Background: Menin represses pancreatic beta cell proliferation.
Results: Menin promotes processing of let-7a, whose target IRS2 plays an important role in insulin signaling and beta cell proliferation.
Conclusion: Menin represses beta cell proliferation partly via regulation of miRNA biogenesis.
Significance: Understanding how menin represses beta cell proliferation will aid toward improving therapies targeting endocrine tumors and metabolic diseases including diabetes.
Keywords: Beta Cell, Cancer Biology, MicroRNA, Pancreas, Pancreatic Islets, Tumor Suppressor Gene, IRS2, Menin, let-7a
Abstract
Multiple endocrine neoplasia type I (MEN1) is an inherited syndrome that includes susceptibility to pancreatic islet hyperplasia. This syndrome results from mutations in the MEN1 gene, which encodes menin protein. Menin interacts with several transcription factors, including JunD, and inhibits their activities. However, the precise mechanism by which menin suppresses gene expression is not well understood. Here, we show that menin interacts with arsenite-resistant protein 2 (ARS2), a component of the nuclear RNA CAP-binding complex that is crucial for biogenesis of certain miRNAs including let-7a. The levels of primary-let-7a (pri-let-7a) are not affected by menin; however, the levels of mature let-7a are substantially decreased upon Men1 excision. Let-7a targets, including Insr and Irs2, pro-proliferative genes that are crucial for insulin-mediated signaling, are up-regulated in Men1-excised cells. Inhibition of let-7a using anti-miRNA in wild type cells is sufficient to enhance the expression of insulin receptor substrate 2 (IRS2) to levels observed in Men1-excised cells. Depletion of menin does not affect the expression of Drosha and CBP80, but substantially impairs the processing of pri-miRNA to pre-miRNA. Ars2 knockdown decreased let-7a processing in menin-expressing cells but had little impact on let-7a levels in menin-excised cells. As IRS2 is known to mediate insulin signaling and insulin/mitogen-induced cell proliferation, these findings collectively unravel a novel mechanism whereby menin suppresses cell proliferation, at least partly by promoting the processing of certain miRNAs, including let-7a, leading to suppression of Irs2 expression and insulin signaling.
Introduction
Multiple endocrine neoplasia type I (MEN1)2 is an inherited syndrome, with development of neoplasia in several endocrine organs including pancreatic islets (1–4). The gene mutated in this syndrome, MEN1, encodes a nuclear protein of 610 amino acids, menin (5, 6). Menin interacts with multiple proteins and is involved in a variety of cellular processes including gene transcription, cell proliferation, apoptosis, and genome stability (7–12), but the precise mechanisms regarding menin-mediated suppression of cell proliferation remain to be elucidated. The recently solved crystal structure of menin reveals that it contains a deep pocket that binds short MLL1 or JunD peptides in the same manner, but has opposite effects on gene transcription (13), supporting the capacity of menin to act as either a context-dependent transcription activator or repressor.
MicroRNAs (miRNAs) are short (20–24 nucleotides), endogenous non-protein-coding RNA molecules that negatively regulate gene expression post-transcription. MiRNAs either bind to their target gene mRNA and promote their degradation and/or inhibit protein translation (14, 15). The primary transcripts of miRNA genes (pri-miRNA) are processed in the nucleus by the Microprocessor complex consisting of DGCR8 and Drosha to ∼70-nucleotide hairpin structures called pre-miRNAs (16–18). Pre-miRNAs are exported out of the nucleus and processed to ∼22-nucleotide double stranded miRNA duplexes by Dicer (19–21). The miRNA duplexes are unwound, and the mature miRNA is incorporated into the RNA-induced silencing complex containing the Argonaute protein (22, 23). MiRNAs are involved in diverse biological processes during development and disease (24, 25) including type 2 diabetes (26–28). Aberrant expression of miRNAs has also been linked to several types of cancer (29, 30).
miRNA-mediated regulation of pancreatic islet cell proliferation and insulin secretion has previously been demonstrated (31–34). Conditional deletion of Dicer1 early in pancreas development results in dramatic reduction of insulin-producing beta cells (31). Similarly, Dicer1-deficient adult beta cells show dramatic decrease in insulin content, insulin-promoter activity, and insulin mRNA levels resulting in development of diabetes in these animals (32). Furthermore, miR-7a inhibition leads to activation of mTOR signaling and promotes adult beta cell replication in mouse primary islets (33). Analysis of miRNAs showing differential expression between normal pancreas and pancreatic endocrine tumors (PET) identified 87 up-regulated and 8 down-regulated miRNA in PETs (34). Among the down-regulated miRNAs, miR-155 showed lack of detectable expression in PETs upon comparison with normal pancreas (34). Whereas inactivation of the MEN1 gene is among the most frequent genetic events identified in sporadic PETs (35), little is known as to whether menin is involved in regulating miRNAs during the pathogenesis of the MEN1 syndrome.
Here, we show that menin interacts with arsenite-resistance protein 2 (ARS2), a component of the nuclear cap-binding complex, and facilitates processing of primary-miRNA (pri-miRNA) including pri-let-7a and pri-miR-155, reducing the levels of Insr and Irs2, both let-7a targets. Moreover, anti-miR-mediated knockdown of let-7a results in increased levels of insulin receptor substrate 2 (IRS2). These findings unravel the role of menin in regulating the biogenesis of miRNAs via interaction with ARS2 and expression of a key component of the insulin-signaling pathway, IRS2.
EXPERIMENTAL PROCEDURES
Plasmids and Cell Culture
Retroviral plasmids expressing FLAG-tagged menin have been described previously (36). Lentiviral constructs expressing Ars2 were obtained from Open Biosystems. Lentiviral packaging plasmids, pMD2G and pAX2G, were purchased from Addgene. MEFs and HEK293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 1% Pen/Strep.
Western Blotting
Cells were lysed in radioimmuneprecipitation assay buffer (Sigma) on ice for 10 min and sonicated to shear the genomic DNA. Protein concentrations were determined by BCA assay, and the samples were subjected to SDS-PAGE. The proteins were transferred to a PVDF membrane. Antibodies used were anti-menin (Bethyl, A300-105A), anti-IRS2 (Cell Signaling, antibody 4502), anti-RAS (Millipore, catalog 05-516), anti-ARS2 (37), anti-Drosha (Cell Signaling, antibody 3364), anti-CBP80 (Bethyl, A301-793A) and anti-β-actin (Sigma, A5441).
