc18-1 in stabilizing functional t-SNARE conformations has been described (81).
Our results obtained in the reconstituted fusion assay are largely consistent with cellular exocytosis studies, describing an essential role of Munc18-1 domain 3a in stimulating exocytosis independent of its inhibitory/chaperoning function (70, 71, 82). Overall, these studies showed that single mutations in the domain 3a loop region like our M334R mutation cause only subtle changes in exocytosis (71). A robust reduction of exocytosis requires large insertion mutations (5 or 39 amino acids) after position 333 or the deletion of 17 amino acids (317–333) (70, 82). Concerning P335A, which resulted in a profound stimulatory effect in the reconstituted assay, this mutant showed only a non-significant increase in constitutive and stimulated exocytosis of a NPY-hPLAP construct in PC12 cells (82). However, it is not clear if the reaction, which is accelerated by the P335A mutation in vitro, is a rate-limiting step in the cellular exocytosis assay, which measures bulk exocytosis of large dense granules in PC12 cells at low temporal resolution. Nevertheless, a similar mutation in the Munc18-2 homologue causes familial hemophagocytic lymphohistiocytosis (FHL) and increases Munc18-2 dimerization without affecting syntaxin 11 binding, consistent with our results (83, 84). Thus, proline residues at the beginning of helix 12 clearly play an important role, likely acting as a hinge point (29, 72, 85).
In current models of the Munc18-1/SNARE complex, the central cavity of Munc18-1 harbors the SNARE complex, similar to syntaxin 1 but involving distinct interaction sites. Several studies suggest such a binding mode, but direct structural evidence is missing (69, 86). Changes in the loop region of domain 3a would be part of a functional cycle and expose critical sites for the binding of VAMP2 and the SNARE complex. These binding sites would face the cavity of Munc18-1. In contrast to these models, our data presented in this study suggest another binding site. The location of Leu-348 on the exposed surface of helix 12 and our result that L348R perturbs VAMP2 binding and the stimulatory effect of Munc18-1 suggest that Munc18-1 also contains a VAMP2 binding site facing away from the Munc18-1 cavity. Thus, we used the crystal structures of the fully assembled SNARE complex and of Munc18-1 containing the extended helix 12 (13, 69, 72) to generate an alternative model (Fig. 8A). In this model the N-terminal part of helix 12 (residues 327–348) is positioned antiparallel to VAMP2 (residues 75–96), in accordance with previous NMR studies and VAMP2-Munc18-1 cross-linking results (52, 69). Leu-348 locates at the border of the interface, and the L348R mutation would abolish the interaction via steric hindrance. In contrast, other point mutations (K332E, K333E, K332E/K333E, Q336A/Y337L, Y337L/Q338A, and Q336A/Y337L/Q338A) would influence but not sterically eliminate the binding (71). Importantly, the extended helix 12 would function as a folding template for VAMP2. Such an interaction would also be consistent with the stimulatory effect of the P335A mutant and the observation that the assembled SNARE complex induces an α-helical structure in an isolated helix 12 peptide (13, 69, 72). NMR data for VAMP2 obtained in the presence of dodecylphosphocholine micelles indicates that membrane lipids can induce two transient helical segments located at the N terminus (residues 36–54) and in a membrane proximal region (residues 77–88) (87). Thus, critical hydrophobic amino acids of VAMP2, which form part of the hydrophobic core of the four-helix SNARE bundle, may be shielded by the membrane environment. Via an initial interaction with the membrane proximal region of VAMP2, Munc18-1 may release VAMP2 from the membrane, making the N-terminal half of VAMP2 available for t-SNARE binding, thereby initiating SNARE complex assembly. Alternatively or in addition, Munc18-1 may help to structure the remaining inherently unstructured region in the C-terminal part of the VAMP2 SNARE motif and the subsequent linker region targeting inherent breakpoints in SNARE complex assembly (11, 12, 88). Thus, structuring the membrane proximal region could help to transduce the mechanical force generated by SNARE complex assembly to the lipid bilayer, favoring membrane fusion (Fig. 8B). Consistently, the post-fusion fully assembled SNARE complex is characterized by continuous helical structures of VAMP2 and syntaxin 1 (13). Interestingly, in our structural model, helix 11 neighboring helix 12 would pair with the membrane proximal region of syntaxin 1. In this context it is remarkable that a Munc18-2 mutation, K314L/R315L, which in the Munc18-1 crystal structure would be localized at the end of helix 11, results in a gain-of-function mutation, stimulating insulin exocytosis in rat pancreatic β-cells (89). Thus, the target of helix 11 or its interplay with helix 12 will require further analyses in the future.
