Abstract
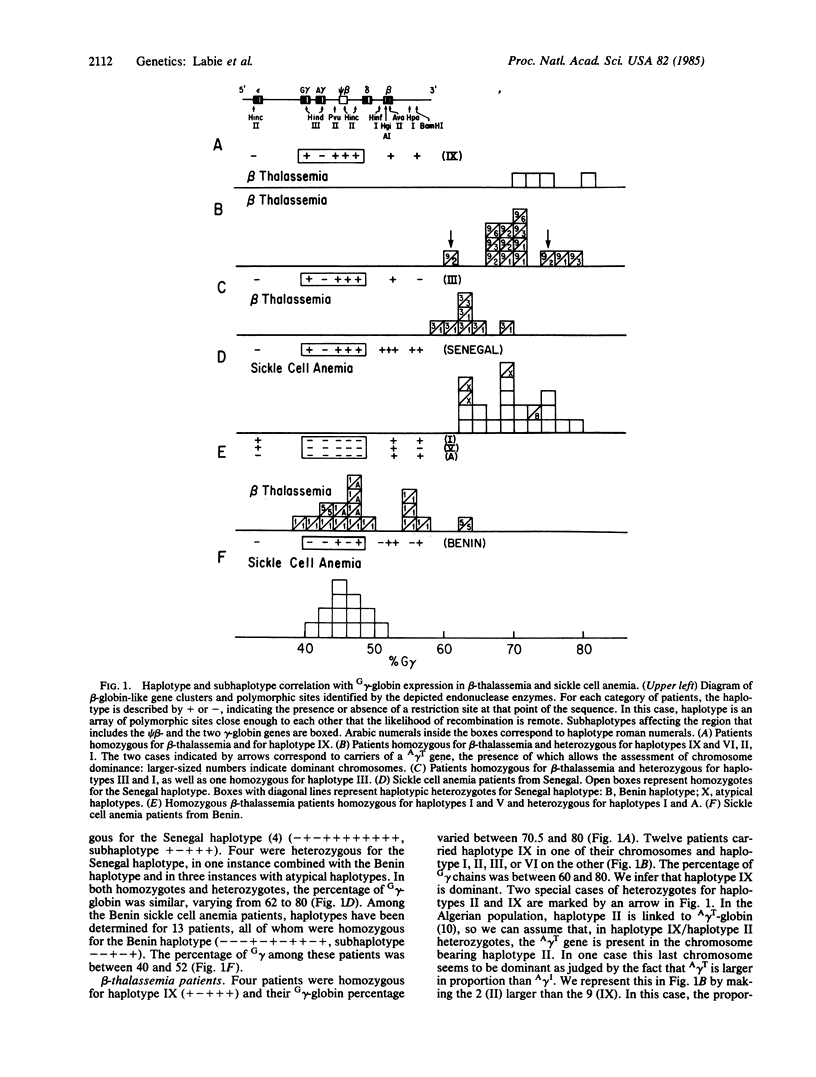
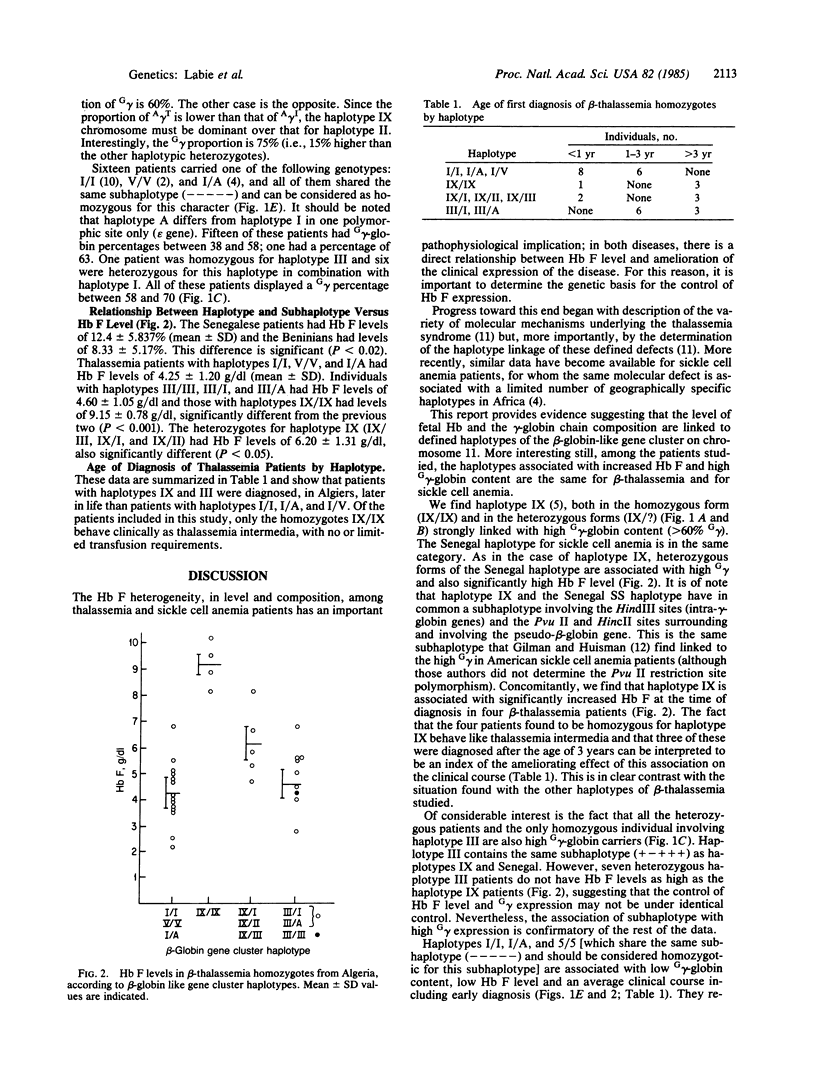
We have studied 42 homozygous beta-thalassemia patients from Algeria and 34 sickle cell anemia patients from Senegal and Benin, determining the relationship between haplotypes, Hb F, and G gamma-globin/A gamma-globin ratios. Populations selected have a high frequency of haplotype homozygotes because of consanguinity (Algeria) and geographic homogeneity (West Africa). We find in beta-thalassemia patients, that haplotype IX in haplotypic homozygotes and heterozygotes, haplotype III in heterozygotes, and the Senegal haplotype in sickle cell anemia patients are all linked to high G gamma-globin expression. In addition, haplotypes IX and Senegal, but not haplotype III, have high Hb F levels. All of these haplotype have a common subhaplotype (+- ) in the gamma-globin gene region. In addition, haplotypes IX, III, and Senegalese sickle cell anemia patients exhibit hematological amelioration of their disease. Conversely, haplotypes I, V, and A in thalassemia patients, which also have a common subhaplotype (-----), and the Benin subhaplotype (--++-) in sickle cell anemia patients are all associated with low G gamma-globin and low Hb F levels. Low G gamma-globin expression in the adult is associated with two haplotypes that are not common between thalassemia and sickle cell anemia patients. We conclude that the determinant for high G gamma-globin expression is haplotype-linked to common and genetically dominant subhaplotypes in the two diseases. The total Hb F level, unlike the high G gamma-globin expression, however, is linked to haplotypes but not to subhaplotypes, thus dissociating the two genetic effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. P., Goff S. C., Efremov G. D., Gravely M. E., Huisman T. H. Globin chain electrophoresis: a new approach to the determination of the G gamma/A gamma ratio in fetal haemoglobin and to studies of globin synthesis. Br J Haematol. 