RNA Extraction and Quantitative Real-time PCR
Total RNA was extracted from cultured cells with TRIzol and an RNeasy extraction kit from Qiagen. One μg of RNA was transcribed into cDNA using Superscript III RT from Invitrogen, and real-time PCR was performed on the 7500 Fast Real Time PCR machine from Applied Biosystems. Either Gapdh or Hprt1 mRNA levels were used as endogenous controls, and analysis was done using the relative quantification method according to instructions from ABI.
Isolation of miRNA and cDNA Synthesis
miRNA was isolated using the mirVana miRNA isolation kit from Invitrogen. Briefly, cells were lysed in lysis/binding solution, and RNA was extracted with acid-phenol:chloroform. One-third volume of ethanol was added to the aqueous phase and passed through a filter cartridge. Another two-third volume of ethanol was added to the filtrate, mixed, and passed through a second filter cartridge. Finally, the miRNA was eluted with 100 μl of elution solution at 95 °C. cDNA was synthesized using the NCode miRNA First-Strand cDNA Synthesis kit from Invitrogen. Briefly, a poly(A) tail was added to the miRNA samples using poly(A) polymerase, followed by incubation at 37 °C for 15 min. First strand cDNA was synthesized from the polyadenylated miRNA by reverse transcription using the supplied Universal RT Primer Superscript III RT/RNaseOUT enzyme mix and incubated at 50 °C for 50 min.
Primary miRNA Processing Assay
To generate pri-let-7a, PCR was performed using primers flanking the pre-let-7a hairpin of the mouse locus. Forward and reverse primers added ssRNA flanks as well as a 5′-XhoI and 3′-XbaI site. The resulting PCR products were digested and cloned into pcDNA3.1. Pri-let-7a template for in vitro transcription was generated from the pri-miRNA-pcDNA3 construct described above. PCR was employed to add on a 5′ T7 promoter (5′-TCG TAA TAC GAC TCA CTA TAG GGA TAT CCA TCA CAC TGG CGG CC-3′) and (5′- GCT GAT CAG CGA GCT CTA GC-3′). Radiolabeled pri-let-7a was generated using the T7 High Yield RNA Synthesis kit (New England Biolabs) in the presence of [32P]UTP according to the manufacturer's instructions. Cell lysates were prepared from MEFs in radioimmuneprecipitation assay buffer followed by sonication and centrifugation. A reaction mixture containing 15 μl of cell lysate, 6.4 mm MgCl2, and radiolabeled pri-let-7a transcript was incubated at 37 °C for 90 min. RNA was extracted using TRIzol and resolved on a 6% urea-polyacrylamide gel, and the bands were detected by autoradiography.
Statistical Analyses
Statistical analysis was performed using GraphPad Prism (v 5.0, GraphPad Software). The data are presented as the mean ± S.D. A two-tailed Student's t test was used for measuring statistical differences.
RESULTS
Menin Interacts with ARS2, a Component of the Nuclear RNA Cap-binding Complex Important for miRNA Biogenesis
Menin acts as a scaffold protein coordinating the function of different proteins (38), but the precise mechanisms by which it represses gene transcription and cell proliferation are not fully understood. We sought to identify novel menin-interacting partners to provide further insight into menin-mediated regulation of cell proliferation. To this end, ectopically expressed menin was purified by affinity chromatography using FLAG M2 beads, and the menin-interacting proteins were excised for characterization by mass spectroscopy. Analysis of the purified proteins showed that ARS2 was among the major eluted proteins (Fig. 1A). ARS2 is a component of the nuclear RNA cap-binding complex that stabilizes the primary miRNA (pri-miRNA) transcript for processing by the Microprocessor complex consisting of Drosha and DGCR8, and thus plays an essential role in miRNA-mediated silencing (37, 39). To confirm ARS2 as a menin-interacting partner, we performed co-immunoprecipitation in cell lysates from HEK293 cells ectopically expressing menin. Immunoprecipitation with an anti-menin antibody effectively pulled down ARS2 (Fig. 1B), indicating ARS2 as a novel menin-interacting partner.
FIGURE 1.
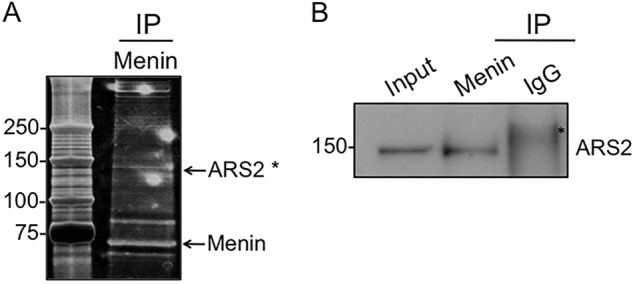
Menin interacts with ARS2. A, silver staining of menin-containing fractions after affinity purification using anti-FLAG M2 beads in HEK293 cells ectopically expressing menin. Visible bands were excised for identification by mass spectroscopy. Asterisk indicates ARS2 peptide fragments identified by mass spectroscopy; MLDAAVIK, LTPLLSVR, NINGITQHK, RGWVTFDR, VALSEPQPER, SKYHPDEVGK, FVTSNTQELGK, VLDKLLLYLR, DLDAPDDVDFF, VRNINGITQHK, VALSEPQPERR, ISHGEVLEWQK, ESLSEEEAQKMGR, EVAFFNNFLTDAK, TFEEKLTPLLSVR, LGSIAEIDLGVPPPVMK, MLDAAVIKMEGGTENDLR, AIVEYRDLDAPDDVDFF, ILEQEEEEEQAGKPGEPSK, and ILEQEEEEEQAGKPGEPSKK. B, nuclear extract from HEK293 cells ectopically expressing menin were immunoprecipitated (IP) with anti-menin antibody, and immunoblotted for ARS2. Asterisk indicates a nonspecific band.