FIGURE 8.
Model of Munc18-1 interacting with the SNARE complex. A, crystal structures of Munc18-1 containing the extended helix 12 in domain 3a (magenta) and the full-length neuronal SNARE complex were aligned using known interaction sites spanning residues 333–339 in helix 12 (cyan, residue 348 shown in the same color) and residues 75–96 in VAMP2 (orange) (13, 69, 72). In addition, helix 11 would interact with syntaxin 1. The membrane area is shown by a gray box. B, functional model of Munc18-1 in regulating SNAREpin assembly (Syt1 and CpxII are omitted for clarity). a, schematic representation of open syntaxin 1 in its SNAP-25/VAMP2 binding mode. b, in the inhibitory Munc18-1·syntaxin 1 binary complex the Habc domain folds back on the H3 domain (SNARE motif), blocking SNARE assembly. c, extension of helix 12 (extended helical hairpin) clashes sterically with the binding of Munc18-1 to closed syntaxin 1. d, the extended helix 12 serves as a scaffold for efficient VAMP2 structuring. Membrane fusion is stimulated either by structuring the membrane proximal region of VAMP2 and/or by facilitating the entry of VAMP2 into the emerging SNAREpin. e, SNAREpin zippering includes the transmembrane domains of VAMP2 and syntaxin 1 and drives full membrane fusion.
In our model we used the Munc18-1 crystal structure containing the bound syntaxin 4 N-peptide and the extended helix 12, which likely represents the active (SNARE complex binding competent) conformation of Munc18-1. In the inhibitory Munc18-1·syntaxin 1 binary complex, the domain 3a loop region would shield the binding site for VAMP2. Upon activation of the binary complex, presumably involving Munc13-1, the structural change in domain 3a would remove the block, and part of the loop region will extend helix 12, functioning as an on/off switch now favoring VAMP2 binding and SNARE complex formation (24, 32). At present, an effect of the L348R mutation on the structural switch cannot be ruled out completely. To unambiguously resolve the molecular mechanism, crystal structures of Munc18-1 bound to VAMP2, the t-SNARE, and the v-/t-SNARE will be required. Nevertheless, our alternative model provides a conceptual framework to resolve the fundamental stimulatory function of Munc18-1.
In summary, our study suggests that the extended helix 12 in domain 3a of Munc18-1 provides a template for the membrane proximal region of VAMP2 to initiate v-/t-SNARE interactions and/or directly accelerated SNAREpin zippering, helping to transduce the force generated by the SNAREpin assembly to the lipid bilayer. Such a reaction mechanism might also be applicable to other cellular SEC1/Munc18-SNARE pairs.
Acknowledgments
Expression vectors encoding the light chains of neurotoxins were kind gifts of Dr. Thomas Binz and the late Dr. Heiner Niemann. The expression vector encoding GST-Munc18-1/nSec1 was a gift of Dr. Richard Scheller.
This work was supported by a German Research Foundation grant (SFB/TRR 83 (to D. P. and T. H. S.).
- Syt1
- synaptotagmin1
- Munc18-1
- mammalian unc18-1
- Cpx
- complexin
- GUV
- giant unilamellar vesicle
- SUV
- small unilamellar vesicle
- [3H]DPPC
- (1,2-3H]dipalmitoyl phosphatidylcholine
- rhodamine-DPPE
- 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl)
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- DOPS
- 1,2-dioleoyl-sn-glycero-3-phosphoserine
- POPE
- (1-hexadecanoyl-2-octadecenoyl-sn-glycero-3-phosphoethanolamine
- NBD
- 12-(N-methyl-N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl))
- NBD-DPPE
- 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-7-nitro-2,1,3-benzoxadiaziole-4-yl
- DiO
- 3,3′-dioctadecyloxacarbocyanine perchlorate/DiOC18(3).
