Abstract
Exposure to pro-inflammatory cytokines, such as angiotensin II, endothelin-1 or TNF leads to endothelial dysfunction, characterized by the reduced production of nitric oxide via endothelial nitric oxide synthase (eNOS). We recently identified the Ca2+ binding protein S100A1 as an essential factor required for eNOS activity. Here we report that pro-inflammatory cytokines down-regulate expression of S100A1 in primary human microvascular endothelial cells (HMVECs) via induction of microRNA-138 (miR-138), in a manner that depends on the stabilization of HIF1-α. We show that loss of S100A1 in ECs reduces stimulus-induced NO production, which can be prevented by inhibition of miR-138. Our study suggests that targeting miR-138 might be beneficial for the treatment of cardiovascular disease.
Keywords: Endothelial dysfunction, Endothelial nitric oxide synthase, Cytokines, Angiotensin II, Endothelin-1, TNF
1. Introduction
Dysfunction of the arterial vascular endothelium is associated with the most significant predisposing risk factors for cardiovascular disease (CVD) such as smoking, hypertension, diabetes, hyperglycemia, hypercholesterolemia and advanced age [1]. The endothelium serves a source of vasoactive factors that maintain – in its healthy state – the endothelium’s anti-inflammatory, anti-thrombotic, anti-proliferative, and stimulus-dependent vasodilatory functions. Induction of chronic vascular inflammation, as characterized by the persistent elevation of circulating inflammatory cytokines, correlates with endothelial dysfunction (ED) and is recognized as a major contributor to the development of atherosclerosis and cardiovascular disease [2-4]. ED can be seen as a shift towards a chronic pro-coagulative and vaso-constrictive state that correlates with, but typically precedes, clinical manifestations of coronary, cerebrovascular and peripheral vascular diseases [1,5]. Central to the quiescent state of healthy endothelium is the stimulus-dependent generation of the vaso-relaxant factor nitric oxide (NO) by the endothelial isoform of the nitric oxide synthase enzymes (eNOS, NOS3). Chronic elevation of pro-inflammatory cytokines, most notably Angiotensin II leads to a reduction in stimulus-induced NO release from the endothelium with a consequent loss of vasoreactivity [6].
S100A1 is a small EF-hand Ca2+ binding protein that relays intracellular Ca2+ oscillations and regulates vascular tone [7-10]. Previously, we were able to show that S100A1 directly interacts with eNOS, prevents PKC-mediated phosphorylation of the eNOS inhibitory Thr-495 site and augments eNOS enzymatic activity in ECs [11]. S100A1 levels are rapidly lost in hypoxic ECs or in ischemic tissues and this correlates with a loss of stimulus-induced NO production [11]. We recently published that endothelial cell-type specific regulation of S100A1 in response to hypoxia is achieved via the HIF1-α dependent induction of miR-138, which specifically targets a conserved 22 nucleotide sequence in the 3’UTR of S100A1. Pro-inflammatory cytokines have been shown to activate HIF1-α in a variety of different cells types important to vascular biology [12-17]. Thus, here we sought to investigate if pro-inflammatory cytokines cause loss of eNOS activity and lead to ECs dysfunction via HIF1-α mediated induction of miR-138 and consequent loss of S100A1.
2. Materials and Methods
2.1 Cell Culture
EA.hy926 endothelial cells ATCC (CRL-2922) were cultured in DMEM (4.5g/L glucose) supplemented with 10% FBS. Human microvascular endothelial cells (HMVEC) were purchased from Lonza (CC-7030) at passage 4-5 and cultured in ATCC vascular cell basal medium (PCS-100-030) supplemented with the ATCC endothelial cell VEGF growth kit (PCS-100-041). For chemical hypoxia cells were treated for 24h with CoCl2 (250 μmol/L). Viability of EA.hy926 ECs or primary human ECs was not compromised at CoCl2 concentrations less than 1 mmol/L for 24h (not shown). Where appropriate cells were incubated with Ang II (10 nmol/L), Et-1 (10 nmol/L) or TNFα (50 ng/ml) for the times indicated.
2.2 Immunoblot assay
Cell samples were lysed in Laemmli buffer and 30 μg/Lane whole cell extract was loaded on 4-20% Tris-Glycine gel, (Life Technologies, CA) and transferred to nitrocellulose membrane. Membranes were probed for S100A1 (Acris, San Diego, CA, cat # SP5355P, β-actin (SantaCruz cat # sc-8432), HIF1-α (SantaCruz cat# sc-10790) total eNOS (BD Biosciences cat# 610297) or phospho Thr-495 eNOS (BD Biosciences cat# 612707). Protein expression was quantitatively assessed using an Odyssey scanner (Li-Cor Biosciences, Lincoln NE). All immunoblot assays were done in duplicate and repeated a minimum of three times.