Menin Affects the Levels of Mature let-7a and miR-155
A previous report showed that depletion of ARS2 results in reduced levels of several miRNAs including miR-21, let-7, and miR-155 (37). To determine whether menin plays a role in the biogenesis of certain miRNAs, we compared mature let-7a and miR-155 levels in Men1l/l;CreER MEFs, a cell line in which Men1 can be effectively excised upon treatment with 4-hydroxytamoxifen (Fig. 2A). qRT-PCR analysis showed that mature let-7a and miR-155 expression levels were substantially reduced upon Men1 excision (Fig. 2, B and C). Furthermore, ectopic expression of menin in BON cells, a human neuroendocrine carcinoid cell line that expresses low levels of endogenous menin (40), resulted in increased levels of let-7a (Fig. 2D), consistent with the idea that menin facilitates the processing of let-7a. To determine whether menin affects miRNA levels at the transcriptional level, we examined the levels of pri-miRNA in both WT and Men1-excised cells. The levels of pri-let-7a (Fig. 3A) and pri-miR-155 (Fig. 3B) in Men1-excised cells were similar to levels in control WT cells. These findings indicate that menin promotes the biogenesis of certain miRNAs, including let-7a and miR-155, post-transcriptionally at the level of miRNA processing.
FIGURE 2.

Mature let-7a miRNA levels are increased in menin-expressing cells. A, Men1l/l and Men1l/l;CreER MEFs were treated with 4-hydroxytamoxifen (4-OHT), and excision of menin was determined by Western blotting. B and C, qRT-PCR showing the levels of let-7a (B) and miR-155 (C) in Men1l/l and Men1l/l;CreER MEFs treated with 4-OHT. D, qRT-PCR showing let-7a levels in BON cells ectopically expressing either vector or wild type (WT) menin; inset, Western blotting showing levels of ectopic menin expression. Error bars indicate ± S.D.
FIGURE 3.
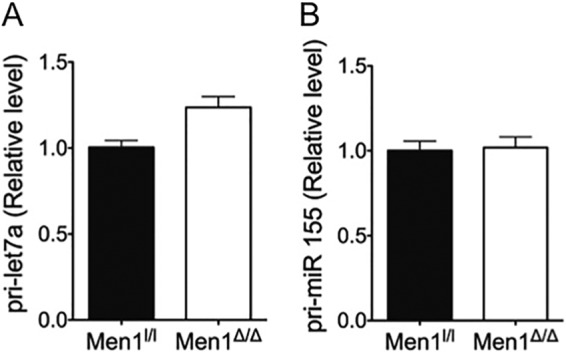
Menin does not affect the levels of the pri-miRNA transcript. Men1l/l and Men1l/l;CreER MEFs were treated with 4-hydroxytamoxifen, and the levels of pri-let7a (A) and pri-miR-155 (B) were determined by qRT-PCR. Error bars indicate ± S.D.
Menin Enhances the Processing of pri-let-7a to pre-let-7a
Because menin does not affect the transcript level of pri-let-7a (Fig. 3A), and ARS2 has been shown to be involved in the processing of pri-miRNA to pre-miRNA, we determined whether menin affects the processing of pri-let-7a to pre-let-7a biochemically. We performed a miRNA-processing assay using 32P-radiolabeled pri-let-7a as the substrate and cell lysates from either menin-expressing or menin-null cells. Lysates from menin-null cells contained diminished processing activity compared with lysates from control WT cells, which generated a cleavage product of ∼94 nucleotides (Fig. 4). This result indicates that menin plays a role in the biogenesis of let-7a by enhancing the processing of pri-let-7a to pre-let-7a.
FIGURE 4.
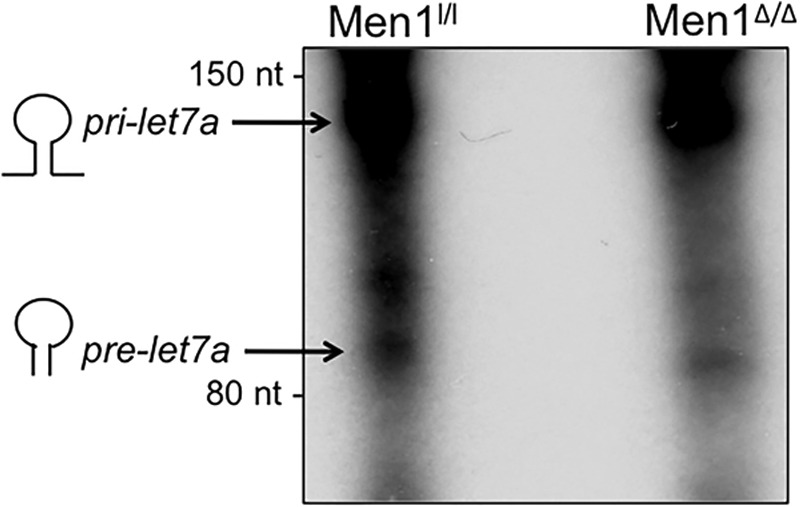
Menin is required for processing of let-7a. Radiolabeled pri-let-7a was incubated with cell extracts from WT and menin-null MEFs, and the RNA was resolved on a 6% urea-polyacrylamide gel followed by autoradiography. The substrate, pri-let-7a, and product, pre-let-7a, are as indicated. nt, nucleotides.
To determine whether the decrease in the processing of pri-let-7a in Men1-excised cells results from decreased levels of components of the pri-miRNA processing machinery, we examined the levels of Drosha and CBP80. Our results show that the protein levels of Drosha, an RNase III enzyme that executes the initial step of miRNA processing in the nucleus along with DGCR8 (17), and CBP80, a nuclear cap-binding protein (37), are comparable in both menin-excised and wild type cells (Fig. 5). This indicates that the role of menin in the processing of pri-let-7a does not occur via regulation of the expression levels of the Microprocessor complex, but rather by enhancing the stability of the pri-let-7a transcript.
FIGURE 5.