REFERENCES
- 1. Südhof T. C., Rothman J. E. (2009) Membrane fusion. Grappling with SNARE and SM proteins. Science 323, 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jahn R., Fasshauer D. (2012) Molecular machines governing exocytosis of synaptic vesicles. Nature 490, 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizo J., Südhof T. C. (2012) The membrane fusion enigma. SNAREs, Sec1/Munc18 proteins, and their accomplices. Guilty as charged? Annu. Rev. Cell Dev. Biol. 28, 279–308 [DOI] [PubMed] [Google Scholar]
- 4. Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324 [DOI] [PubMed] [Google Scholar]
- 5. Weber T., Zemelman B. V., McNew J. A., Westermann B., Gmachl M., Parlati F., Söllner T. H., Rothman J. E. (1998) SNAREpins. Minimal machinery for membrane fusion. Cell 92, 759–772 [DOI] [PubMed] [Google Scholar]
- 6. Sutton R. B., Fasshauer D., Jahn R., Brunger A. T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 [DOI] [PubMed] [Google Scholar]
- 7. Fasshauer D., Margittai M. (2004) A transient N-terminal interaction of SNAP-25 and syntaxin nucleates SNARE assembly. J. Biol. Chem. 279, 7613–7621 [DOI] [PubMed] [Google Scholar]
- 8. Melia T. J., Weber T., McNew J. A., Fisher L. E., Johnston R. J., Parlati F., Mahal L. K., Sollner T. H., Rothman J. E. (2002) Regulation of membrane fusion by the membrane-proximal coil of the t-SNARE during zippering of SNAREpins. J. Cell Biol. 158, 929–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sørensen J. B., Wiederhold K., Müller E. M., Milosevic I., Nagy G., de Groot B. L., Grubmüller H., Fasshauer D. (2006) Sequential N- to C-terminal SNARE complex assembly drives priming and fusion of secretory vesicles. EMBO J. 25, 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pobbati A. V., Stein A., Fasshauer D. (2006) N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science 313, 673–676 [DOI] [PubMed] [Google Scholar]
- 11. Gao Y., Zorman S., Gundersen G., Xi Z., Ma L., Sirinakis G., Rothman J. E., Zhang Y. (2012) Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science 337, 1340–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Min D., Kim K., Hyeon C., Cho Y. H., Shin Y. K., Yoon T. Y. (2013) Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism. Nat. Commun. 4, 1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stein A., Weber G., Wahl M. C., Jahn R. (2009) Helical extension of the neuronal SNARE complex into the membrane. Nature 460, 525–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malsam J., Kreye S., Söllner T. H. (2008) Membrane fusion. SNAREs and regulation. Cell. Mol. Life Sci. 65, 2814–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harrison S. D., Broadie K., van de Goor J., Rubin G. M. (1994) Mutations in the Drosophila Rop gene suggest a function in general secretion and synaptic transmission. Neuron 13, 555–566 [DOI] [PubMed] [Google Scholar]
- 16. Verhage M., Maia A. S., Plomp J. J., Brussaard A. B., Heeroma J. H., Vermeer H., Toonen R. F., Hammer R. E., van den Berg T. K., Missler M., Geuze H. J., Südhof T. C. (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287, 864–869 [DOI] [PubMed] [Google Scholar]
- 17. Voets T., Toonen R. F., Brian E. C., de Wit H., Moser T., Rettig J., Südhof T. C., Neher E., Verhage M. (2001) Munc18-1 promotes large dense-core vesicle docking. Neuron 31, 581–591 [DOI] [PubMed] [Google Scholar]
- 18. Weimer R. M., Richmond J. E., Davis W. S., Hadwiger G., Nonet M. L., Jorgensen E. M. (2003) Defects in synaptic vesicle docking in unc-18 mutants. Nat. Neurosci. 6, 1023–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosono R., Hekimi S., Kamiya Y., Sassa T., Murakami S., Nishiwaki K., Miwa J., Taketo A., Kodaira K. I. (1992) The unc-18 gene encodes a novel protein affecting the kinetics of acetylcholine metabolism in the nematode Caenorhabditis elegans. J. Neurochem. 58, 1517–1525 [DOI] [PubMed] [Google Scholar]