2.3 Plasmids
All constructs were as described [18]. The S100A1–3’UTR reporter construct of comprised the 3’UTR of the human S100A1 gene cloned downstream of a constitutive ribosomal protein L10 (RPL10) promoter and renilla luciferase gene. A 3’UTR control plasmid with SV40 T antigen 3’UTR and β-actin control plasmid with β-actin promoter were used as controls for transfection efficiency. A gene block (IDT) comprising the entire S100A1–3’UTR, but lacking specifically the 22 nucleotide putative miR-138 target site, was subcloned NheI to XhoI into the luciferase reporter vector to generate the ΔmiR-138 construct as described [18]. All constructs were verified by sequencing using Jefferson’s genomics facility.
2.4 Transfection
EA.hy926 ECs were cultured in 24 well plates in DMEM with 10% FBS. Cells were transfected with 1μg of luciferase reporter constructs mixed with Lipofectamine 2000 in serum-free DMEM, according to the manufacturer’s instruction (Life Technologies). Transfection media was changed after 2-3 hours and replaced with DMEM, 10% FBS. Cells were harvested 40h after transfection. HIF1-α siRNA was purchased from SantaCruz (Dallas, TX, cat # SC-35561). To inhibit activity of microRNA-138, we used a cholesterol-conjugated antagomir-138 (10 μg/ml, Fidelity Systems, Gaithersburg, MD) [19] to inhibit miR-138. To test activation of MAPKs via miR-138, primary HMVECs were transfected in DMEM with 20 nmoles (per well of a 6-well dish) microRNA mimic (Dharmacon ThermoFisher, cat # C- 300605-05-0005) or Control mimic (cat # CP-004500-01-05) using Lipofectamine 2000 and media was changed after 1–2 hours. Cell extracts were prepared 24h later.
2.5 Real time PCR for miR-138 expression
RNA was isolated from cell samples using RNAzol RT according to manufacturer’s instruction (MRC, Inc cat# RN190). 1 μg of input RNA was used to reverse transcribe cDNA using Origene (Rockville, MD) kit (cat # HP100042). Human miR-138 real time PCR primer pair (cat # HP300151) was from Origene which consisted of a universal primer- 5’-CTCTATGCGTCTGTACAAG-3’ and microRNA-138 specific primer 5’-AGCUGGUGUUGUGAAUCAGGCCG-3’. Quantitative real time PCR was performed using BioRad (Hercules,CA) SYBR Green master mix and a Stratagene (La Jolla, CA) real time PCR cycler. Expression of U6 RNA, which did not change by cytokine treatment, was used to normalize for input RNA. In order to allow for better comparison between different batches of primary HMVECs the maximum expression of miR-138 induced by 24h treatment with Ang II (10nmol/L) in HMVECs was arbitrarily set as 1.
2.6 Luciferase assays
Light Switch luciferase assay reagents (cat # LS100) were from SwitchGear Genomics. ECs were cultured and transfected in 24 well plates. To measure luciferase expression, 100 μl of assay reagents were added to each well and enzyme activity was measured, according to the manufacturer’s instruction (SwitchGear Genomics) using a Wallac Victor3 Multilabel counter (Perkin Elmer, MA). Luciferase expression is given as relative light units (RLU). All luciferase experiments were done in triplicate and repeated a minimum of 3 times.
2.7 Matrigel tube formation assay
Growth-factor reduced Matrigel Matrix (BD Biosciences, cat# 356231) was diluted 1:1 with ATCC vascular cell basal medium (no additives). HMVEC (approx 200,000 cells) were treated with Angiotensin II (10 nmol/L) and either incubated with antagomir-138 or control or infected with recombinant adenovirus expressing S100A1 and GFP from a bicistronic insert [10] or control (ctr) as described [18] at a multiplicity of infection (MOI) of 17. After 24h cells were detached from the plastic support with trypsin and seeded onto the Matrigel matrix in ATCC medium supplemented with ATCC endothelial cell VEGF growth kit (PCS-100-041) and Ang II (10 nmol/L). Images were taken 24h later and digitized using NIH-ImageJ software. Original images of the matrigel tube formation assays are included as online supplement Figure S11.