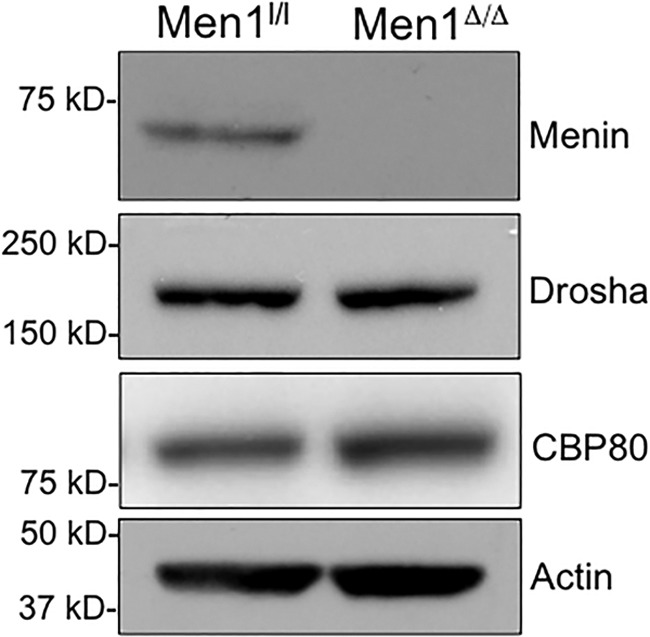
Men1 excision does not affect the levels of pri-miRNA processing machinery. Men1l/l and Men1l/l;CreER MEFs were treated with 4-hydroxytamoxifen, and the protein levels of Drosha and nuclear cap-binding protein subunit 1 (CBP80) were analyzed by immunoblotting. Actin was used as a loading control.
Functional Interaction between Menin and ARS2 Is Crucial for Processing of pri-let-7a
To elucidate the causal relationship between the interaction of menin and ARS2 in miRNA processing, we knocked down Ars2 expression in both WT and menin-null cells. A significant reduction in the levels of ARS2 protein was observed in both cell types using three independent clones of shRNA targeting Ars2 (Fig. 6A). qRT-PCR analysis indicated a 40% decrease in let-7a levels in WT cells for all three shRNAs examined (Fig. 6B). On the contrary, Ars2 knockdown did not affect let-7a levels in Men1-excised cells (Fig. 6B). These findings indicate that the impact of ARS2 on miRNA processing depends on the presence of menin, strongly suggesting that menin and ARS2 interact functionally to control processing of let-7a.
FIGURE 6.
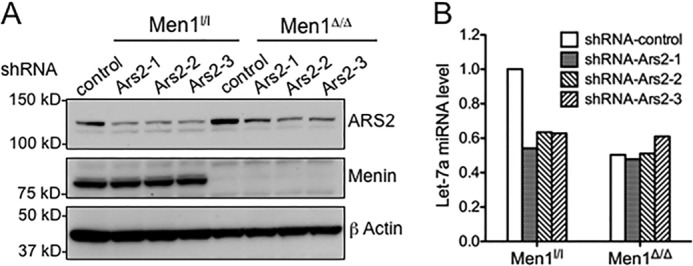
Ars2 knockdown results in decreased let-7a processing in menin-expressing cells. A, immunoblotting for ARS2 in menin-expressing (Men1l/l) and menin-null cells (Men1Δ/Δ) expressing either control or Ars2-targeting shRNAs. Immunoblotting for actin is included as a loading control. B, qRT-PCR showing the levels of let-7a in Men1l/l and Men1Δ/Δ cells expressing either control or Ars2-targeting shRNAs.
IRS2 Levels Are Enhanced upon Men1 Excision
Using prediction algorithms, Insr and Irs2 were previously identified as let-7 targets based on sequence complementarity between the miRNA and the 3′-UTR of target genes (41). Inhibition of let-7a using anti-miRs prevented down-regulation of IRS2 expression in the liver of mice on a high fat diet, confirming IRS2 as a let-7a target (41). Importantly, IRS2 plays a central role in peripheral insulin signaling and pancreatic beta cell proliferation, and disruption of IRS2 causes type 2 diabetes in mice (42). We have shown previously that excision of the Men1 gene ameliorated pre-existing glucose intolerance and increased both glucose-stimulated insulin release and circulating insulin levels in mouse models for diabetes (43). We therefore examined whether a decrease in let-7a levels upon Men1 excision leads to increased IRS2 expression. Our results show that upon Men1 excision, Irs2 expression is increased ∼2-fold at the mRNA level (Fig. 7A), with substantial increase at the protein level (Fig. 7B). This is consistent with the role of miRNAs in regulating gene expression by degradation of target gene mRNA and/or inhibiting translation (14, 15). Furthermore, Men1 excision increased levels of insulin receptor, Insr, another known target of let-7a that phosphorylates and activates IRS2 (41) (data not shown). In agreement with this finding, ectopic expression of menin in mouse βHC9 cells, insulin-producing cells derived from pancreatic islets with beta cell hyperplasia, decreases Irs2 mRNA and protein levels (Fig. 7C). Additionally, decreased levels of IRS2 protein levels were observed in BON cells ectopically expressing menin (Fig. 7D). These findings suggest that the elevated level of mature let-7a in menin-expressing cells targets the Irs2 transcript for degradation, and excision of Men1 subsequently relieves this degradation, resulting in enhanced expression of IRS2.
FIGURE 7.