- 20. Hata Y., Slaughter C. A., Südhof T. C. (1993) Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature 366, 347–351 [DOI] [PubMed] [Google Scholar]
- 21. Dulubova I., Sugita S., Hill S., Hosaka M., Fernandez I., Südhof T. C., Rizo J. (1999) A conformational switch in syntaxin during exocytosis. Role of munc18. EMBO J. 18, 4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen X., Lu J., Dulubova I., Rizo J. (2008) NMR analysis of the closed conformation of syntaxin-1. J Biomol NMR 41, 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burkhardt P., Hattendorf D. A., Weis W. I., Fasshauer D. (2008) Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 27, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma C., Li W., Xu Y., Rizo J. (2011) Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat. Struct. Mol. Biol. 18, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rickman C., Medine C. N., Bergmann A., Duncan R. R. (2007) Functionally and spatially distinct modes of munc18-syntaxin 1 interaction. J. Biol. Chem. 282, 12097–12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rowe J., Corradi N., Malosio M. L., Taverna E., Halban P., Meldolesi J., Rosa P. (1999) Blockade of membrane transport and disassembly of the Golgi complex by expression of syntaxin 1A in neurosecretion-incompetent cells. Prevention by rbSEC1. J. Cell Sci. 112, 1865–1877 [DOI] [PubMed] [Google Scholar]
- 27. McEwen J. M., Kaplan J. M. (2008) UNC-18 promotes both the anterograde trafficking and synaptic function of syntaxin. Mol. Biol. Cell 19, 3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han L., Jiang T., Han G. A., Malintan N. T., Xie L., Wang L., Tse F. W., Gaisano H. Y., Collins B. M., Meunier F. A., Sugita S. (2009) Rescue of Munc18-1 and -2 double knockdown reveals the essential functions of interaction between Munc18 and closed syntaxin in PC12 cells. Mol. Biol. Cell 20, 4962–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Misura K. M., Scheller R. H., Weis W. I. (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404, 355–362 [DOI] [PubMed] [Google Scholar]
- 30. Richmond J. E., Davis W. S., Jorgensen E. M. (1999) UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat. Neurosci. 2, 959–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Augustin I., Rosenmund C., Südhof T. C., Brose N. (1999) Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400, 457–461 [DOI] [PubMed] [Google Scholar]
- 32. Ma C., Su L., Seven A. B., Xu Y., Rizo J. (2013) Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science 339, 421–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gerber S. H., Rah J. C., Min S. W., Liu X., de Wit H., Dulubova I., Meyer A. C., Rizo J., Arancillo M., Hammer R. E., Verhage M., Rosenmund C., Südhof T. C. (2008) Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science 321, 1507–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen J., Tareste D. C., Paumet F., Rothman J. E., Melia T. J. (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128, 183–195 [DOI] [PubMed] [Google Scholar]
- 35. Schollmeier Y., Krause J. M., Kreye S., Malsam J., Söllner T. H. (2011) Resolving the function of distinct Munc18-1/SNARE protein interaction modes in a reconstituted membrane fusion assay. J. Biol. Chem. 286, 30582–30590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shen J., Rathore S. S., Khandan L., Rothman J. E. (2010) SNARE bundle and syntaxin N-peptide constitute a minimal complement for Munc18-1 activation of membrane fusion. J. Cell Biol. 190, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dulubova I., Khvotchev M., Liu S., Huryeva I., Südhof T. C., Rizo J. (2007) Munc18-1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. U.S.A. 104, 2697–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khvotchev M., Dulubova I., Sun J., Dai H., Rizo J., Südhof T. C. (2007) Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J. Neurosci. 27, 12147–12155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johnson J. R., Ferdek P., Lian L. Y., Barclay J. W., Burgoyne R. D., Morgan A. (2009) Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem. J. 418, 73–80 [DOI] [PubMed] [Google Scholar]