2.8 Nitric oxide assay
HMVEC (approx 100,000 cells) were incubated with antagomir-138 or control and either Ang II (10 nmol/L) or TNFα (50 ng/ml) for 24h. Following this, cells were starved for 4h in ATCC vascular cell basal medium without supplements. Cells were then incubated with ATCC vascular cell basal medium supplemented with all components of the ATCC endothelial cell growth kit (PCS-100-041) except VEGF. NO production was then stimulated with the addition of 50 ng/ml VEGF (R&D systems) and Ang II or TNFα. Medium was collected 24h later and NO levels were measured using a fluorescent NO/Nitrite/Nitrate assay (Cayman Chemical, cat# 780051) according to manufacturer’s instructions. In other experiments HMVECs were infected with recombinant Adenovirus expressing S100A1 and GFP from a bicistronic insert, or control, at a MOI of 17. The infection medium was removed 8h later and incubation was continued overnight. Next day cells were treated with Ang II (10nmol/L) or TNFα (50 ng/ml). After 24h cells were starved for 4h in ATCC medium without supplements. Cells were then incubated with ATCC vascular cell basal medium supplemented with all components of the ATCC endothelial cell growth kit (PCS-100-041) except VEGF. NO production was then stimulated with the addition of 50 ng/ml VEGF (R&D systems) and Ang II or TNFα. Medium was collected 24h later and NO levels were measured using a fluorescent NO/Nitrite/Nitrate assay (Cayman Chemical, cat# 780051) according to manufacturer’s instructions.
2.9 Statistical Analysis
One-way ANOVA with Tukey’s post-test for multiple comparisons was used to analyze the appropriate data using GraphPad PRISM software. Data are shown as ± SEM in the figures. A P value of < 0.05 was considered statistically significant. Experiments were done independently a minimum of three times. Each set-up was done at least in duplicate for each repetition.
3. Results
3.1 Pro-inflammatory cytokines reduce S100A1 expression and cause dysfunction in ECs
Endothelial cells isolated from patients with Heart Failure (HF) display reduced levels of S100A1 [10], while increased expression of vasoactive pro-inflammatory cytokines is known to correlate with HF [20,21]. In order to investigate if pro-inflammatory cytokines would change expression of S100A1 we treated primary human microvascular endothelial cells (HMVECs) with angiotensin II (Ang II), endothelin-1 (Et-1) or tumor necrosis factor (TNFα) for 24h. All of these cytokines reduced S100A1 protein levels to less than 25% of untreated controls, while simultaneously increasing expression of the intercellular adhesion molecule-1 (ICAM-1), a molecule known to participate in vascular pathologies by promoting the attachment of leucocytes to injured endothelium [22,23] (Figure 1A, see Figure S1 for quantification of Immunoblot). The magnitude of the effect was comparable to that induced by hypoxia (induced by treatment with CoCl2) in ECs, a treatment we have recently shown to cause EC dysfunction via the reduced expression of S100A1 [18].
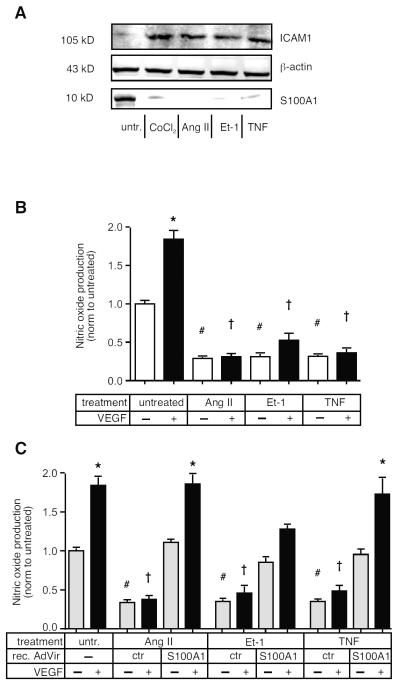
Figure 1. Pro-inflammatory cytokines reduce S100A1 expression and cause dysfunction in ECs.
A) Primary HMVECs were incubated with Ang II (10 nmol/L), Et-1 (10 nmol/L), TNFα (50 ng/ml) or CoCl2 (250 μmol/L) for 24h before extract preparation and SDS-PAGE analysis for ICAM and S100A1. Representative image is shown. Experiment was repeated twice more, for quantification see Figure S1. B) HMVECs were incubated with cytokines for 24h, then treated ± VEGF (50 ng/ml) as indicated, for a further 24h. eNOS produced nitric oxide levels in the supernatant were measured using a nitrate/nitrite assay. Experiment was done 3 times, each at least in duplicate. C) HMVECs were infected with recombinant adenovirus expression S100A1 (or ctr. Adenovirus) and treated for 24h with Ang II, Et-1 or TNF as indicated. Cells were then stimulated, or not, with VEGF (50 ng/ml) for 24h before assaying supernatant nitrate/nitrite levels. *, P<0.05 vs untreated, no VEGF. # P<0.05 vs untreated, no VEGF; ✠, P<0.02 vs untreated + VEGF. Experiment was performed 3 times, each at least in duplicate.