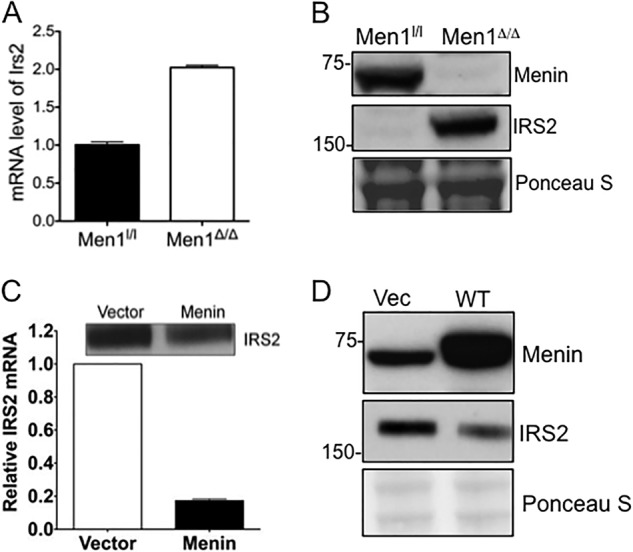
IRS2 levels are reduced in menin-expressing cells. A, Men1l/l and Men1l/l;CreER MEFs were treated with 4-hydroxytamoxifen, and the mRNA levels of Irs2 were determined by qRT-PCR. B, immunoblotting for IRS2 in cell lysates from Men1l/l and Men1l/l;CreER MEFs is shown. Ponceau S is included as a loading control. C, retrovirus expressing either control or menin was stably transduced into βHC9, and the mRNA levels of Irs2 were determined by qRT-PCR; inset, Western blotting showing protein levels of IRS2. D, cell lysates from BON cells ectopically expressing either vector or menin were immunoblotted for menin and IRS2. Ponceau S staining is included as a loading control. Error bars indicate ± S.D.
Anti-miR-induced Knockdown of let-7a Restores the Levels of IRS2
To determine whether menin-mediated biogenesis of let-7a plays a key role in menin-induced repression of Irs2 expression, we examined the effect of let-7a inhibition on Irs2 levels. To this end, cells were transfected with either let-7a or control anti-miR, and the levels of IRS2 were quantitated 5 days after transfection. A significant increase in the protein levels of IRS2 was observed in menin-expressing MEFs transfected with let-7a anti-miR compared with control anti-miR-treated cells (Fig. 8, second panel, lane 2 versus 1). As positive controls, the protein level of RAS, a known target of the let-7 microRNA family (44), was also up-regulated in menin-expressing MEFs treated with let-7a anti-miRNA compared with control anti-miR-treated cells, as expected (Fig. 8, third panel, lane 2 versus 1). These results clearly demonstrate that the decreased levels of IRS2 in menin-expressing MEFs compared with Men1-excised cells can be, at least partly, attributed to the presence of elevated levels of let-7a. To examine whether the impact of the let-7a anti-miR is menin/let-7a-specific, we treated menin-null cells with let-7a anti-miR. Our results indicate that the level of IRS2 in Men1-excised cells was not affected by let-7a anti-miR treatment (Fig. 8, lane 4 versus 3), possibly due, in part, to the relatively low levels of let-7a present in the menin-null cells, as expected. Similarly, the protein levels of RAS were unchanged after let-7a anti-miR treatment in Men1-excised cells compared with control anti-miR-treated cells. Collectively, these results indicate that the difference in IRS2 protein levels in menin-null and menin-expressing cells is largely attributed to menin-regulated production of mature let-7a, which degrades Irs2 mRNA and inhibits protein translation.
FIGURE 8.
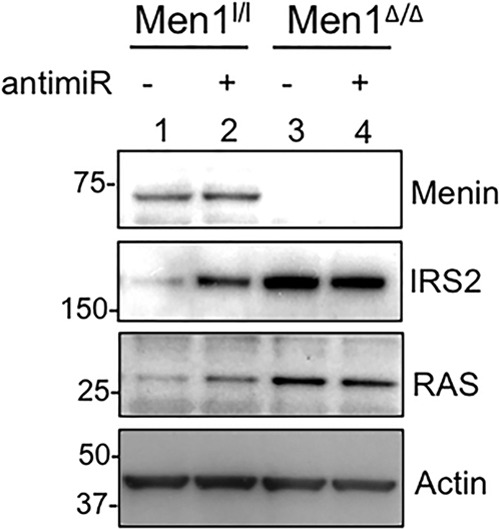
Anti-miR-induced repression of let-7a expression results in increased levels of IRS2. Men1l/l and Men1Δ/Δ MEFs were harvested 5 days after transfection with either control (−) or let-7a (+) anti-miR, and the protein levels of IRS2 and RAS were determined by Western blotting. Immunoblotting for actin is included as a loading control.
DISCUSSION
Here, we identify the role of menin in the biogenesis of certain miRNAs including let-7a and miR-155. We show that menin interacts with ARS2, a protein previously known to contribute toward stability and delivery of capped pri-miRNA transcripts to the Microprocessor complex containing Drosha and DGCR8 (37). Excision of Men1 results in decreased levels of several miRNAs including let-7a and miR-155 (Fig. 2). Analysis of the transcript levels indicated that the levels of pri-miRNA were not altered by menin (Fig. 3). Additionally, radiolabeled processing assay indicates that menin plays a role in the processing of pri-miRNA to pre-miRNA (Fig. 4). The recently solved crystal structure of menin (13) suggests that it acts as a scaffold protein and regulates gene expression by interacting with distinct partners including transcription factors JunD (7, 13), NF-κB (45), PPARγ (46), and various histone modification enzymes including MLL (8, 13, 47, 48), PRMT5 (12), EZH2 (49) and HDACs (50). Furthermore, it has previously been shown that menin binds to the promoter of miR-26-a and induces its expression, resulting in decreased SMAD1 levels during osteoblastic differentiation of human adipose tissue-derived stem cells (51). Ars2 knockdown resulted in significant decrease of let-7a levels in menin-expressing cells whereas it had little effect in menin-null cells. This indicates that the functional interaction between menin and ARS2, in addition to the miRNA processing machinery, is essential for proper processing of the let-7a miRNA (Fig. 6). Additionally, this is the first report detailing a post-transcriptional role for menin in regulating gene expression and cell proliferation via regulation of miRNA processing.
