Background: NELL1, an oligomeric glycoprotein, is an osteoinductive factor.
Results: The C-terminal cysteine-rich region of NELL1 has cell-binding activity. Intermolecular, but not intramolecular, disulfide bonding is important for the oligomerization and cell adhesion activity of NELL1.
Conclusion: NELL1 requires oligomerization-induced conformational change for efficient mediation of cell adhesion and spreading.
Significance: The structural basis of NELL1-mediated cell adhesion is identified.
Keywords: Cell Adhesion, Disulfide, Extracellular Matrix, Osteoblasts, Protein Conformation
Abstract
NELL1 is a large oligomeric secretory glycoprotein that functions as an osteoinductive factor. NELL1 contains several conserved domains, has structural similarities to thrombospondin 1, and supports osteoblastic cell adhesion through integrins. To define the structural requirements for NELL1-mediated cell adhesion, we prepared a series of recombinant NELL1 proteins (intact, deleted, and cysteine-mutant) from a mammalian expression system and tested their activities. A deletion analysis demonstrated that the C-terminal cysteine-rich region of NELL1 is critical for the cell adhesion activity of NELL1. Reducing agent treatment decreased the cell adhesion activity of full-length NELL1 but not of its C-terminal fragments, suggesting that the intramolecular disulfide bonds within this region are not functionally necessary but that other disulfide linkages in the N-terminal region of NELL1 may be involved in cell adhesion activity. By replacing cysteine residues with serines around the coiled-coil domain of NELL1, which is responsible for oligomerization, we created a mutant NELL1 protein that was unable to form homo-oligomers, and this monomeric mutant showed substantially lower cell adhesion activity than intact NELL1. These results suggest that an oligomerization-induced conformational change in the C-terminal region of NELL1 is important for the efficient mediation of cell adhesion and spreading by NELL1.
Introduction
NELL1 (Nel-like molecule 1) is a large secretory glycoprotein that belongs to the NELL3 family of proteins, together with NELL2 (1–4). The NELL1 and NELL2 genes are predominantly expressed in the brain and also show partly overlapping expression patterns (5). These genes have 72% similarity in their deduced amino acid sequences. However, the biological functions of the proteins they encode are greatly different. NELL2-deficient mice have a significant enhancement of long-term potentiation in the dentate gyrus, suggesting that NELL2 is a negative regulator of neuronal activity (6). NELL2 has also been shown to support the survival of neurons from the hippocampus and cerebral cortex (7). In contrast, NELL1-deficient mice show cranial and vertebral skeletal defects, suggesting that NELL1 plays a key role as a regulator of craniofacial skeletal morphogenesis (8, 9). NELL1 has also been shown to induce osteogenic differentiation and bone formation in various models (10–19). Taking advantage of the potent osteoinductive activity of NELL1 with a relatively high specificity to the osteochondral lineage, NELL1-based therapies for bone regeneration are under development for future applications. However, the molecular mechanisms underlying NELL1-induced osteogenic differentiation are not fully understood, and no specific receptors for NELL1 have been identified.
Soluble factors involved in osteoblast differentiation and bone regeneration, such as bone morphogenetic protein 2 (BMP-2) and FGF-2, act on osteoblasts by binding to their specific cell surface receptors to activate various signaling pathways (20). For example, BMP-2 activates both the Smad and MAPK pathways through binding to the BMP receptor I/II complex, whereas FGF-2 activates the MAPK pathway through binding to the FGF receptor. Similarly, NELL1 transduces osteogenic signals via the MAPK pathway (21). Upon treatment of primary rat fetal calvarial cells with NELL1, ERK1/2 kinases were transiently activated after only 10 min of stimulation, and then Runx2, a master regulator of osteoblast differentiation, was phosphorylated to induce the expression of osteogenic marker genes. A pulldown experiment with the lysates of rat fetal calvarial cells treated with epitope-tagged NELL1 revealed the rapid accumulation of ∼45-kDa and 55-kDa tyrosine-phosphorylated proteins in the cells as NELL1-binding proteins. However, it is not clear whether they are NELL1 receptors. We demonstrated previously that NELL1 promoted integrin-dependent cell adhesion through several cell-binding sites localized to the C-terminal half of the NELL1 protein (22). Because integrin-dependent cell adhesion activates focal adhesion kinase (FAK), followed by MAPK activation, we suggested that NELL1 modulates osteoblast differentiation via the integrin-FAK-MAPK pathway. In a direct demonstration by Shen et al. (23), murine mesenchymal cells cultured on NELL1 showed both enhanced cell attachment and phosphorylation of FAK that are dependent on integrin β1, thereby promoting osteogenic differentiation. These findings point to integrin β1 as an attractive candidate as the cell surface receptor for NELL1.
The human NELL1 gene encodes a polypeptide of 810 amino acids with structural similarities to thrombospondin 1(TSP-1), a multifunctional extracellular matrix protein. NELL1 contains several structural motifs, including an N-terminal TSP-1-like (TSPN) domain, a coiled-coil (CC) domain, four von Willebrand factor type C (VWC) domains, and six EGF-like domains. The TSPN domain of NELL1 has been shown to have a heparin-binding activity that may be important for interaction with heparan sulfate proteoglycans to modulate cell-matrix interactions or cell function (3, 5). The EGF-like domains of NELL1 were identified as binding sites for the protein kinase C βI subunit, suggesting a novel mode of action of NELL1; that is, functions in the cytoplasm (24). The VWC domain, also called chordin-like cysteine-rich domain, has been characterized for its binding to BMPs (25). However, no such function has been identified in the VWC domains of NELL14 Similar to TSP-1, NELL1 expressed in mammalian cells forms homo-oligomers, presumably through the coiled-coil domain, and has been suggested to be stabilized by intermolecular disulfide bonds (26). However, TSP-1 forms only homotrimers (27), whereas NELL1 forms similar amounts of homodimers and homotrimers (26). Although these forms of NELL1 may have different roles in regulating osteoblastic differentiation, little is known about the relevance of the structure of NELL1 to the cellular response.
In this study, we used a series of recombinant proteins to more closely define the cell-binding sites of NELL1. Through deletion analysis, we found that the C-terminal, most cysteine-rich region is critical for the cell adhesion activity of NELL1. Interestingly, the cell adhesion activity of full-length NELL1, but not of its C-terminal fragments, was decreased dramatically by treatment with a reducing agent, suggesting that intramolecular disulfide bonds within this region are not functionally necessary but that other disulfide linkages in the N-terminal region of NELL1 may be involved in cell adhesion activity. Further deletion analysis revealed that NELL1 forms homo-oligomers through the coiled-coil domain. By analyzing cysteine point mutants, we identified four cysteine residues around the coiled-coil domain that are involved in intermolecular disulfide bonds and are required not only for the oligomerization of NELL1 but also for the full cell adhesion activity of NELL1. We conclude that NELL1 oligomerization is necessary for efficient cell adhesion by intact NELL1.
EXPERIMENTAL PROCEDURES
Antibodies
Mouse anti-NELL1 polyclonal antibody (B01P) was purchased from Abnova (Taipei, Taiwan). Mouse monoclonal antibodies against FLAG (catalog no. F3165) and vinculin (catalog no. V9131) were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal antibodies against FAK, phospho-FAK (Tyr397), ERK1/2, and phospho-ERK1/2 (Thr202/Tyr204) were purchased from Cell Signaling Technology (Danvers, MA). Rabbit polyclonal antibody against human β-actin was purchased from GeneTex (Irvine, CA). Rabbit polyclonal antibodies against integrin α3 (catalog no. AB1920) and integrin β1 (catalog no. AB1952) were purchased from Millipore (Billerica, MA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgGs were purchased from GE Healthcare. Alexa Fluor 488-conjugated anti-mouse and anti-rabbit IgGs were purchased from Invitrogen. Function-blocking anti-integrin monoclonal antibodies against the α3 (Ralph 3.2, Santa Cruz Biotechnology, Santa Cruz, CA), α6 (GoH3, eBioscience, San Diego, CA), α7 (6A11, MBL, Nagoya, Japan), αV (RMV-7, Millipore), and β1 (Ha2/5, BD Biosciences) subunits were used for adhesion inhibition assays.
Plasmid Construction
Expression vectors for human NELL1 with an N-terminal FLAG tag and a C-terminal hexahistidine tag were prepared as follows. cDNA encoding full-length human NELL1 without the signal peptide (residues 17–810) was amplified by RT-PCR from total RNA isolated from human brain (Invitrogen). The PCR products were digested with BamHI and XhoI, cloned into the pSecTag2-FLAG vector (28), and designated NELL1-FL. cDNA fragments encoding the C-terminal deletion mutants of human NELL1 (residues 17–634, 17–388, 17–272, 17–226, 17–749, 17–688, 17–266, 17–230, and 17–219) were amplified by PCR using NELL1-FL as a template, cloned into the pSecTag2-FLAG vector, and designated NELL1-ΔC1, NELL1-ΔC2, NELL1-ΔC3 (also called NELL1-N), NELL1-ΔC4 (also called NELL1-N-ΔCC3), NELL1-ΔC5, NELL1-ΔC6, NELL1-N-ΔCC1, NELL1-N-ΔCC2, and NELL1-N-ΔCC4, respectively. Deletion mutants of the full-length NELL1 expression plasmid lacking the coding sequence for one or both of the C-terminal VWC domains (residues 635–688, 689–749, and 635–749) were generated by inverse PCR and designated NELL1-ΔVWC3, NELL1-ΔVWC4, and NELL1-ΔVWC34, respectively. cDNA fragments encoding the C-terminal VWC domains of human NELL1 (residues 635–810, 635–688, 689–749, and 750–810) were amplified by PCR using NELL1-FL as a template, cloned into the pSecTag2-FLAG vector, and designated NELL1-VWC345, NELL1-VWC3, NELL1-VWC4, and NELL1-VWC5, respectively. Deletion mutants of the full-length NELL1 expression plasmid lacking the coding sequence for the coiled-coil domain (residues 240–267 and 227–269) were generated by inverse PCR and designated NELL1-ΔCC1 and NELL1-ΔCC2, respectively. The codons of five cysteine residues within and around the coiled-coil domain (at positions 220, 227, 230, 267, and 269) were changed to those of serine in the full-length NELL1 expression plasmid by site-directed mutagenesis by inverse PCR. After the desired mutations were confirmed by sequencing, cDNA fragments encoding the TSPN and coiled-coil domains were amplified by PCR using the mutant plasmids as templates, cloned into the pSecTag2-FLAG vector, and designated NELL1-N-mut1 through NELL1-N-mut2345 (according to the relative positions of the mutated cysteine residues). All primer sequences used in this study are available upon request.