Reduced bioavailablity of nitric oxide (NO) is a hallmark of cytokine-induced EC dysfunction [24]. We have previously shown that S100A1 is an essential regulator of eNOS (NOS3) activity in ECs [11] and that lack of S100A1 impairs endothelial NO production and endothelial-dependent vasodilation [10]. Treatment of HMVECs with Ang II, Et-1 or TNFα all strongly impaired both basal as well as stimulus-induced NO production (Figure 1B). Re-expression of S100A1 via infection with recombinant adenovirus restored both basal as well as VEGF-induced NO production in cytokine-treated ECs, demonstrating the essential role of S100A1 to eNOS activation (Figure 1C).
3.2 Pro-inflammatory cytokines decrease S100A1 via increased HIF1-α and miR-138 levels in HMVECs
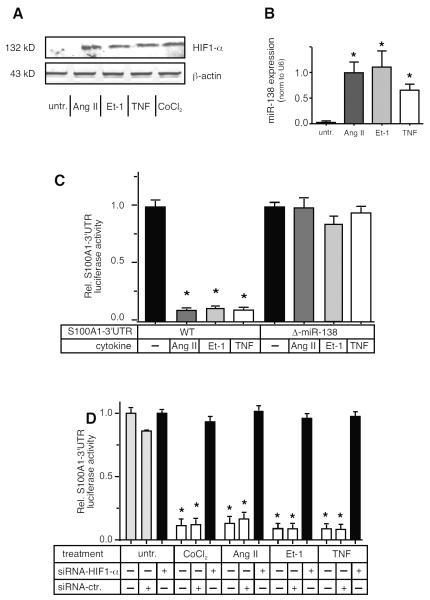
We recently reported that hypoxia-induced HIF1-α stabilization in ECs led to the induction of miR-138 with consequent loss of S100A1 [18]. Ang II as well as Et-1 and TNF have been shown to increase the stability of HIF1-α in different cell types, [25-27] albeit not primary human ECs. All three different cytokines tested increased HIF1-α levels (Figure 2A, see also Figure S2 for quantification) in HMVECs, comparably to those induced by chemical hypoxia via CoCl2, and greatly induced miR-138 (Figure 2B), (see Figure S3 for a timecourse of miR-138 induction in ECs). The importance of the HIF1-α - miR-138 axis to the expression of S100A1 in ECs was demonstrated by measuring expression of a luciferase reporter construct that contained the human S100A1–3’untranslated region (3’UTR) [18] in cytokine treated EA.hy926 cells, which are easily transfectable, immortalized human endothelial cells. Expression of this reporter construct was greatly diminished when cells were exposed to Ang II , Et-1 or TNF (Figure 2C) whereas expression of a control construct having the 3’UTR of the SV40-T-ag instead of S100A1 was not affected by cytokine treatment (Figure S4). The 3’UTR of the S100A1 mRNA has a strong miR-138 binding site [18]. Deletion of the 22 nucleotide miR-138 target site within the S100A1–3’UTR also completely prevented any downregulation of reporter gene expression by cytokine treatment (Figure 2C). Finally, siRNA mediated knockdown of HIF1-α reduced miR-138 expression in Ang II treated ECs (Figure S5) As expected, this inhibition of HIF1-α by siRNA knockdown restored expression of the S100A1–3’UTR reporter construct in cytokine or CoCl2 treated cells (Figure 2D), while expression of a control 3’UTR luciferase reporter was not changed by HIF1-α inhibition (Figure S6).
Figure 2. Pro-inflammatory cytokines decrease S100A1 via increased HIF1-α and miR-138 levels in ECs.
A) Primary HMVECs were incubated with Ang II, Et-1, TNFα or CoCl2 (250 μmol/L) for 24h before extract preparation and SDS-PAGE analysis for HIF1-α. Representative immunoblot is shown. Experiment was repeated twice more, for quantification see Figure S2. B) Expression levels of miR-138 were assessed by qPCR in EC extracts subjected to 24h treatment with Angiotensin II (Ang II, 10 nmol/L), Endothelin-1 (Et-1, 10 nmol/L) or human TNFα (TNF, 10ng/ml). Expression levels of the small nuclear RNA U6 were assessed in parallel. U6 levels were not changed by cytokine treatment. *, P<0.05 vs untreated. The experiment was performed 4 times, each at least in duplicate. C) EA.hy926 ECs were transfected with the WT S100A1 luciferase reporter construct or the miR-138 binding site deleted reporter (Δ-miR-138) and treated for 24h with Ang II, Et-1 or TNFα, prior to luciferase assay. *, P<0.05 vs untreated. Experiment was done 3 times, each in triplicate. D) EA.hy926 ECs were transfected with the S100A1–3’UTR reporter gene construct and co-transfected with either siRNA against HIF1-α (Dharmacon) or scramble control and subsequently treated with cytokines as indicated. Treatment with CoCl2, a chemical inducer of the hypoxia response was included as a control. *, P<0.02 vs untreated. Experiment was done 3 times, each at least in duplicate.