The role of miRNAs in regulating pancreatic beta cell proliferation and insulin secretion has been demonstrated extensively (27, 28, 31–33). We show here that menin plays a role in the processing of let-7a, a member of the let-7 family of miRNAs that regulates glucose metabolism in multiple organs (41). Furthermore, global knockdown of let-7a in mice prevents and treats impaired glucose tolerance in diet-induced obese mice, partly by increasing the levels of IRS2 and INSR (41). Similarly, we observed decreased levels of let-7a in Men1-excised cells with significant increase in IRS2 expression (Fig. 7), and inhibition of let-7a using specific anti-miR resulted in de-repression of IRS2 (Fig. 8) suggesting that the increased levels of Irs2 upon Men1 excision can be attributed, in part, to reduced let-7a levels. It has previously been reported that IRS2 is markedly overexpressed in rat insulinoma cells compared with rat primary islet β cells (52). Furthermore, the importance of IRS2 in insulin signaling has been demonstrated using transgenic knock-out mouse models where Irs2−/− mice show a marked decrease in beta cell mass and develop type 2 diabetes (53). It is thus conceivable that elevated serum insulin and decreased blood glucose levels in Men1-null transgenic mice (54) can be attributed partly to decreased let-7a with subsequent increase in IRS2 and increased pancreatic beta cell mass. As a corollary, it can also be conceived that disruption of the menin-let-7a-IRS2 axis contributes, at least in part, to tumorigenesis caused by menin mutations in MEN1 syndrome. Moreover, it is likely that menin-mediated processing of miRNAs such as let-7a also contributes partly to the suppression of endocrine tumors.
A recent report described insulin-mediated down-regulation of menin and localization in the cytoplasm in a time-dependent manner via the human insulin receptor (55). It is conceivable that activation of the insulin signaling pathway by insulin leads to reduction in menin levels particularly in the nucleus, consequently resulting in decreased processing of pri-let-7a and increased Irs2 expression. However, in the cell lines used for our study, we did not observe any decrease in global menin levels or increased localization in the cytoplasm upon treatment with insulin for either 15 min or 24 h (data not shown). However, increased p-AKT levels were detected in cells treated with insulin (data not shown). It is possible that the effect of insulin on menin expression was not observed in our cells because of the lack of ectopic expression of human insulin receptor.
In addition to let-7a, we observed that menin plays a role in the biogenesis of miR-155 with reduced levels upon Men1 excision (Fig. 2C). A previous screen for miRNAs aberrantly regulated in pancreatic neuroendocrine tumors identified significant down-regulation of miR-155 expression in pancreatic endocrine tumors compared with normal pancreas (34). As >40% of pancreatic endocrine tumors harbor somatic mutations in the Men1 gene (56), it is possible that the decreased levels of miR-155 observed in pancreatic endocrine tumors can be partly attributed to the dysregulation of miR-155 biogenesis upon Men1 mutation.
In conclusion, we have uncovered a novel mechanism by which menin regulates gene expression post-transcriptionally via regulating biogenesis of miRNAs including let-7a and miR-155, and provide a rationale for possible use of anti-miR-based therapy for alleviating symptoms associated with MEN1 syndrome and metabolic diseases including diabetes.
Acknowledgments
We thank Dr. Chaoxing Yuan at Penn Proteomics facility for mass spectrometry analysis and Dr. Craig Thompson at Memorial Sloan-Kettering Cancer Center for the anti-ARS2 antibodies.
This work was supported, in whole or in part, by National Institutes of Health Grants R01-CA-113962 and R01-DK085121 (to X. H.). This work was also supported by Caring for Carcinoid Foundation-AACR Grant Care for Carcinoid Foundation 11-60-33XH, Institute for Translational Medicine and Therapeutics Grant 400-4130-2-0.4001, and Juvenile Diabetes Research Foundation Grant 5-2012-221.
- MEN1
- multiple endocrine neoplasia type I
- ARS2
- arsenic resistance protein-2
- IRS2
- insulin receptor substrate-2
- MEF
- mouse embryonic fibroblast
- miRNA
- microRNA
- PET
- pancreatic endocrine tumor
- pre-miRNA
- precursor microRNA
- pri-miRNA
- primary microRNA
- qRT-PCR
- quantitative real-time PCR.
REFERENCES
- 1. Libé R., Bertherat J. (2005) Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur. J. Endocrinol. 153, 477–487 [DOI] [PubMed] [Google Scholar]
- 2. Burgess J. R., Nord B., David R., Greenaway T. M., Parameswaran V., Larsson C., Shepherd J. J., Teh B. T. (2000) Phenotype and phenocopy: the relationship between genotype and clinical phenotype in a single large family with multiple endocrine neoplasia type 1 (MEN 1). Clin. Endocrinol. 53, 205–211 [DOI] [PubMed] [Google Scholar]
- 3. Marx S. J. (2005) Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat. Rev. Cancer 5, 367–375 [DOI] [PubMed] [Google Scholar]
- 4. Bertolino P., Radovanovic I., Casse H., Aguzzi A., Wang Z. Q., Zhang C. X. (2003) Genetic ablation of the tumor suppressor menin causes lethality at mid-gestation with defects in multiple organs. Mech. Dev. 120, 549–560 [DOI] [PubMed] [Google Scholar]
- 5. Chandrasekharappa S. C., Guru S. C., Manickam P., Olufemi S. E., Collins F. S., Emmert-Buck M. R., Debelenko L. V., Zhuang Z., Lubensky I. A., Liotta L. A., Crabtree J. S., Wang Y., Roe B. A., Weisemann J., Boguski M. S., Agarwal S. K., Kester M. B., Kim Y. S., Heppner C., Dong Q., Spiegel A. M., Burns A. L., Marx S. J. (1997) Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276, 404–407 [DOI] [PubMed] [Google Scholar]
- 6. Lemmens I., Van de Ven W. J., Kas K., Zhang C. X., Giraud S., Wautot V., Buisson N., De Witte K., Salandre J., Lenoir G., Pugeat M., Calender A., Parente F., Quincey D., Gaudray P., De Wit M. J., Lips C. J., Höppener J. W., Khodaei S., Grant A. L., Weber G., Kytölä S., Teh B. T., Farnebo F., Thakker R. V. (1997) Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol. Genet. 6, 1177–1183 [DOI] [PubMed] [Google Scholar]