Protein Expression and Purification
Recombinant NELL1 proteins were produced by using the FreeStyle MAX 293 expression system (Invitrogen) according to the instructions of the manufacturer. Briefly, 293-F cells were transfected with the expression plasmids using the FreeStyle MAX reagent (Invitrogen) and grown in serum-free FreeStyle 293 expression medium (Invitrogen) for 6 days. Recombinant proteins were purified from the conditioned media using HisTrap HP columns (GE Healthcare) as described previously (26). Purified proteins were separated by SDS-PAGE and visualized by silver staining.
Cell Culture
The murine preosteoblast cell line MC3T3-E1 clone 4 was obtained from the Riken Cell Bank (Tsukuba, Japan) and was maintained in α-minimum Eagle's medium (MEM) containing 10% (v/v) FCS. The human neuroblastoma cell line IMR-32 was obtained from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan) and was maintained in MEM containing 10% (v/v) FCS and 1% (v/v) MEM non-essential amino acid solution (Sigma-Aldrich).
Cell Adhesion Assays
Cell adhesion assays were performed using 96-well microtiter plates (Maxisorp, Nunc, Roskilde, Denmark). Briefly, plates were coated with the indicated concentration of human plasma fibronectin (Millipore) or NELL1 at 4 °C overnight and then blocked with 1% (w/v) BSA for 1 h at room temperature. Cells were harvested with 1 mm EDTA, suspended in serum-free α-MEM at a density of 3 × 105 cells/ml, and then plated at 3 × 104 cells/well. After incubation in a CO2 incubator at 37 °C for 1.5 h, non-adherent cells were washed from the wells, and adherent cells were fixed and stained for 30 min with 0.4% (w/v) crystal violet in 50% (v/v) methanol. After washing with distilled water, attached cells were counted by microscopic examination of random fields in two independent wells.
The coating efficiency of each NELL1 protein on microtiter plates was determined by quantification of residual protein remaining in the coating solution after incubation. There were no major differences in coating efficiency among the NELL1 proteins used.
Immunoblot Analysis
Conditioned media were separated on SDS-PAGE or non-denaturing-PAGE (native PAGE), followed by transfer onto Hybond ECL nitrocellulose membranes (GE Healthcare). The membranes were blocked with 5% (w/v) nonfat dry milk in PBS containing 0.1% (v/v) Tween 20, followed by incubation with primary antibodies in Can Get Signal Immunoreaction Enhancer Solution 1 (Toyobo, Osaka, Japan) for 1 h at room temperature. The membranes were then washed with PBS containing 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibodies in Can Get Signal Solution 2 (Toyobo) at room temperature for 1 h. The membranes were developed with ECL Western blotting detection reagents (GE Healthcare) and imaged on an LAS-4000mini luminescent image analyzer (GE Healthcare).
Atomic Force Microscopy Analysis
For each NELL1 protein analyzed, an aliquot of the purified protein (25 μg/ml) was deposited onto a mica chip and allowed to set for 20 min in air. The samples were then washed with distilled water, further dried for 20 min, and analyzed with an atomic force microscope (model SPA-400, SII NanoTechnology Inc., Chiba, Japan). Images were taken in dynamic force microscopy mode in air at room temperature.
Immunofluorescence Staining
Glass coverslips (12 mm in diameter; Fisher Scientific, Pittsburgh, PA) were coated with 40 μg/ml fibronectin, 100 nm laminin 332 (LM-332) (28), or 100 nm NELL1 at 4 °C overnight and then blocked with 3% (w/v) BSA for 1 h at room temperature. EDTA-detached MC3T3-E1 cells were plated onto glass coverslips and cultured in serum-free α-MEM at 37 °C for 1.5 h. Non-adherent cells were washed from the coverslips, and adherent cells were fixed with 4% (w/v) paraformaldehyde in TBS for 30 min at room temperature. Cells were permeabilized with 0.5% (v/v) Triton X-100 for 5 min at room temperature, blocked with 3% (w/v) BSA for 1 h at room temperature, and then incubated with primary antibodies for 1 h at room temperature. The cells were washed three times with TBS containing 0.03% Triton X-100, incubated with Alexa Fluor 488-conjugated secondary antibodies for 1 h, washed three times with TBS, and then incubated with rhodamine-phalloidin (Cytoskeleton, Inc., Denver, CO) for 30 min. Nuclei were stained with DAPI before mounting on glass slides with ProLong Gold antifade reagent (Invitrogen). Specimens were observed by using an FV1000 confocal laser-scanning microscope (Olympus, Tokyo, Japan).
FAK and ERK Phosphorylation Assays
FAK and ERK1/2 phosphorylation was detected as follows. MC3T3-E1 cells were subjected to serum starvation for 16 h. The cells were harvested with 1 mm EDTA, suspended in serum-free α-MEM with or without 1 μm FAK inhibitor 14 (1,2,4,5-benzenetetraminetetrahydrochloride, Santa Cruz Biotechnology), and kept in suspension for 30 min at 37 °C. Then the cells were placed on 24-well cell culture plates coated with 100 nm NELL1-FL, NELL1-VWC345, NELL1-mut2345, or 0.01% (w/v) poly-l-lysine (Wako Pure Chemical, Osaka, Japan) at 2 × 105 cells/well and allowed to adhere for 1.5 h. After washing with ice-cold PBS, the cells were lysed with CelLytic M cell lysis reagent (Sigma-Aldrich) containing 1 × PhosSTOP phosphatase inhibitor mixture (Roche). Protein samples were separated on SDS-PAGE and transferred onto Hybond ECL nitrocellulose membranes (GE Healthcare). The membranes were blocked with 5% (w/v) PhosphoBLOCKER blocking reagent (Cell Biolabs, San Diego, CA) in TBS containing 0.1% (v/v) Tween 20, followed by incubation with primary antibodies in Can Get Signal Immunoreaction Enhancer Solution 1 (Toyobo) at 4 °C overnight. The membranes were then washed with TBS containing 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibodies in Can Get Signal Solution 2 (Toyobo) at room temperature for 1 h. The membranes were developed with ECL Prime Western blotting detection reagents (GE Healthcare) and imaged on an LAS-4000mini luminescent image analyzer (GE Healthcare).
RESULTS
The C-terminal Region of NELL1 Is Critical for Cell Adhesion Activity
We reported previously that NELL1 promoted integrin-dependent cell adhesion through several cell-binding sites localized to the C-terminal half of NELL1 (22). However, because the C-terminal half of the NELL1 protein tended to degrade rapidly in a mammalian expression system, thereafter we analyzed the cell-binding sites using proteins prepared from a bacterial expression system, raising the question whether the proteins exhibited proper folding. In fact, a relatively high concentration of these bacterially produced NELL1 proteins was required for cell adhesion. In this study, we used a series of recombinant NELL1 proteins prepared in a mammalian expression system to more closely define the cell-binding sites of NELL1.
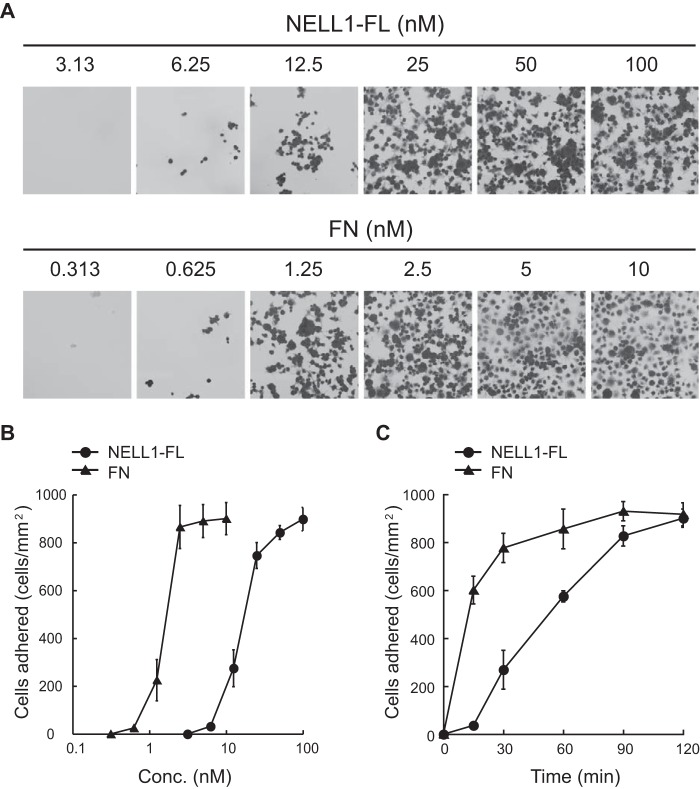
To better characterize the nature of NELL1-mediated cell adhesion, the dose dependence and kinetics of cell adhesion to full-length NELL1 (NELL1-FL) were first examined using the murine preosteoblastic MC3T3-E1 cell line (Fig. 1). NELL1 promoted cell adhesion in a dose-dependent manner, with maximum adhesion occurring at a NELL1 coating concentration of ∼100 nm. This adhesion activity was ≥10 times lower than that of the major structural extracellular matrix protein fibronectin (Fig. 1, A and B). The kinetics of cell adhesion to NELL1 versus to fibronectin indicated that the cells on NELL1 coating required more time than those on fibronectin coating to reach maximum adhesion (Fig. 1C). These findings suggest that the cell adhesion properties of NELL1 are relatively weak compared with those of structural extracellular matrix proteins such as fibronectin.
FIGURE 1.
Cell adhesion activity of recombinant NELL1. A and B, dose-dependent adhesion of MC3T3-E1 cells to full-length NELL1 (NELL1-FL) and fibronectin (FN). These proteins were coated onto 96-well plates at the indicated concentrations (Conc.). MC3T3-E1 cells were allowed to adhere for 1.5 h at 37 °C. Non-adherent cells were washed from the wells, and adherent cells were then fixed and stained with crystal violet. Representative images (A) and the number of adhered cells per area (B) are shown for cells adhering to NELL1-FL and fibronectin. C, kinetics of cell adhesion to NELL1-FL and fibronectin. NELL1-FL (100 nm) and fibronectin (10 nm) were coated onto 96-well plates, and MC3T3-E1 cells were allowed to adhere for the indicated times at 37 °C before the adhered cells were counted. Each value represents the mean ± S.E. of triplicate results.