- 40. Meijer M., Burkhardt P., de Wit H., Toonen R. F., Fasshauer D., Verhage M. (2012) Munc18-1 mutations that strongly impair SNARE-complex binding support normal synaptic transmission. EMBO J. 31, 2156–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rickman C., Duncan R. R. (2010) Munc18/Syntaxin interaction kinetics control secretory vesicle dynamics. J. Biol. Chem. 285, 3965–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Malintan N. T., Nguyen T. H., Han L., Latham C. F., Osborne S. L., Wen P. J., Lim S. J., Sugita S., Collins B. M., Meunier F. A. (2009) Abrogating Munc18-1-SNARE complex interaction has limited impact on exocytosis in PC12 cells. J. Biol. Chem. 284, 21637–21646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Toonen R. F., Kochubey O., de Wit H., Gulyas-Kovacs A., Konijnenburg B., Sørensen J. B., Klingauf J., Verhage M. (2006) Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J. 25, 3725–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tareste D., Shen J., Melia T. J., Rothman J. E. (2008) SNAREpin/Munc18 promotes adhesion and fusion of large vesicles to giant membranes. Proc. Natl. Acad. Sci. U.S.A. 105, 2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Wit H., Walter A. M., Milosevic I., Gulyás-Kovács A., Riedel D., Sørensen J. B., Verhage M. (2009) Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell 138, 935–946 [DOI] [PubMed] [Google Scholar]
- 46. Brose N., Petrenko A. G., Südhof T. C., Jahn R. (1992) Synaptotagmin. A calcium sensor on the synaptic vesicle surface. Science 256, 1021–1025 [DOI] [PubMed] [Google Scholar]
- 47. Reist N. E., Buchanan J., Li J., DiAntonio A., Buxton E. M., Schwarz T. L. (1998) Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J. Neurosci. 18, 7662–7673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Z., Liu H., Gu Y., Chapman E. R. (2011) Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. J. Cell Biol. 195, 1159–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim J. Y., Choi B. K., Choi M. G., Kim S. A., Lai Y., Shin Y. K., Lee N. K. (2012) Solution single-vesicle assay reveals PIP2-mediated sequential actions of synaptotagmin-1 on SNAREs. EMBO J. 31, 2144–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malsam J., Parisotto D., Bharat T. A., Scheutzow A., Krause J. M., Briggs J. A., Söllner T. H. (2012) Complexin arrests a pool of docked vesicles for fast Ca2+-dependent release. EMBO J. 31, 3270–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Honigmann A., van den Bogaart G., Iraheta E., Risselada H. J., Milovanovic D., Mueller V., Müllar S., Diederichsen U., Fasshauer D., Grubmüller H., Hell S. W., Eggeling C., Kühnel K., Jahn R. (2013) Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat. Struct. Mol. Biol. 20, 679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parisotto D., Malsam J., Scheutzow A., Krause J. M., Söllner T. H. (2012) SNAREpin assembly by Munc18-1 requires previous vesicle docking by synaptotagmin 1. J. Biol. Chem. 287, 31041–31049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Südhof T. C. (1994) Synaptotagmin I. A major Ca2+ sensor for transmitter release at a central synapse. Cell 79, 717–727 [DOI] [PubMed] [Google Scholar]
- 54. Reim K., Mansour M., Varoqueaux F., McMahon H. T., Südhof T. C., Brose N., Rosenmund C. (2001) Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104, 71–81 [DOI] [PubMed] [Google Scholar]
- 55. Huntwork S., Littleton J. T. (2007) A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat. Neurosci. 10, 1235–1237 [DOI] [PubMed] [Google Scholar]