- 7. Agarwal S. K., Guru S. C., Heppner C., Erdos M. R., Collins R. M., Park S. Y., Saggar S., Chandrasekharappa S. C., Collins F. S., Spiegel A. M., Marx S. J., Burns A. L. (1999) Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 96, 143–152 [DOI] [PubMed] [Google Scholar]
- 8. Hughes C. M., Rozenblatt-Rosen O., Milne T. A., Copeland T. D., Levine S. S., Lee J. C., Hayes D. N., Shanmugam K. S., Bhattacharjee A., Biondi C. A., Kay G. F., Hayward N. K., Hess J. L., Meyerson M. (2004) Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol. Cell 13, 587–597 [DOI] [PubMed] [Google Scholar]
- 9. Jin S., Mao H., Schnepp R. W., Sykes S. M., Silva A. C., D'Andrea A. D., Hua X. (2003) Menin associates with FANCD2, a protein involved in repair of DNA damage. Cancer Res. 63, 4204–4210 [PubMed] [Google Scholar]
- 10. Yang Y., Hua X. (2007) In search of tumor suppressing functions of menin. Mol. Cell. Endocrinol. 265–266, 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen G., A. J., Wang M., Farley S., Lee L. Y., Lee L. C., Sawicki M. P. (2008) Menin promotes the Wnt signaling pathway in pancreatic endocrine cells. Mol. Cancer Res. 6, 1894–1907 [DOI] [PubMed] [Google Scholar]
- 12. Gurung B., Feng Z., Iwamoto D. V., Thiel A., Jin G., Fan C. M., Ng J. M., Curran T., Hua X. (2013) Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome. Cancer Res. 73, 2650–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang J., Gurung B., Wan B., Matkar S., Veniaminova N. A., Wan K., Merchant J. L., Hua X., Lei M. (2012) The same pocket in menin binds both MLL and JunD but has opposite effects on transcription. Nature 482, 542–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartel D. P. (2009) MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kusenda B., Mraz M., Mayer J., Pospisilova S. (2006) MicroRNA biogenesis, functionality and cancer relevance. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 150, 205–215 [DOI] [PubMed] [Google Scholar]
- 16. Denli A. M., Tops B. B., Plasterk R. H., Ketting R. F., Hannon G. J. (2004) Processing of primary microRNAs by the Microprocessor complex. Nature 432, 231–235 [DOI] [PubMed] [Google Scholar]
- 17. Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Rådmark O., Kim S., Kim V. N. (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425, 415–419 [DOI] [PubMed] [Google Scholar]
- 18. Gregory R. I., Yan K. P., Amuthan G., Chendrimada T., Doratotaj B., Cooch N., Shiekhattar R. (2004) The Microprocessor complex mediates the genesis of microRNAs. Nature 432, 235–240 [DOI] [PubMed] [Google Scholar]
- 19. Hutvágner G., McLachlan J., Pasquinelli A. E., Bálint E., Tuschl T., Zamore P. D. (2001) A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838 [DOI] [PubMed] [Google Scholar]
- 20. Ketting R. F., Fischer S. E., Bernstein E., Sijen T., Hannon G. J., Plasterk R. H. (2001) Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15, 2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Knight S. W., Bass B. L. (2001) A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293, 2269–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krol J., Loedige I., Filipowicz W. (2010) The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet 11, 597–610 [DOI] [PubMed] [Google Scholar]
- 23. Gregory R. I., Chendrimada T. P., Cooch N., Shiekhattar R. (2005) Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123, 631–640 [DOI] [PubMed] [Google Scholar]
- 24. Sayed D., Abdellatif M. (2011) MicroRNAs in development and disease. Physiol. Rev. 91, 827–887 [DOI] [PubMed] [Google Scholar]
- 25. Small E. M., Olson E. N. (2011) Pervasive roles of microRNAs in cardiovascular biology. Nature 469, 336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fernandez-Valverde S. L., Taft R. J., Mattick J. S. (2011) MicroRNAs in beta-cell biology, insulin resistance, diabetes and its complications. Diabetes 60, 1825–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poy M. N., Eliasson L., Krutzfeldt J., Kuwajima S., Ma X., Macdonald P. E., Pfeffer S., Tuschl T., Rajewsky N., Rorsman P., Stoffel M. (2004) A pancreatic islet-specific microRNA regulates insulin secretion. Nature 432, 226–230 [DOI] [PubMed] [Google Scholar]
- 28. Trajkovski M., Hausser J., Soutschek J., Bhat B., Akin A., Zavolan M., Heim M. H., Stoffel M. (2011) MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 474, 649–653 [DOI] [PubMed] [Google Scholar]
- 29. Caldas C., Brenton J. D. (2005) Sizing up miRNAs as cancer genes. Nat. Med. 11, 712–714 [DOI] [PubMed] [Google Scholar]
- 30. Di Leva G., Croce C. M. (2013) miRNA profiling of cancer. Curr. Opin. Genet. Dev. 23, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lynn F. C., Skewes-Cox P., Kosaka Y., McManus M. T., Harfe B. D., German M. S. (2007) MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes 56, 2938–2945 [DOI] [PubMed] [Google Scholar]
- 32. Melkman-Zehavi T., Oren R., Kredo-Russo S., Shapira T., Mandelbaum A. D., Rivkin N., Nir T., Lennox K. A., Behlke M. A., Dor Y., Hornstein E. (2011) miRNAs control insulin content in pancreatic beta-cells via down-regulation of transcriptional repressors. EMBO J. 30, 835–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y., Liu J., Liu C., Naji A., Stoffers D. A. (2013) MicroRNA-7 regulates the mTOR pathway and proliferation in adult pancreatic beta-cells. Diabetes 62, 887–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roldo C., Missiaglia E., Hagan J. P., Falconi M., Capelli P., Bersani S., Calin G. A., Volinia S., Liu C. G., Scarpa A., Croce C. M. (2006) MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J. Clin. Oncol. 24, 4677–4684 [DOI] [PubMed] [Google Scholar]
- 35. Perren A., Komminoth P., Heitz P. U. (2004) Molecular genetics of gastroenteropancreatic endocrine tumors. Ann. N.Y. Acad. Sci. 1014, 199–208 [DOI] [PubMed] [Google Scholar]