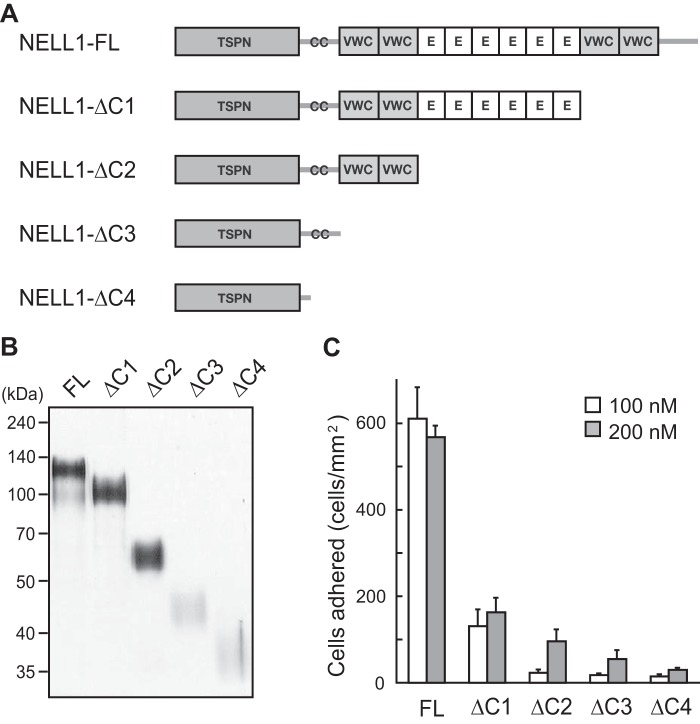
Next, to determine which NELL1 domain is critical for promoting cell adhesion, we prepared a series of C-terminally truncated NELL1 proteins in the mammalian 293-F cells (Fig. 2A). These proteins were purified by nickel-chelate affinity chromatography and resolved by SDS-PAGE under reducing conditions (Fig. 2B). The cell adhesion activity of these proteins was evaluated relative to that of full-length NELL1 using MC3T3-E1 cells (Fig. 2C). When compared on a molar basis, cell adhesion activity was reduced significantly by the deletion of the C-terminal VWC domains and was decreased further by increasing C-terminal truncations at a NELL1 coating concentration of either 100 nm or 200 nm. These results suggest that the C-terminal region around the VWC domains is a major cell-binding site of NELL1.
FIGURE 2.
Deletion of the C-terminal region of NELL1 reduces cell adhesion activity. A, schematic of NELL1-FL and a series of C-terminal deletion mutants. NELL1-FL contains one TSPN domain, one CC domain, four VWC domains, and six EGF-like (E) repeats. B, the intact and mutant NELL1s were expressed in 293-F cells, and the purified proteins were resolved by SDS-PAGE and visualized by silver staining. Molecular weight markers are indicated at the left. C, NELL1-FL and its deletion mutants were coated onto 96-well plates at a concentration of 100 or 200 nm, and MC3T3-E1 cells were allowed to adhere for 1.5 h at 37 °C before adhered cells in the wells were processed for cell counting. Each value represents the mean ± S.E. of triplicate results.
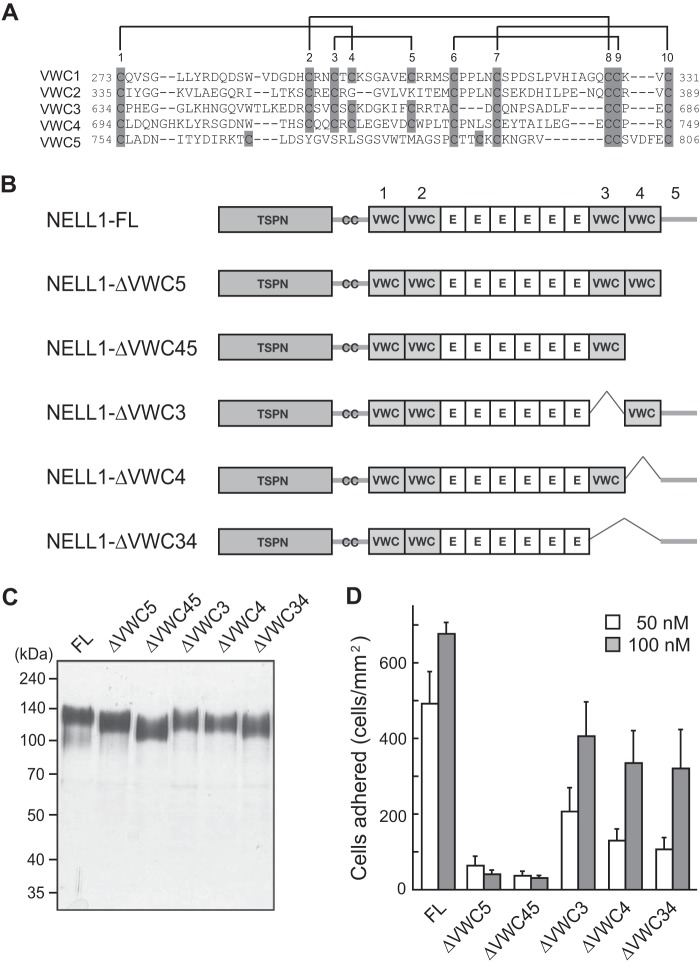
A VWC domain usually contains 10 conserved cysteines that form five intramolecular disulfide bonds (29). As shown in Fig. 3A, most of these cysteines are present in the four VWC domains of NELL1 (designated VWC1 through VWC4). Although some of these cysteines are absent from the C-terminal-most region (residues 750–810) of NELL1, this region is designated VWC5 for the sake of convenience. To further localize the cell-binding sites within the C-terminal region, VWC3, VWC4, and VWC5 were deleted either singly or in pairs (Fig. 3, B and C), and the cell adhesion activity of the resulting proteins was evaluated (Fig. 3D). Removing the C-terminal-most region by itself (NELL1-ΔVWC5) or along with the adjacent VWC domain (NELL1-ΔVWC45) drastically reduced the activity. The activity was reduced moderately when either or both of the C-terminal VWC domains (VWC3 and VWC4) were removed. These results suggest that the C-terminal-most region is critical for the cell adhesion activity of NELL1 and that the two C-terminal VWC domains could also contribute to the activity.
FIGURE 3.
The C-terminal VWC domains are necessary for NELL1-mediated cell adhesion. A, amino acid sequence alignment of the VWC domains of NELL1. The 10 positions at which conserved cysteine residues occur in the VWC domains are numbered above the sequences, with the cysteine residues boxed in gray. Possible intramolecular disulfide bonds are designated as solid lines. Note that several conserved cysteines are lacking in the C-terminal-most region (residues 754–806, designated VWC5). B, schematic representation of deletion mutants of the C-terminal VWC domains of NELL1. E, EGF-like repeat. C, the intact and mutant NELL1s were expressed in 293-F cells, and the purified proteins were resolved by SDS-PAGE and visualized by silver staining. Molecular weight markers are indicated at the left. D, NELL1-FL and its deletion mutants were coated onto 96-well plates at a concentration of 50 nm or 100 nm, and MC3T3-E1 cells were allowed to adhere for 1.5 h at 37 °C before the adhered cells were counted. Each value represents the mean ± S.E. of triplicate results.
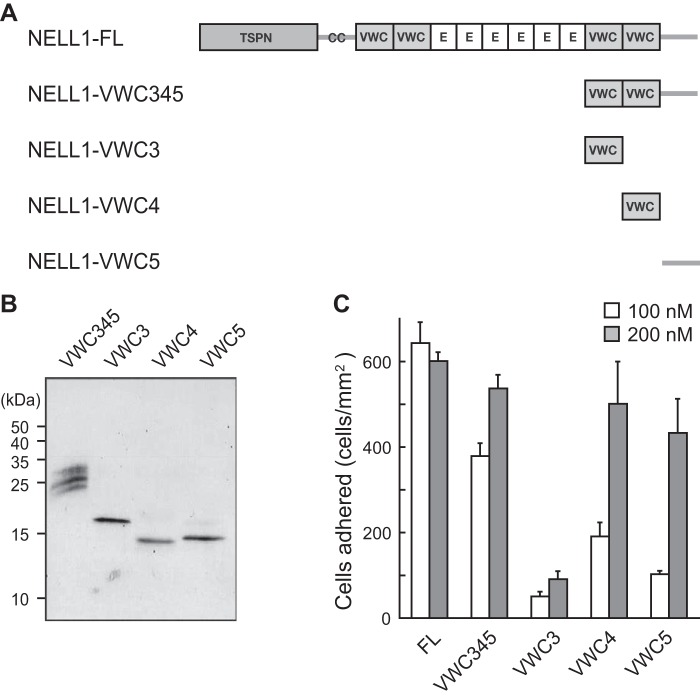
Next, we examined the cell adhesion activity of individual domains of the NELL1 C-terminal region. Four recombinant proteins comprising one or all of VWC3, VWC4, and VWC5 were expressed successfully in 293-F cells. The purified proteins were visualized by SDS-PAGE under reducing conditions (Fig. 4, A and B), and their cell adhesion activity was evaluated (Fig. 4C). The C-terminal fragment containing VWC3 through VWC5 (NELL1-VWC345) showed a similar cell adhesion activity as full-length NELL1 (NELL1-FL) at a 200 nm coating concentration, but the activity was reduced to about 60% of that of NELL1-FL at a 100 nm coating concentration. The fourth VWC domain (NELL1-VWC4) and the C-terminal-most region (NELL1-VWC5) showed slightly weaker activities than NELL1-VWC345, whereas the third VWC domain (NELL1-VWC3) had little activity. These results suggest that NELL1 has two cell-binding sites within the C-terminal region, one in the fourth VWC domain and the other in the C-terminal-most region. We also tested whether NELL1 directly binds to cells via the C-terminal region. A competition analysis was performed by preincubating cells with the NELL1 C-terminal fragment before the cells were allowed to adhere to full-length NELL1. Surprisingly, no inhibition of cell adhesion was observed when cells were preincubated with up to 1 μm of NELL1-VWC345 (data not shown). A higher concentration of the NELL1 fragment may be required for efficient competition. Alternatively, soluble NELL1 fragments in solution may have reduced cell adhesion activity compared with immobilized NELL1.
FIGURE 4.
NELL1 contains two cell-binding sites within the C-terminal region. A, schematic of four C-terminal fragments of NELL1. E, EGF-like repeat. B, the C-terminal fragments of NELL1 were expressed in 293-F cells, and the purified proteins were resolved by SDS-PAGE and visualized by silver staining. Molecular weight markers are indicated at the left. C, NELL1-FL and its C-terminal fragments were coated onto 96-well plates at a concentration of 100 or 200 nm, and MC3T3-E1 cells were allowed to adhere for 1.5 h at 37 °C before adhered cells in the wells were processed for counting. Each value represents the mean ± S.E. of triplicate results.