- 56. Yoon T. Y., Lu X., Diao J., Lee S. M., Ha T., Shin Y. K. (2008) Complexin and Ca2+ stimulate SNARE-mediated membrane fusion. Nat. Struct. Mol. Biol. 15, 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maximov A., Tang J., Yang X., Pang Z. P., Südhof T. C. (2009) Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323, 516–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Malsam J., Seiler F., Schollmeier Y., Rusu P., Krause J. M., Söllner T. H. (2009) The carboxy-terminal domain of complexin I stimulates liposome fusion. Proc. Natl. Acad. Sci. U.S.A. 106, 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kümmel D., Krishnakumar S. S., Radoff D. T., Li F., Giraudo C. G., Pincet F., Rothman J. E., Reinisch K. M. (2011) Complexin cross-links prefusion SNAREs into a zigzag array. Nat. Struct. Mol. Biol. 18, 927–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li F., Pincet F., Perez E., Giraudo C. G., Tareste D., Rothman J. E. (2011) Complexin activates and clamps SNAREpins by a common mechanism involving an intermediate energetic state. Nat. Struct. Mol. Biol. 18, 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tang J., Maximov A., Shin O. H., Dai H., Rizo J., Südhof T. C. (2006) A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell 126, 1175–1187 [DOI] [PubMed] [Google Scholar]
- 62. Lee H. K., Yang Y., Su Z., Hyeon C., Lee T. S., Lee H. W., Kweon D. H., Shin Y. K., Yoon T. Y. (2010) Dynamic Ca2+-dependent stimulation of vesicle fusion by membrane-anchored synaptotagmin 1. Science 328, 760–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kyoung M., Srivastava A., Zhang Y., Diao J., Vrljic M., Grob P., Nogales E., Chu S., Brunger A. T. (2011) In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc. Natl. Acad. Sci. U.S.A. 108, E304–E313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jorquera R. A., Huntwork-Rodriguez S., Akbergenova Y., Cho R. W., Littleton J. T. (2012) Complexin controls spontaneous and evoked neurotransmitter release by regulating the timing and properties of synaptotagmin activity. J. Neurosci. 32, 18234–18245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martens S., Kozlov M. M., McMahon H. T. (2007) How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 [DOI] [PubMed] [Google Scholar]
- 66. Krishnakumar S. S., Kümmel D., Jones S. J., Radoff D. T., Reinisch K. M., Rothman J. E. (2013) Conformational dynamics of calcium-triggered activation of fusion by synaptotagmin. Biophys. J. 105, 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Deák F., Xu Y., Chang W. P., Dulubova I., Khvotchev M., Liu X., Südhof T. C., Rizo J. (2009) Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J. Cell Biol. 184, 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Emsley P., Lohkamp B., Scott W. G., Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu Y., Su L., Rizo J. (2010) Binding of Munc18-1 to synaptobrevin and to the SNARE four-helix bundle. Biochemistry 49, 1568–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boyd A., Ciufo L. F., Barclay J. W., Graham M. E., Haynes L. P., Doherty M. K., Riesen M., Burgoyne R. D., Morgan A. (2008) A random mutagenesis approach to isolate dominant-negative yeast sec1 mutants reveals a functional role for domain 3a in yeast and mammalian Sec1/Munc18 proteins. Genetics 180, 165–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Han G. A., Bin N. R., Kang S. Y., Han L., Sugita S. (2013) The domain-3a of Munc18–1 plays a crucial role at the priming stage of exocytosis. J. Cell Sci. 126, 2361–2371 [DOI] [PubMed] [Google Scholar]
- 72. Hu S. H., Christie M. P., Saez N. J., Latham C. F., Jarrott R., Lua L. H., Collins B. M., Martin J. L. (2011) Possible roles for Munc18-1 domain 3a and Syntaxin1 N-peptide and C-terminal anchor in SNARE complex formation. Proc. Natl. Acad. Sci. U.S.A. 108, 1040–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Takamori S., Holt M., Stenius K., Lemke E. A., Grønborg M., Riedel D., Urlaub H., Schenck S., Brügger B., Ringler P., Müller S. A., Rammner B., Gräter F., Hub J. S., De Groot B. L., Mieskes G., Moriyama Y., Klingauf J., Grubmüller H., Heuser J., Wieland F., Jahn R. (2006) Molecular anatomy of a trafficking organelle. Cell 127, 831–846 [DOI] [PubMed] [Google Scholar]