1980 Apr;44(4):527–534. doi: 10.1111/j.1365-2141.1980.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Beldjord C., Lapouméroulie C., Baird M. L., Girot R., Adjrad L., Lenoir G., Benabadji M., Labie D. Four new haplotypes observed in Algerian beta-thalassemia patients. Hum Genet. 1983;65(2):204–206. doi: 10.1007/BF00286665. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Efremov G. D., Duma H., Mladenovski B., Hyman C. B., Rachmilewitz E. A., Bouver N., Miller A., Brodie A. The present status of the heterogeneity of fetal hemoglobin in beta-thalassemia: an attempt to unify some observations in thalassemia and related conditions. Ann N Y Acad Sci. 1974;232(0):107–124. doi: 10.1111/j.1749-6632.1974.tb20576.x. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Antonarakis S. E., Kazazian H. H., Jr Polymorphism and molecular pathology of the human beta-globin gene. Prog Hematol. 1983;13:49–73. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Pagnier J., Mears J. G., Dunda-Belkhodja O., Schaefer-Rego K. E., Beldjord C., Nagel R. L., Labie D. Evidence for the multicentric origin of the sickle cell hemoglobin gene in Africa. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1771–1773. doi: 10.1073/pnas.81.6.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H. Hemoglobin F in beta thalassemia and related conditions. Ann N Y Acad Sci. 1980;344:240–252. doi: 10.1111/j.1749-6632.1980.tb33665.x. [DOI] [PubMed] [Google Scholar]
- Schroeder W. A., Huisman T. H., Shelton J. R., Shelton J. B., Kleihauer E. F., Dozy A. M., Robberson B. Evidence for multiple structural genes for the gamma chain of human fetal hemoglobin. Proc Natl Acad Sci U S A. 1968 Jun;60(2):537–544. doi: 10.1073/pnas.60.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serjeant G. R. Fetal haemoglobin in homozygous sickle cell disease. Clin Haematol. 1975 Feb;4(1):109–122. [PubMed] [Google Scholar]