Intramolecular Disulfide Bonds of the VWC Domain Are Not Necessary for Cell Adhesion Activity
As described above, because a VWC domain contains several intramolecular disulfide bonds, the tertiary structure of the VWC domain is thought to be important for recognition of cell surface receptors (integrins). To examine whether the disulfide bonds of the VWC domains are necessary for the cell adhesion activity of NELL1, we performed cell adhesion assays using NELL1 proteins pretreated with DTT (Fig. 5). The DTT treatment did not affect the coating efficiency of any NELL1 proteins. Interestingly, DTT treatment had no effect on the cell adhesion activity of the two small C-terminal fragments (VWC4 and VWC5), slightly reduced the activity of the larger C-terminal fragment (VWC345), but caused a dramatic decrease in the activity of full-length NELL1. These results indicate that intramolecular disulfide bonds within the VWC domains are not functionally necessary but that other disulfide linkages in the N-terminal region of NELL1 may be involved in cell adhesion activity. Therefore, we examined the role of oligomerization of NELL1 in the cell adhesion activity of the protein.
FIGURE 5.
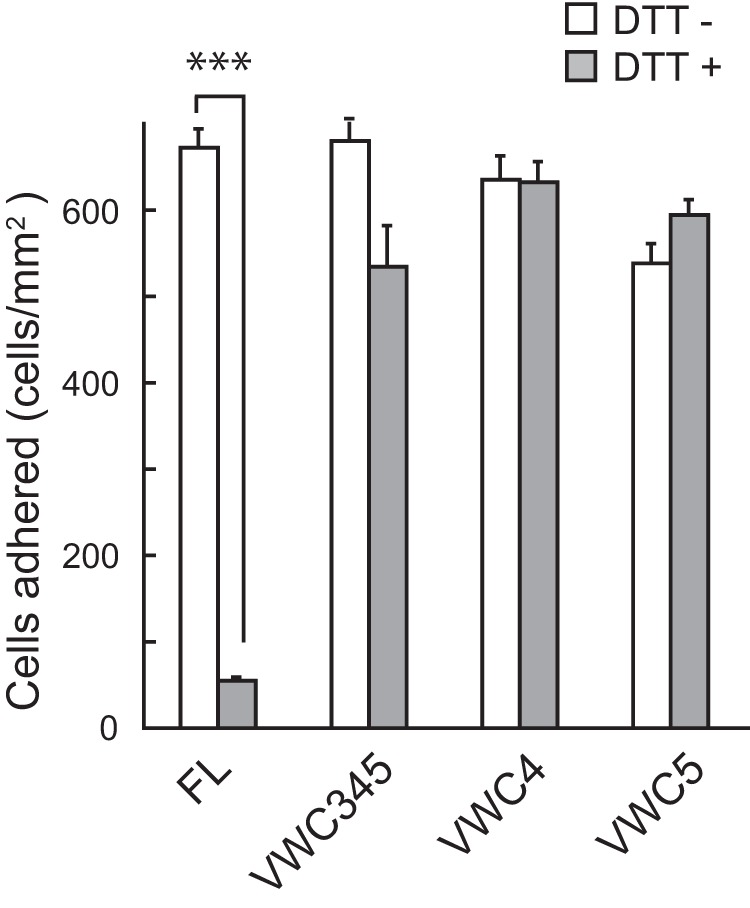
Cell adhesion activity of the full-length NELL1, but not of the C-terminal fragments, is reduction-sensitive. NELL1-FL and its C-terminal fragments were pretreated with 20 mm DTT and then coated onto 96-well plates at a concentration of 200 nm. MC3T3-E1 cells were allowed to adhere to the wells for 1.5 h at 37 °C before adhered cells in the wells were processed for counting. Each value represents the mean ± S.E. of triplicate results. ***, p < 0.001 (Student's t test).
NELL1 Forms Homo-oligomers through the Coiled-coil Domain
NELL1 proteins overexpressed in 293-F cells have been shown to form homodimers and homotrimers with intermolecular disulfide bonds (26). We first examined the oligomerization of endogenous NELL1 in the human neuroblastoma cell line IMR-32 (Fig. 6A) (30). We found NELL1 homotrimers and homodimers and even monomers at low levels secreted from IMR-32 cells. To investigate whether the oligomeric forms of NELL1 have a different activity from the monomeric forms, we first examined the mechanisms of oligomerization of NELL1. Because TSP-1 forms homotrimers through the coiled-coil domain (27), the oligomerization of NELL1 was thought to occur in the same manner. To examine the role of the coiled-coil domain in NELL1 oligomerization, we prepared expression constructs encoding full-length NELL1 and its deletion mutants lacking the coiled-coil domain (Fig. 6B). These constructs were transfected into 293-F cells, and the conditioned media were resolved by SDS-PAGE under non-reducing or reducing conditions and immunoblotted with anti-FLAG antibody (Fig. 6C). Under reducing conditions, all three NELL1 proteins resolved as monomeric bands of about 140 kDa. Under non-reducing conditions, intact NELL1 was detected predominantly as 280-kDa homodimers and 420-kDa homotrimers. However, deletion of the coiled-coil domain increased not only the proportion of the monomeric bands but also the extent of smearing in the high molecular weight region of the gel. These results indicate that deletion of the coiled-coil domain from NELL1 caused a failure of proper oligomerization.
FIGURE 6.
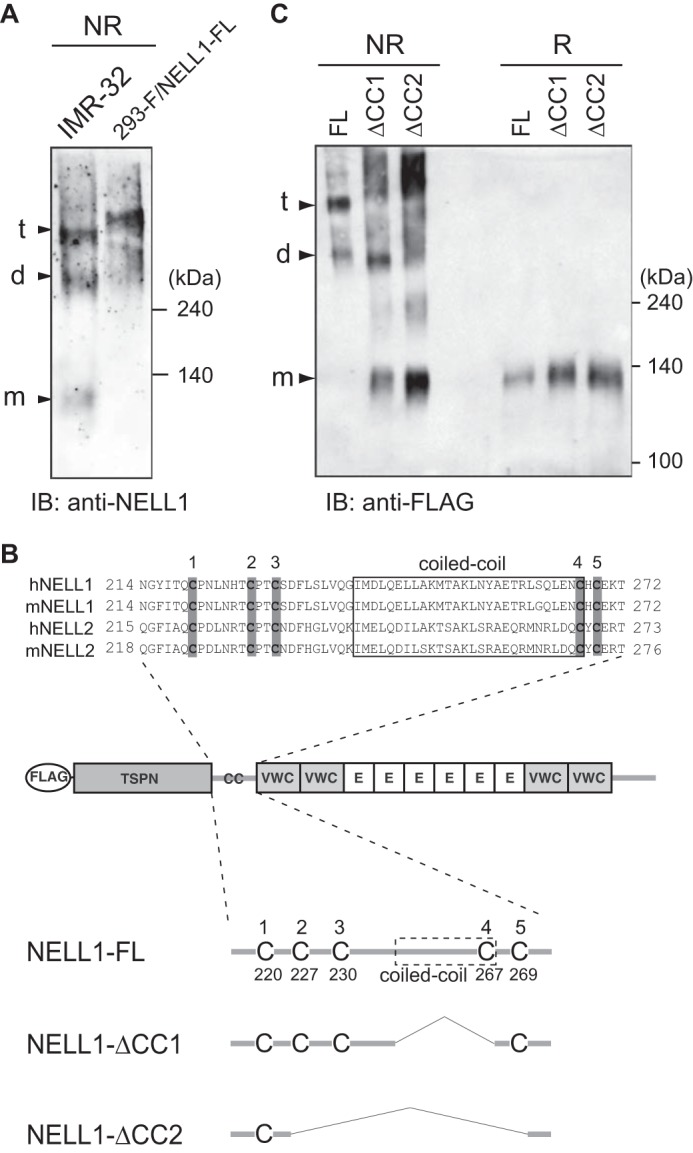
NELL1 forms homo-oligomers through the coiled-coil domain. A, oligomerization of an endogenous NELL1 protein. The conditioned media of IMR-32 cells and NELL1-FL-expressing 293-F cells were resolved by SDS-PAGE and immunoblotted (IB) with anti-NELL1 antibody under non-reducing (NR) conditions. The bands assumed to be monomers (m), dimers (d), and trimers (t) are indicated at the left. Molecular weight markers are indicated at the right. B, schematic of full-length NELL1 protein and its deletion mutants lacking the coiled-coil domain. A FLAG tag was fused to the N terminus of NELL1. The amino acid sequences around the coiled-coil domains of human and mouse NELL proteins were aligned at the top. The coiled-coil domains, which were identified using the SMART program (Simple Molecular Architecture Research Tool) (44, 45), are boxed. The five conserved cysteine residues are shaded in gray. E, EGF-like repeat. C, oligomerization of NELL1-FL and its deletion mutants. NELL1-FL and its deletion mutants were expressed in 293-F cells. The conditioned media were resolved by SDS-PAGE and immunoblotted with anti-FLAG antibody under non-reducing or reducing (R) conditions.
Next, to examine more precisely the role of the coiled-coil domain in NELL1 oligomerization, we prepared a C-terminal deletion construct comprising the TSPN and coiled-coil domains of NELL1 (NELL1-N) and a series of further C-terminally truncated constructs (Fig. 7A). These constructs were transfected into 293-F cells, and the extent of oligomerization was evaluated as described above (Fig. 7B). Under reducing conditions, all proteins appeared as 40- to 50-kDa monomers. Under non-reducing conditions, NELL1-N was detected as a faint band at about 100 kDa and major bands at about 150 kDa and >200 kDa, assumed to be homodimers, homotrimers, and homotetramers, respectively. When only six amino acids were further deleted from NELL1-N, the tetrameric band disappeared, and the monomeric band was detected under non-reducing conditions (Fig. 7B, lane ΔCC1). Furthermore, when the coiled-coil domain was deleted from NELL1-N, the trimeric band disappeared completely, and the monomeric bands became the major band species (Fig. 7B, lanes ΔCC2 and ΔCC3). Taken together, our results thus far demonstrate that the coiled-coil domain is responsible for the oligomerization of NELL1. No band was detected for the construct lacking Cys-220 (NELL1-N-ΔCC4) (Fig. 7B), although the protein was detected in cell lysates (data not shown), suggesting that Cys-220 is essential for the secretion or stability of NELL1 protein. According to the typical structure of the TSPN domain (31), Cys-220 of NELL1 may form an intramolecular disulfide bond with Cys-167, the only cysteine residue within the TSPN domain.
FIGURE 7.