- 74. Struck D. K., Hoekstra D., Pagano R. E. (1981) Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20, 4093–4099 [DOI] [PubMed] [Google Scholar]
- 75. Cuff J. A., Barton G. J. (1999) Evaluation and improvement of multiple sequence methods for protein secondary structure prediction. Proteins 34, 508–519 [DOI] [PubMed] [Google Scholar]
- 76. Ouali M., King R. D. (2000) Cascaded multiple classifiers for secondary structure prediction. Protein Sci. 9, 1162–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jones D. T. (1999) Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292, 195–202 [DOI] [PubMed] [Google Scholar]
- 78. Lin J. C., Barua B., Andersen N. H. (2004) The helical alanine controversy. An Ala-6 insertion dramatically increases helicity. J. Am. Chem. Soc. 126, 13679–13684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aoki S., Epand R. M. (2012) Caveolin-1 hydrophobic segment peptides insertion into membrane mimetic systems. Role of proline residue. Biochim. Biophys. Acta 1818, 12–18 [DOI] [PubMed] [Google Scholar]
- 80. Suh J. Y., Lee Y. T., Park C. B., Lee K. H., Kim S. C., Choi B. S. (1999) Structural and functional implications of a proline residue in the antimicrobial peptide gaegurin. Eur. J. Biochem. 266, 665–674 [DOI] [PubMed] [Google Scholar]
- 81. Weninger K., Bowen M. E., Choi U. B., Chu S., Brunger A. T. (2008) Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure 16, 308–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Martin S., Tomatis V. M., Papadopulos A., Christie M. P., Malintan N. T., Gormal R. S., Sugita S., Martin J. L., Collins B. M., Meunier F. A. (2013) The Munc18-1 domain 3a loop is essential for neuroexocytosis but not for syntaxin-1A transport to the plasma membrane. J. Cell Sci. 126, 2353–2360 [DOI] [PubMed] [Google Scholar]
- 83. Hackmann Y., Graham S. C., Ehl S., Höning S., Lehmberg K., Aricò M., Owen D. J., Griffiths G. M. (2013) Syntaxin binding mechanism and disease-causing mutations in Munc18-2. Proc. Natl. Acad. Sci. U.S.A. 110, E4482–E4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Al Hawas R., Ren Q., Ye S., Karim Z. A., Filipovich A. H., Whiteheart S. W. (2012) Munc18b/STXBP2 is required for platelet secretion. Blood 120, 2493–2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Baker R. W., Jeffrey P. D., Hughson F. M. (2013) Crystal structures of the Sec1/Munc18 (SM) protein Vps33, alone and bound to the homotypic fusion and vacuolar protein sorting (HOPS) subunit Vps16*. PLoS ONE 8, e67409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi L., Kümmel D., Coleman J., Melia T. J., Giraudo C. G. (2011) Dual roles of Munc18-1 rely on distinct binding modes of the central cavity with Stx1A and SNARE complex. Mol. Biol. Cell. 22, 4150–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ellena J. F., Liang B., Wiktor M., Stein A., Cafiso D. S., Jahn R., Tamm L. K. (2009) Dynamic structure of lipid-bound synaptobrevin suggests a nucleation-propagation mechanism for trans-SNARE complex formation. Proc. Natl. Acad. Sci. U.S.A. 106, 20306–20311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Li F., Pincet F., Perez E., Eng W. S., Melia T. J., Rothman J. E., Tareste D. (2007) Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 14, 890–896 [DOI] [PubMed] [Google Scholar]
- 89. Lam P. P., Ohno M., Dolai S., He Y., Qin T., Liang T., Zhu D., Kang Y., Liu Y., Kauppi M., Xie L., Wan W. C., Bin N. R., Sugita S., Olkkonen V. M., Takahashi N., Kasai H., Gaisano H. Y. (2013) Munc18b is a major mediator of insulin exocytosis in rat pancreatic β-cells. Diabetes 62, 2416–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]