- 36. Schnepp R. W., Chen Y. X., Wang H., Cash T., Silva A., Diehl J. A., Brown E., Hua X. (2006) Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res. 66, 5707–5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gruber J. J., Zatechka D. S., Sabin L. R., Yong J., Lum J. J., Kong M., Zong W. X., Zhang Z., Lau C. K., Rawlings J., Cherry S., Ihle J. N., Dreyfuss G., Thompson C. B. (2009) Ars2 links the nuclear cap-binding complex to RNA interference and cell proliferation. Cell 138, 328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yokoyama A., Cleary M. L. (2008) Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell 14, 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sabin L. R., Zhou R., Gruber J. J., Lukinova N., Bambina S., Berman A., Lau C. K., Thompson C. B., Cherry S. (2009) Ars2 regulates both miRNA- and siRNA-dependent silencing and suppresses RNA virus infection in Drosophila. Cell 138, 340–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stålberg P., Grimfjärd P., Santesson M., Zhou Y., Lindberg D., Gobl A., Oberg K., Westin G., Rastad J., Wang S., Skogseid B. (2004) Transfection of the multiple endocrine neoplasia type 1 gene to a human endocrine pancreatic tumor cell line inhibits cell growth and affects expression of JunD, delta-like protein 1/preadipocyte factor-1, proliferating cell nuclear antigen, and QM/Jif-1. J. Clin. Endocrinol. Metab. 89, 2326–2337 [DOI] [PubMed] [Google Scholar]
- 41. Frost R. J., Olson E. N. (2011) Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc. Natl. Acad. Sci. U.S.A. 108, 21075–21080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Withers D. J., Gutierrez J. S., Towery H., Burks D. J., Ren J. M., Previs S., Zhang Y., Bernal D., Pons S., Shulman G. I., Bonner-Weir S., White M. F. (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391, 900–904 [DOI] [PubMed] [Google Scholar]
- 43. Yang Y., Gurung B., Wu T., Wang H., Stoffers D. A., Hua X. (2010) Reversal of preexisting hyperglycemia in diabetic mice by acute deletion of the Men1 gene. Proc. Natl. Acad. Sci. U.S.A. 107, 20358–20363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson S. M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K. L., Brown D., Slack F. J. (2005) RAS is regulated by the let-7 microRNA family. Cell 120, 635–647 [DOI] [PubMed] [Google Scholar]
- 45. Heppner C., Bilimoria K. Y., Agarwal S. K., Kester M., Whitty L. J., Guru S. C., Chandrasekharappa S. C., Collins F. S., Spiegel A. M., Marx S. J., Burns A. L. (2001) The tumor suppressor protein menin interacts with NF-κB proteins and inhibits NF-κB-mediated transactivation. Oncogene 20, 4917–4925 [DOI] [PubMed] [Google Scholar]
- 46. Dreijerink K. M., Varier R. A., van Beekum O., Jeninga E. H., Höppener J. W., Lips C. J., Kummer J. A., Kalkhoven E., Timmers H. T. (2009) The multiple endocrine neoplasia type 1 (MEN1) tumor suppressor regulates peroxisome proliferator-activated receptor γ-dependent adipocyte differentiation. Mol. Cell. Biol. 29, 5060–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Milne T. A., Hughes C. M., Lloyd R., Yang Z., Rozenblatt-Rosen O., Dou Y., Schnepp R. W., Krankel C., Livolsi V. A., Gibbs D., Hua X., Roeder R. G., Meyerson M., Hess J. L. (2005) Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc. Natl. Acad. Sci. U.S.A. 102, 749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yokoyama A., Wang Z., Wysocka J., Sanyal M., Aufiero D. J., Kitabayashi I., Herr W., Cleary M. L. (2004) Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol. Cell. Biol. 24, 5639–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thiel A. T., Feng Z., Pant D. K., Chodosh L. A., Hua X. (2013) The trithorax protein partner menin acts in tandem with EZH2 to suppress C/EBPα and differentiation in MLL-AF9 leukemia. Haematologica 98, 918–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gobl A. E., Berg M., Lopez-Egido J. R., Oberg K., Skogseid B., Westin G. (1999) Menin represses JunD-activated transcription by a histone deacetylase-dependent mechanism. Biochim. Biophys. Acta 1447, 51–56 [DOI] [PubMed] [Google Scholar]
- 51. Luzi E., Marini F., Tognarini I., Galli G., Falchetti A., Brandi M. L. (2012) The regulatory network menin-microRNA 26a as a possible target for RNA-based therapy of bone diseases. Nucleic Acid Ther. 22, 103–108 [DOI] [PubMed] [Google Scholar]
- 52. Schuppin G. T., Pons S., Hügl S., Aiello L. P., King G. L., White M., Rhodes C. J. (1998) A specific increased expression of insulin receptor substrate 2 in pancreatic beta-cell lines is involved in mediating serum-stimulated beta-cell growth. Diabetes 47, 1074–1085 [DOI] [PubMed] [Google Scholar]
- 53. Kubota N., Tobe K., Terauchi Y., Eto K., Yamauchi T., Suzuki R., Tsubamoto Y., Komeda K., Nakano R., Miki H., Satoh S., Sekihara H., Sciacchitano S., Lesniak M., Aizawa S., Nagai R., Kimura S., Akanuma Y., Taylor S. I., Kadowaki T. (2000) Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 49, 1880–1889 [DOI] [PubMed] [Google Scholar]
- 54. Crabtree J. S., Scacheri P. C., Ward J. M., McNally S. R., Swain G. P., Montagna C., Hager J. H., Hanahan D., Edlund H., Magnuson M. A., Garrett-Beal L., Burns A. L., Ried T., Chandrasekharappa S. C., Marx S. J., Spiegel A. M., Collins F. S. (2003) Of mice and MEN1: insulinomas in a conditional mouse knockout. Mol. Cell. Biol. 23, 6075–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wuescher L., Angevine K., Hinds T., Ramakrishnan S., Najjar S. M., Mensah-Osman E. J. (2011) Insulin regulates menin expression, cytoplasmic localization, and interaction with FOXO1. Am. J. Physiol. Endocrinol. Metab. 301, E474–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jiao Y., Shi C., Edil B. H., de Wilde R. F., Klimstra D. S., Maitra A., Schulick R. D., Tang L. H., Wolfgang C. L., Choti M. A., Velculescu V. E., Diaz L. A., Jr., Vogelstein B., Kinzler K. W., Hruban R. H., Papadopoulos N. (2011) DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331, 1199–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]