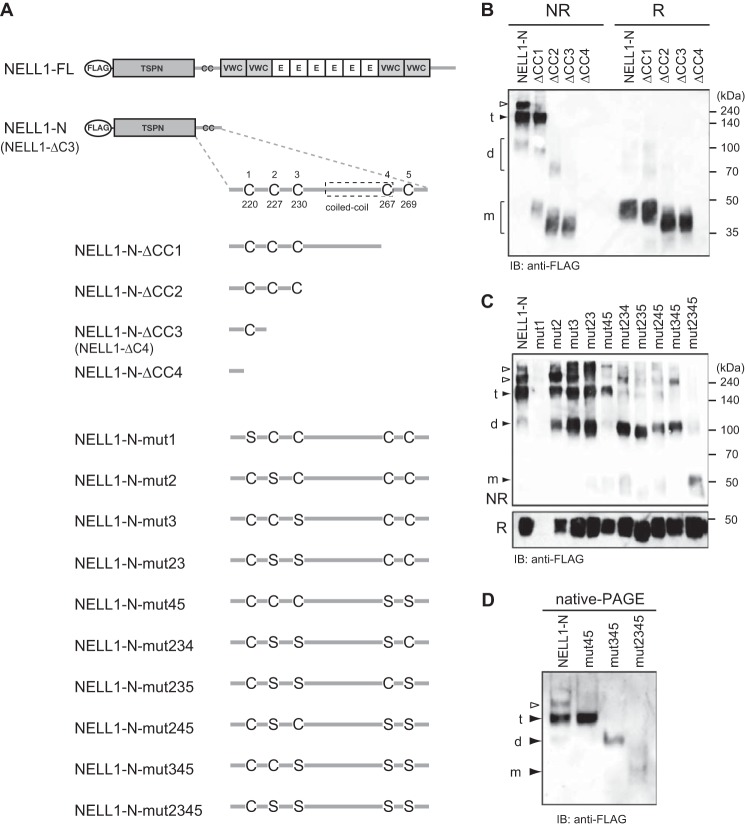
Four cysteine residues are involved in intermolecular disulfide bond formation for NELL1 oligomerization. A, schematic of the N-terminal fragment of NELL1 (NELL1-N) and its C-terminal deletion or cysteine-to-serine substitution mutants. E, EGF-like repeat. B and C, oligomerization of the N-terminal fragments of NELL1. NELL1-N and its deletion (B) or cysteine-to-serine substitution (C) mutants were expressed in 293-F cells. The conditioned media were resolved by SDS-PAGE and immunoblotted (IB) with anti-FLAG antibody under non-reducing (NR) or reducing (R) conditions. The bands assumed to be monomers (m), dimers (d), and trimers (t) are indicated at the left. The bands indicated with empty arrowheads are likely to be multimers and/or aggregates. Molecular weight markers are indicated at the right. D, intermolecular disulfide bonds are necessary for NELL1 oligomerization. NELL1-N and its cysteine-to-serine substitution mutants were expressed in 293-F cells. The conditioned media were resolved by native PAGE and immunoblotted with anti-FLAG antibody.
Intermolecular Disulfide Bonds Are Essential for the Oligomerization of NELL1
We next investigated the role of intermolecular disulfide bonds in the oligomerization of NELL1. There are five conserved cysteine residues around the coiled-coil domain in mouse and human NELL proteins (Fig. 6B). To identify the cysteine residues potentially involved in intermolecular disulfide bond formation, we substituted one to four cysteine residues around the coiled-coil domain with serines (Fig. 7A) and performed an analysis as before (Fig. 7C). Replacement of Cys220 with serine (mut1) did not produce a band, which is consistent with the result in Fig. 6B (lane ΔCC4). Replacing one or two cysteine residues with serines (mut2, mut3, mut23, and mut45) produced a similar band pattern of dimers, trimers, and tetramers as that of the parent NELL1-N protein, but the dimer bands tended to be increased. However, replacing three cysteine residues with serines (mut234, mut235, mut245, and mut345) resulted in a much larger decrease in the trimer bands, whereas replacement of four cysteine residues (mut2345) caused a failure of oligomerization. These results indicate that four cysteine residues (Cys227, Cys230, Cys267, and Cys269) are involved in intermolecular disulfide bond formation by NELL1. When the oligomerization of these mutants was examined by native-PAGE, mut345 and mut2345 were detected as a dimer and a monomer, respectively, which is in agreement with the result of SDS-PAGE analysis under non-reducing conditions (Fig. 7D). These results support the idea that the coiled-coil domain of NELL1, with relatively short heptad repeats, could not maintain oligomeric forms because of low hydrophobic interactions, and indicate that intermolecular disulfide bonds are essential for stabilizing the oligomeric forms of NELL1.
Oligomeric NELL1 Shows Higher Cell Adhesion Activity Than Monomeric NELL1
To investigate whether the oligomeric forms of NELL1 have a different activity from the monomeric forms, we prepared a quadruple cysteine mutant of the full-length NELL1 (NELL1-mut2345) (Fig. 8A). The intact and mutant proteins were purified and resolved by SDS-PAGE under reducing or non-reducing conditions (Fig. 8B). Under reducing conditions, both proteins were detected as 140-kDa monomers, and the loading of an equal amount of protein was confirmed. Under non-reducing conditions, intact NELL1 was detected as homodimers and homotrimers, whereas the quadruple cysteine mutant produced a monomeric band and a faint larger band similar to the size of homodimers. The latter band does not seem to correspond to authentic homodimers but may have been produced by aggregates cross-linked by intermolecular disulfide bonding between free cysteine residues within the VWC or EGF-like domains. Representative atomic force microscopy images of these proteins are shown in Fig. 8C. Intact NELL1 molecules had a rugged appearance with thickness, whereas the monomeric mutant molecules exhibited a flat and shrunken morphology. We examined the cell adhesion activity of these proteins in MC3T3-E1 cells (Fig. 8D) and found that the quadruple cysteine mutant had a significantly reduced cell adhesion activity. These results suggest that the monomeric mutant of NELL1 may be unable to expose its cell-binding sites to the cells as effectively as the oligomeric forms.
FIGURE 8.
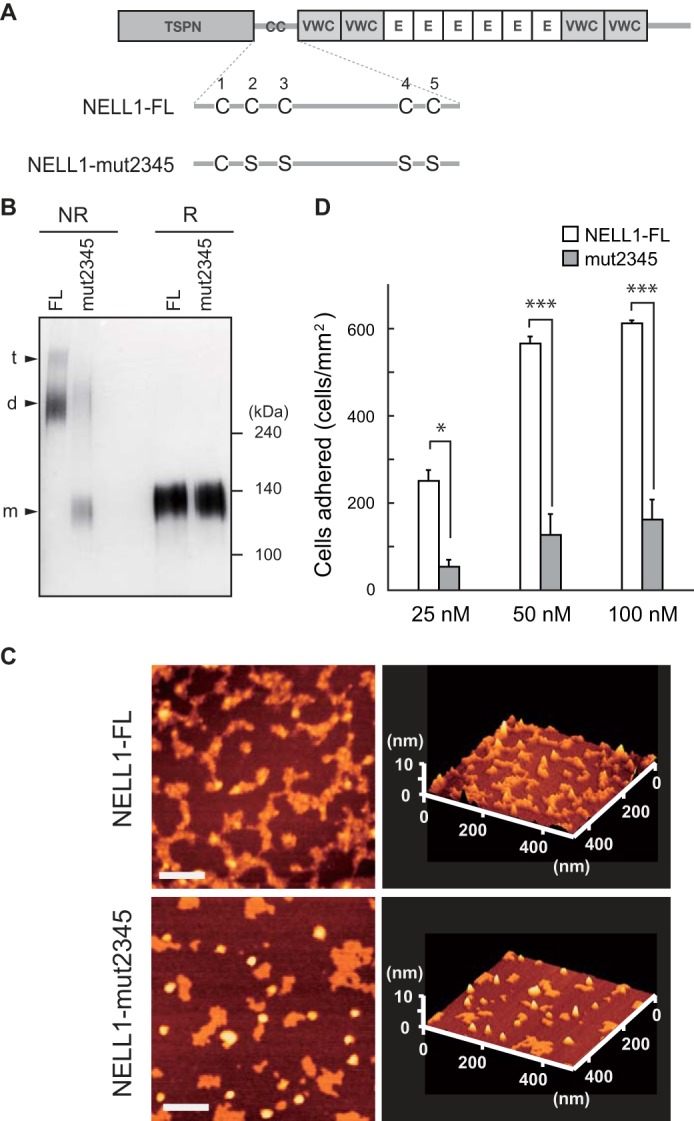
A quadruple cysteine-to-serine mutant of NELL1 shows reduced cell adhesion activity. A, schematic of intact (NELL1-FL) and quadruple cysteine-to-serine substitution mutant (NELL1-mut2345) NELL1s. E, EGF-like repeat. B, the intact and mutant NELL1 proteins were expressed in 293-F cells, and the purified proteins were resolved by SDS-PAGE under non-reducing (NR) or reducing (R) conditions and visualized by silver staining. The bands assumed to be monomers (m), dimers (d), and trimers (t) are indicated at the left. Molecular weight markers are indicated at the right. C, atomic force microscopy images of NELL1-FL and NELL1-mut2345. Samples were adsorbed onto a mica chip, dried, and then scanned in dynamic force microscopy mode. Scale bars = 100 nm. D, NELL1-FL and NELL1-mut2345 were coated onto 96-well plates at a concentration of 25, 50, or 100 nm, and MC3T3-E1 cells were allowed to adhere for 1.5 h at 37 °C before adhered cells in the wells were processed for counting. Each value represents the mean ± S.E. of triplicate results. *, p < 0.05; ***, p < 0.001 (Student's t test).
Intact NELL1 and Its C-terminal Fragment Induce Cell Adhesion and Spreading
It has been shown that cell shape regulates the commitment of mesenchymal stem cells to osteoblast or adipocyte fate (32). The cells that were allowed to adhere and spread underwent osteogenesis, whereas unspread and round cells became adipocytes. To visualize cell morphology on the NELL1 substrate, MC3T3-E1 cells adhering to NELL1-, fibronectin-, or LM-332-coated glass coverslips were stained with rhodamine-phalloidin and anti-vinculin antibody (Fig. 9). Cells plated on uncoated glass coverslips exhibited little adhesion and spreading, whereas cells seeded on fibronectin and LM-332 coverslips showed a well spread morphology but with distinct polymerization patterns within the cytoskeleton. A well developed network of actin stress fibers was observed in fibronectin-adhered cells but not in LM-332-adhered cells. Labeling with antibody against vinculin showed a punctate-like pattern at the ends of the actin microfibrils in some fibronectin-adhered cells but not in LM-332-adhered cells. Cells that adhered on coverslips coated with either full-length NELL1 (NELL1-FL) or the C-terminal NELL1 fragment (NELL1-VWC345) showed less spreading than cells on fibronectin and LM-332 but with several filopodial extensions. These cells showed an organization of F-actin at the cell periphery but no organization of stress fibers across the cell body, similar to cells on LM-332, suggesting that cell adhesion to NELL1 or LM-332 induces the same set of integrins and focal adhesion components.
FIGURE 9.
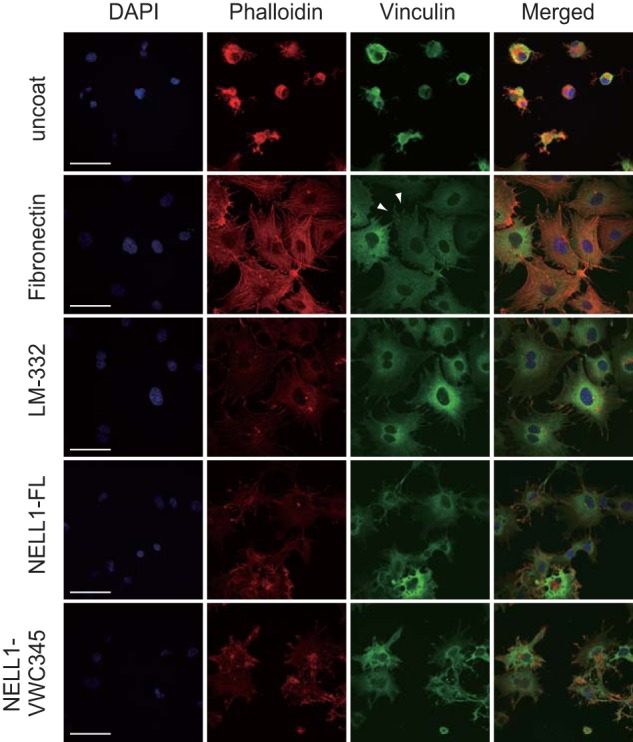
Intact NELL1 and its C-terminal fragment induce cell adhesion and spreading. MC3T3-E1 cells were seeded on glass coverslips coated with fibronectin (40 μg/ml), LM-332 (100 nm), NELL1-FL (100 nm), or NELL1-VWC345 (100 nm) and allowed to adhere for 1.5 h at 37 °C. After a washing step, adherent cells were fixed and labeled with DAPI to stain nuclei (blue), phalloidin to stain F-actin (red), and anti-vinculin antibody to locate focal adhesions (green). Scale bars = 50 μm. Arrowheads point to focal adhesion sites.
Signal Transduction Mediated by Cell Adhesion to NELL1
We previously identified integrin α3β1 as a candidate receptor for NELL1 using the human osteosarcoma cell line Saos-2 (22). Similar results were obtained for MC3T3-E1 cells from adhesion inhibition assays, in which function-blocking monoclonal antibodies against mouse integrins blocked the adherence of the cells to the C-terminal NELL1 fragment as well as to full-length NELL1 (Fig. 10), suggesting again that integrin α3β1 could mediate the adhesive effect of NELL1. It has been shown that the murine bone marrow-derived stromal cell line ST2 cultured on NELL1 exhibited integrin-dependent, increased phosphorylation of FAK (23). Therefore, we examined whether cell adhesion to the C-terminal NELL1 fragment stimulates protein tyrosine phosphorylation in MC3T3-E1 cells. We observed increased tyrosine phosphorylation of FAK and ERK1/2 in cells adhering to full-length NELL1 and the C-terminal NELL1 fragment compared with cells adhering to poly-l-lysine-coated plates (Fig. 11A). Treatment of cells with a specific inhibitor of FAK reduced the tyrosine phosphorylation of not only FAK but also ERK1/2. These results suggest that NELL1-mediated cell adhesion can induce FAK and ERK1/2 activation through integrin receptors. The quadruple cysteine mutant showed reduced tyrosine phosphorylation of these molecules, probably because of its lower cell adhesion activity (Fig. 11B).
FIGURE 10.
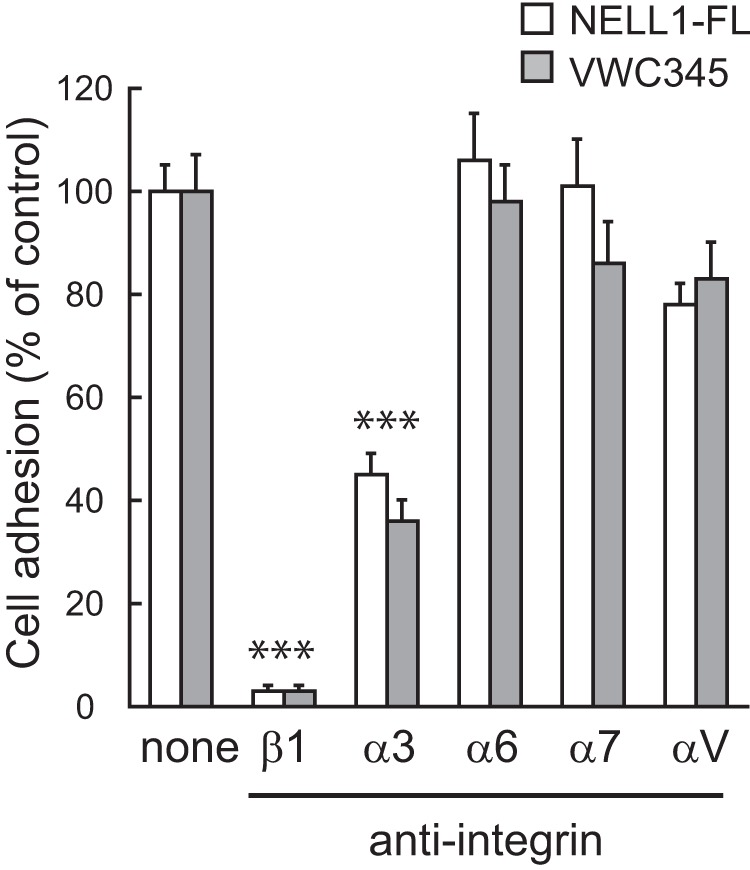
Integrin α3β1 mediates MC3T3-E1 cell adhesion to NELL1. NELL1-FL or NELL1-VWC345 was coated onto 96-well plates at a concentration of 100 nm. MC3T3-E1 cells were preincubated with function-blocking anti-integrin antibodies (5 μg/ml) for 15 min and then allowed to adhere for 1.5 h at 37 °C before the adhered cells were counted. Each value represents the mean ± S.E. of triplicate results relative to those of untreated cells set to 100%. ***p < 0.001 (Student's t test).
FIGURE 11.
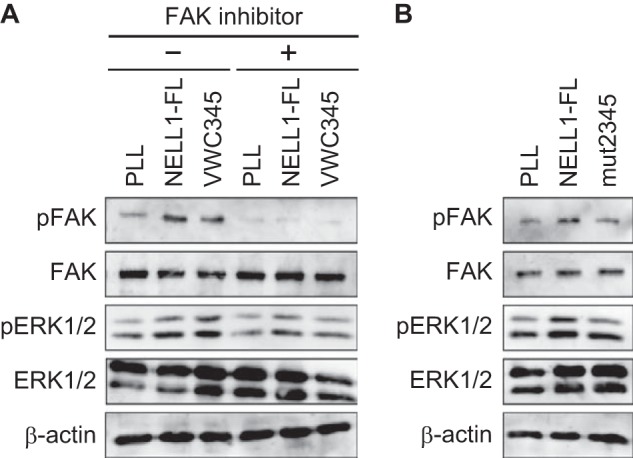
FAK and ERK1/2 phosphorylation in MC3T3-E1 cells adhering to NELL1. A, serum-starved MC3T3-E1 cells were detached and treated with or without FAK inhibitor 14 (1 μm) for 30 min and then plated on dishes coated with 100 nm NELL1-FL, 100 nm NELL1-VWC345, or 0.01% poly-l-lysine (PLL) for 1.5 h. Cell lysates were analyzed by immunoblotting with anti-phosphorylated-FAK and anti-phosphorylated-ERK1/2 antibodies. Anti-FAK, anti-ERK1/2, and anti-β-actin blots were used as internal controls. B, serum-starved MC3T3-E1 cells were detached and plated on dishes coated with 100 nm NELL1-FL, 100 nm NELL1-mut2345, or 0.01% PLL for 1.5 h. Cell lysates were analyzed by immunoblotting with anti-phosphorylated-FAK and anti-phosphorylated-ERK1/2 antibodies.
DISCUSSION
NELL1, a potent growth factor that is highly specific to the osteochondral lineage, is also thought to function as a matricellular protein that influences cell function by modulating cell-matrix interactions. The interaction of NELL1 with cells or with other extracellular matrix components could act on the proliferation and survival of cells or on the differentiation to osteoblasts. We have reported previously that NELL1 promoted cell adhesion through at least three cell-binding sites localized to the C-terminal half of the NELL1 protein (22). In this study, we have dissected the cell-binding sites of NELL1 by using a series of recombinant proteins prepared from mammalian cells. We have identified two major cell-binding sites: one in the fourth VWC domain and the other in the C-terminal-most region. However, we did not find significant cell adhesion activity in the N-terminal VWC and EGF-like domains, which is inconsistent with previous results obtained by using recombinant proteins prepared from a bacterial host (22). One likely reason for the difference is that the cell adhesion assays in this study were performed at relatively low concentrations of NELL1 (about one-fifth of those of the bacterially prepared proteins used), suggesting that NELL1 proteins prepared in mammalian cells undergo appropriate protein folding and disulfide bond formation. Although additional cell-binding sites may be found by using a higher concentration of NELL1, they could be much weaker cell-binding sites than the C-terminal-most region. Another reason for the difference is that the individual fragments of the N-terminal VWC or EGF-like domains have not been investigated in this study because these fragments could not be purified from the mammalian expression host. Accordingly, it is possible that these domains have cryptic cell-binding sites that can be exposed by denaturation or enzymatic cleavage.
Because the C-terminal region of NELL1 contains many cysteine residues that presumably form intramolecular disulfide bonds, integrins may recognize not only short peptide sequences but also the tertiary structure around the cell-binding site. Studies on disintegrins and phage display analysis have suggested that two cysteine residues flanking the core binding sequence form a disulfide bond to present the binding site as a loop, thereby enhancing the binding affinity to integrins (33–37). However, cell adhesion assays using reduced NELL1 proteins have revealed that intramolecular disulfide bonds within the VWC domains are not essential for the cell adhesion activity of NELL1. Surprisingly, the cell adhesion activity of full-length NELL1 was almost abolished by DTT treatment, suggesting that intermolecular disulfide bond formation is involved in the cell adhesion activity of NELL1. To test this possibility, we prepared a quadruple cysteine mutant to produce monomeric forms of NELL1. This monomeric NELL1 mutant showed a much lower cell adhesion activity than intact NELL1, indicating that oligomerization modulates the conformation of the C-terminal region containing the cell-binding sites.
Oligomerization of secreted proteins has often been shown to be required for the reinforcement of their bioactivity. For example, adiponectin exists as a series of oligomers ranging from trimers to hexamers and high molecular weight multimers (12–18 subunits), and the high molecular weight multimers are the more bioactive forms (38). In a study involving recombinant proteins of TSP-1, the cell adhesion activity of the monomeric unit is low compared with that of the trimeric forms, and cell spreading occurs in response to the trimeric forms but not to the monomeric unit, suggesting that the clustering of the C-terminal cell-binding site by the trimerization of TSP-1 may underlie such functional differences (39). Similar to that of TSP-1, the clustering of the C-terminal cell-binding sites by the oligomerization of NELL1 may be important for efficient binding to integrins. However, in the case of NELL1, the monomeric C-terminal fragments (NELL1-VWC345, -VWC4, and -VWC5) have similar cell adhesion activities as intact NELL1 (Fig. 5), suggesting that the protein conformation around the cell-binding site(s) is more important than the clustering of the cell-binding site(s). We have shown that the deletion of each domain in the C-terminal region is likely to influence the activity of the neighboring domains (Fig. 3). This also suggests that the conformation around the cell-binding site(s) is critical for the cell adhesion activity of NELL1. Further studies are required to dissect the mechanisms underlying NELL1-mediated cell adhesion and their relevance to osteogenic differentiation.
We analyzed the mechanisms of NELL1 oligomerization by using a series of expression constructs lacking the coiled-coil domain or by replacing cysteine residues around the domain with serines. A deletion analysis has directly demonstrated that NELL1 forms homo-oligomers mainly through the coiled-coil domain. In the absence of the domain, such mutants remain as monomers, but some small subpopulations of the mutant molecules formed oligomers/multimers, probably because of intermolecular disulfide bond formation between free cysteine residues elsewhere on the monomers (Fig. 6B). This non-native oligomerization does not seem likely to occur in intact NELL1. In the subsequent experiments, we used NELL1 fragments comprising the TSPN and coiled-coil domains to reduce the influence of the free cysteine residues in the VWC or EGF-like domains. However, an immunoblot analysis under non-reducing conditions showed bands higher in molecular weight than homotrimers that probably corresponded to homotetramers and homopentamers (Fig. 7, B and C). This result may not be artificial and may reflect the fact that NELL1 has a potential for forming homotetramers and homopentamers because it has been reported that NELL1 may be secreted as a pentamer in CHO cells (4).
Analysis of cysteine-to-serine substitutions has revealed that four cysteine residues around the coiled-coil domain are involved in intermolecular disulfide bond formation. In contrast, two cysteine residues are required in all five members of the TSP family. Because two cysteine residues in a molecule are theoretically and practically sufficient to form multimers, we cannot exclude the possibility that our observations might be the results of compensation by the remaining free cysteine residues of the mutant proteins. However, deletion or substitution of both Cys267 and Cys269 caused a failure to form homotetramers (Fig. 7, B and C), raising the possibility that cysteine residues located in either the N- or C-terminal end of the coiled-coil have a different role in oligomerization. It has been shown that TSPs form only one homo-oligomer species. TSP-1 and TSP-2 form only homotrimers, whereas TSP-3 to TSP-5 form only homopentamers (40). In contrast, NELL1 has no such restrictions. Therefore, we postulate that the four cysteine residues might be associated with a degree of oligomerization, although it is generally determined by the nature and geometry of the amino acids of the coiled-coil. Further investigation could help to elucidate the exact role of dimeric and trimeric forms of NELL1.
FAK plays a pivotal role in linking cell surface integrins to intracellular signaling pathways (41). FAK associated with several different signaling proteins, thereby leading to the activation of MAPK pathways. Shen et al. (23) demonstrated that murine mesenchymal cells cultured on NELL1 showed integrin-dependent, increased phosphorylation of FAK. In this study, we observed moderate but increased tyrosine phosphorylation of FAK and ERK1/2 in cells adhering to NELL1, suggesting that NELL1-mediated cell adhesion can activate FAK and MAPK pathways through integrin α3β1. However, immunofluorescence staining for vinculin showed that no induction of well organized actin stress fibers and clear focal adhesion plaques was observed in cells cultured on NELL1 (Fig. 9). Both integrin α3 and β1 antibodies produced similar staining patterns, indicating the localization of the integrin not at the periphery of the cells but in the whole cell body (data not shown). These results seem consistent with the report by Gu et al. (42) that α5β1 integrin-mediated cell adhesion to fibronectin induces the formation of stress fibers and focal contacts, whereas α3β1 integrin-mediated cell adhesion to LM-511/521 does not. LM-511/521 preferentially activates the phosphatidylinositol 3-kinase/Akt pathway, rather than the FAK-MAPK pathway, through α3β1 integrin-dependent adhesion (43). Thus, these may suggest that the FAK-MAPK pathway is not the main signaling pathway for NELL1-mediated cell adhesion. The other signaling pathways involved in NELL1-mediated cell adhesion and the relevance of the conformation of the C-terminal region of NELL1 remain to be determined.
In summary, the findings of this study support a mechanism of NELL1 oligomerization and a manner of cell attachment by NELL1 substrates, as illustrated in our schematic model (Fig. 12). In the absence of a reductant, NELL1 forms homodimers or homotrimers through α-helical coiled-coil formation with intermolecular disulfide bond interactions at Cys227, Cys230, Cys267, and Cys269. In contrast, a quadruple cysteine-to-serine substitution mutant exists exclusively as monomers. The monomeric forms of NELL1 show lower cell adhesion activity than oligomeric forms. However, the excised C-terminal fragments show similar cell adhesion and spreading activities as intact NELL1. An oligomerization-induced conformational change in the C-terminal region of NELL1 may be responsible for the interaction with integrins. These aspects need to be considered when designing strategies for the use of NELL1 in bone regeneration therapy.
FIGURE 12.
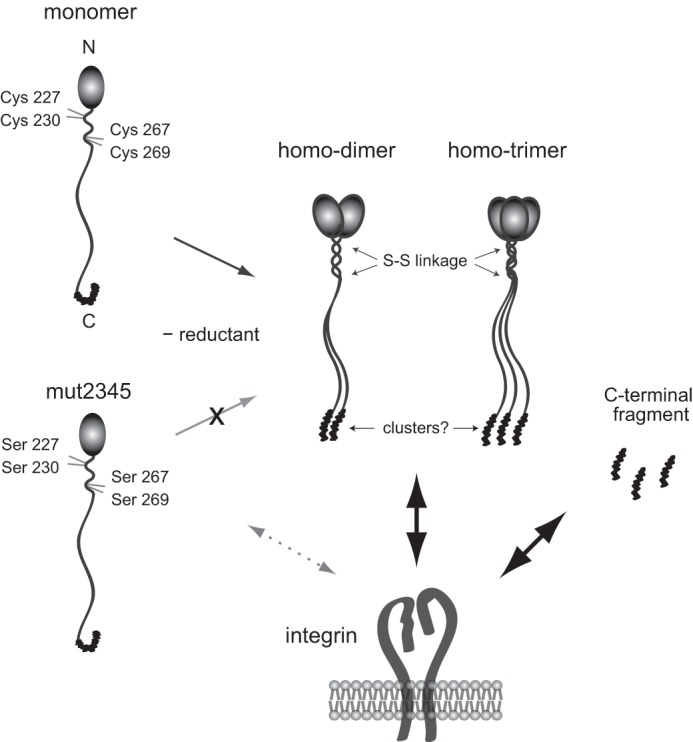
Schematic for the oligomerization and cell-binding properties of NELL1. In the absence of reductant, NELL1 forms homodimers or homotrimers through an α-helical coiled-coil formation with intermolecular disulfide bond interactions at Cys227, Cys230, Cys267, and Cys269. In contrast, the quadruple cysteine-to-serine substitution mutant exists exclusively as monomers. The C-terminal fragments and the oligomeric forms, but not monomeric forms, of NELL1 exhibit similar cell adhesion activities. An oligomerization-induced conformational change in the C-terminal region of NELL1 may be necessary for the interaction of NELL1with integrins.
This work was supported by Ministry of Education, Culture, Sports, Science, and Technology of Japan Grant-in-aid for Scientific Research 23510255.
S. Kuroda, unpublished observation.
- NELL
- Nel-like
- BMP
- bone morphogenetic protein
- FAK
- focal adhesion kinase
- TSP
- thrombospondin
- TSPN
- N-terminal thrombospondin-1-like
- CC
- coiled-coil
- VWC
- von Willebrand factor type C
- FL
- full-length.
REFERENCES
- 1. Watanabe T. K., Katagiri T., Suzuki M., Shimizu F., Fujiwara T., Kanemoto N., Nakamura Y., Hirai Y., Maekawa H., Takahashi E. (1996) Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics 38, 273–276 [DOI] [PubMed] [Google Scholar]
- 2. Ting K., Vastardis H., Mulliken J. B., Soo C., Tieu A., Do H., Kwong E., Bertolami C. N., Kawamoto H., Kuroda S., Longaker M. T. (1999) Human NELL-1 expressed in unilateral coronal synostosis. J. Bone Miner. Res. 14, 80–89 [DOI] [PubMed] [Google Scholar]
- 3. Kuroda S., Oyasu M., Kawakami M., Kanayama N., Tanizawa K., Saito N., Abe T., Matsuhashi S., Ting K. (1999) Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem. Biophys. Res. Commun. 265, 79–86 [DOI] [PubMed] [Google Scholar]
- 4. Zhang X., Zara J., Siu R. K., Ting K., Soo C. (2010) The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J. Dent. Res. 89, 865–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura R., Nakamoto C., Obama H., Durward E., Nakamoto M. (2012) Structure-function analysis of Nel, a thrombospondin-1-like glycoprotein involved in neural development and functions. J. Biol. Chem. 287, 3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsuyama S., Aihara K., Nishino N., Takeda S., Tanizawa K., Kuroda S., Horie M. (2004) Enhanced long-term potentiation in vivo in dentate gyrus of NELL2-deficient mice. Neuroreport 15, 417–420 [DOI] [PubMed] [Google Scholar]
- 7. Aihara K., Kuroda S., Kanayama N., Matsuyama S., Tanizawa K., Horie M. (2003) A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res. Mol. Brain Res. 116, 86–93 [DOI] [PubMed] [Google Scholar]
- 8. Desai J., Shannon M. E., Johnson M. D., Ruff D. W., Hughes L. A., Kerley M. K., Carpenter D. A., Johnson D. K., Rinchik E. M., Culiat C. T. (2006) Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum. Mol. Genet. 15, 1329–1341 [DOI] [PubMed] [Google Scholar]
- 9. Zhang X., Ting K., Pathmanathan D., Ko T., Chen W., Chen F., Lee H., James A. W., Siu R. K., Shen J., Culiat C. T., Soo C. (2012) Calvarial cleidocraniodysplasia-like defects with ENU-induced Nell-1 deficiency. J. Craniofac. Surg. 23, 61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X., Kuroda S., Carpenter D., Nishimura I., Soo C., Moats R., Iida K., Wisner E., Hu F. Y., Miao S., Beanes S., Dang C., Vastardis H., Longaker M., Tanizawa K., Kanayama N., Saito N., Ting K. (2002) Craniosynostosis in transgenic mice overexpressing Nell-1. J. Clin. Invest. 110, 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X., Carpenter D., Bokui N., Soo C., Miao S., Truong T., Wu B., Chen I., Vastardis H., Tanizawa K., Kuroda S., Ting K. (2003) Overexpression of Nell-1, a craniosynostosis-associated gene, induces apoptosis in osteoblasts during craniofacial development. J. Bone Miner. Res. 18, 2126–2134 [DOI] [PubMed] [Google Scholar]
- 12. Aghaloo T., Cowan C. M., Chou Y. F., Zhang X., Lee H., Miao S., Hong N., Kuroda S., Wu B., Ting K., Soo C. (2006) Nell-1-induced bone regeneration in calvarial defects. Am. J. Pathol. 169, 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cowan C. M., Cheng S., Ting K., Soo C., Walder B., Wu B., Kuroda S., Zhang X. (2006) Nell-1 induced bone formation within the distracted intermaxillary suture. Bone 38, 48–58 [DOI] [PubMed] [Google Scholar]
- 14. Cowan C. M., Jiang X., Hsu T., Soo C., Zhang B., Wang J. Z., Kuroda S., Wu B., Zhang Z., Zhang X., Ting K. (2007) Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J. Bone Miner. Res. 22, 918–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu S. S., Zhang X., Soo C., Hsu T., Napoli A., Aghaloo T., Wu B. M., Tsou P., Ting K., Wang J. C. (2007) The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 7, 50–60 [DOI] [PubMed] [Google Scholar]
- 16. Aghaloo T., Cowan C. M., Zhang X., Freymiller E., Soo C., Wu B., Ting K., Zhang Z. (2010) The effect of NELL1 and bone morphogenetic protein-2 on calvarial bone regeneration. J. Oral Maxillofac. Surg. 68, 300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xue J., Peng J., Yuan M., Wang A., Zhang L., Liu S., Fan M., Wang Y., Xu W., Ting K., Zhang X., Lu S. (2011) NELL1 promotes high-quality bone regeneration in rat femoral distraction osteogenesis model. Bone 48, 485–495 [DOI] [PubMed] [Google Scholar]
- 18. James A. W., Pang S., Askarinam A., Corselli M., Zara J. N., Goyal R., Chang L., Pan A., Shen J., Yuan W., Stoker D., Zhang X., Adams J. S., Ting K., Soo C. (2012) Additive effects of sonic hedgehog and Nell-1 signaling in osteogenic versus adipogenic differentiation of human adipose-derived stromal cells. Stem Cells Dev. 21, 2170–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siu R. K., Zara J. N., Hou Y., James A. W., Kwak J., Zhang X., Ting K., Wu B. M., Soo C., Lee M. (2012) NELL-1 promotes cartilage regeneration in an in vivo rabbit model. Tissue Eng. Part A 18, 252–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes F. J., Turner W., Belibasakis G., Martuscelli G. (2006) Effects of growth factors and cytokines on osteoblast differentiation. Periodontol. 2000 41, 48–72 [DOI] [PubMed] [Google Scholar]
- 21. Bokui N., Otani T., Igarashi K., Kaku J., Oda M., Nagaoka T., Seno M., Tatematsu K., Okajima T., Matsuzaki T., Ting K., Tanizawa K., Kuroda S. (2008) Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. 582, 365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasebe A., Nakamura Y., Tashima H., Takahashi K., Iijima M., Yoshimoto N., Ting K., Kuroda S., Niimi T. (2012) The C-terminal region of NELL1 mediates osteoblastic cell adhesion through integrin α3β1. FEBS Lett. 586, 2500–2506 [DOI] [PubMed] [Google Scholar]
- 23. Shen J., James A. W., Chung J., Lee K., Zhang J. B., Ho S., Lee K. S., Kim T. M., Niimi T., Kuroda S., Ting K., Soo C. (2012) NELL-1 promotes cell adhesion and differentiation via Integrinβ1. J. Cell. Biochem. 113, 3620–3628 [DOI] [PubMed] [Google Scholar]
- 24. Kuroda S., Tanizawa K. (1999) Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem. Biophys. Res. Commun. 265, 752–757 [DOI] [PubMed] [Google Scholar]
- 25. Garcia Abreu J., Coffinier C., Larraín J., Oelgeschläger M., De Robertis E. M. (2002) Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene 287, 39–47 [DOI] [PubMed] [Google Scholar]
- 26. Hasebe A., Tashima H., Ide T., Iijima M., Yoshimoto N., Ting K., Kuroda S., Niimi T. (2012) Efficient production and characterization of recombinant human NELL1 protein in human embryonic kidney 293-F cells. Mol. Biotechnol. 51, 58–66 [DOI] [PubMed] [Google Scholar]
- 27. Bornstein P. (1995) Diversity of function is inherent in matricellular proteins. An appraisal of thrombospondin 1. J. Cell Biol. 130, 503–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phan H. P., Sugino M., Niimi T. (2009) The production of recombinant human laminin-332 in a Leishmania tarentolae expression system. Protein Expr. Purif. 68, 79–84 [DOI] [PubMed] [Google Scholar]
- 29. Zhou Y. F., Eng E. T., Zhu J., Lu C., Walz T., Springer T. A. (2012) Sequence and structure relationships within von Willebrand factor. Blood 120, 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maeda K., Matsuhashi S., Tabuchi K., Watanabe T., Katagiri T., Oyasu M., Saito N., Kuroda S. (2001) Brain specific human genes, NELL1 and NELL2, are predominantly expressed in neuroblastoma and other embryonal neuroepithelial tumors. Neurol. Med.-Chir. 41, 582–589 [DOI] [PubMed] [Google Scholar]
- 31. Tan K., Duquette M., Liu J. H., Zhang R., Joachimiak A., Wang J. H., Lawler J. (2006) The structures of the thrombospondin-1 N-terminal domain and its complex with a synthetic pentameric heparin. Structure 14, 33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S. (2004) Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 [DOI] [PubMed] [Google Scholar]
- 33. Koivunen E., Gay D. A., Ruoslahti E. (1993) Selection of peptides binding to the α5β1 integrin from phage display library. J. Biol. Chem. 268, 20205–20210 [PubMed] [Google Scholar]
- 34. Koivunen E., Wang B., Ruoslahti E. (1994) Isolation of a highly specific ligand for the α5β1 integrin from a phage display library. J. Cell Biol. 124, 373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Healy J. M., Murayama O., Maeda T., Yoshino K., Sekiguchi K., Kikuchi M. (1995) Peptide ligands for integrin αvβ3 selected from random phage display libraries. Biochemistry 34, 3948–3955 [DOI] [PubMed] [Google Scholar]
- 36. McLane M. A., Vijay-Kumar S., Marcinkiewicz C., Calvete J. J., Niewiarowski S. (1996) Importance of the structure of the RGD-containing loop in the disintegrins echistatin and eristostatin for recognition of αIIbβ3 and αvβ3 integrins. FEBS Lett. 391, 139–143 [DOI] [PubMed] [Google Scholar]
- 37. Yamada T., Kidera A. (1996) Tailoring echistatin to possess higher affinity for integrin αIIbβ3. FEBS Lett. 387, 11–15 [DOI] [PubMed] [Google Scholar]
- 38. Pajvani U. B., Hawkins M., Combs T. P., Rajala M. W., Doebber T., Berger J. P., Wagner J. A., Wu M., Knopps A., Xiang A. H., Utzschneider K. M., Kahn S. E., Olefsky J. M., Buchanan T. A., Scherer P. E. (2004) Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 279, 12152–12162 [DOI] [PubMed] [Google Scholar]
- 39. Anilkumar N., Annis D. S., Mosher D. F., Adams J. C. (2002) Trimeric assembly of the C-terminal region of thrombospondin-1 or thrombospondin-2 is necessary for cell spreading and fascin spike organisation. J. Cell Sci. 115, 2357–2366 [DOI] [PubMed] [Google Scholar]
- 40. Adams J. C. (2001) Thrombospondins. Multifunctional regulators of cell interactions. Annu. Rev. Cell Dev. Biol. 17, 25–51 [DOI] [PubMed] [Google Scholar]
- 41. Schlaepfer D. D., Hauck C. R., Sieg D. J. (1999) Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71, 435–478 [DOI] [PubMed] [Google Scholar]
- 42. Gu J., Sumida Y., Sanzen N., Sekiguchi K. (2001) Laminin-10/11 and fibronectin differentially regulate integrin-dependent Rho and Rac activation via p130 (Cas)-CrkII-DOCK180 pathway. J. Biol. Chem. 276, 27090–27097 [DOI] [PubMed] [Google Scholar]
- 43. Gu J., Fujibayashi A., Yamada K. M., Sekiguchi K. (2002) Laminin-10/11 and fibronectin differentially prevent apoptosis induced by serum removal via phosphatidylinositol 3-kinase/Akt- and MEK1/ERK-dependent pathways. J. Biol. Chem. 277, 19922–19928 [DOI] [PubMed] [Google Scholar]
- 44. Schultz J., Milpetz F., Bork P., Ponting C. P. (1998) SMART, a simple modular architecture research tool. Identification of signaling domains. Proc. Natl. Acad. Sci. U.S.A. 95, 5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Letunic I., Doerks T., Bork P. (2012) SMART 7. Recent updates to the protein domain annotation resource. Nucleic Acids Res. 40, D302–305 [DOI] [PMC free article] [PubMed] [Google Scholar]