3.3 Inhibition of miR-138 restores normal function to cytokine-treated ECs
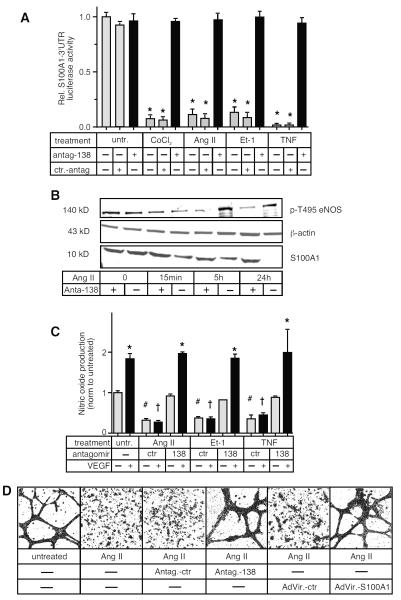
The importance of miR-138 to cytokine-induced EC dysfunction was also demonstrated by direct inhibition of miR-138. When miR-138 function was silenced by incubation of cytokine-treated ECs with a cell-permeable, cholesterol-conjugated antimir (antagomir-138) [18] expression of a luciferase S100A1–3’UTR reporter gene was restored to normal (Figure 3A) in a dose dependent fashion (Figure S7), while expression of a control–3’UTR was unaffected (Figure S8). Moreover, the antagomir-138 restored normal S100A1 protein expression levels to cytokine-treated HMVECs (Figure 3B). We had previously shown that loss of S100A1 reduced NO production by ECs via the increased phosphorylation of eNOS on Thr495 [11], a known inhibitory site [28]. Incubation of Ang II treated HMVECs with the antagomir-138 reduced the increased phosphorylation of eNOS pT495 within 5h (Figure 3B, see Figure S9 for quantification of immunoblots), and was able to restore both basal as well as VEGF-stimulated NO production in cytokine-treated HMVECs (Figure 3C) and reduced expression levels of ICAM back to normal (Figure S10). In addition, using the EC tube formation assay on matrigel matrix, we were able to show that both inhibition of miR-138 with the antagomir-138, as well as restoration of S100A1 levels by recombinant adenovirus, was sufficient to restore tube formation capability to Ang II treated HMVECs. (Figure 3D, original, non-digitized images are provided as Figure S11).
Figure 3. Inhibition of miR1-38 restores normal function to cytokine-treated ECs.
A) EA.hy926 ECs were transfected with the S100A1–3’UTR luciferase reporter construct and incubated with either antagomir-138 or control and stimulated for 24h with cytokines or CoCl2 as indicted prior to luciferase assay. *, P<0.02 vs untreated. B) Primary HMVECs were treated with Ang II ± antagomir-138 for times indicated and S100A1 and phospho-T495 eNOS levels were assessed by Immunoblot analysis. β-actin was assessed as loading control. Representative images are shown. Experiment was repeated twice more, for quantification see Figure S9. C) HMVECs were incubated with cytokines for 24h and co-incubated with either antagomir-138 or control, then treated ± VEGF (50 ng/ml) as indicated for a further 24h. eNOS produced nitric oxide levels in the supernatant were measured using a nitrate/nitrite assay. *, P<0.05 vs untreated. # P<0.05 vs untreated, no VEGF; ✠, P<0.05 vs untreated + VEGF. Experiment was performed 3 times, each at least in duplicate. D) Primary HMVEC were incubated with antagomir-138 or control and treated with Ang II for 24h. Next day later cells were infected (MOI=17) with either control Adenovirus or Adenovirus expressing S100A1. 24h later cells were seeded onto Matrigel matrix. Images of EC tube formation were taken 24h later and digitized using Image J (Original pictures of EC tube formation are included as supplemental Figure S11. Representative images are shown. The experiment was done 3 times, each in duplicate.
3.4 Inhibition of miR-138 prevents chronic activation of cytokine-induced MAPK phosphorylation
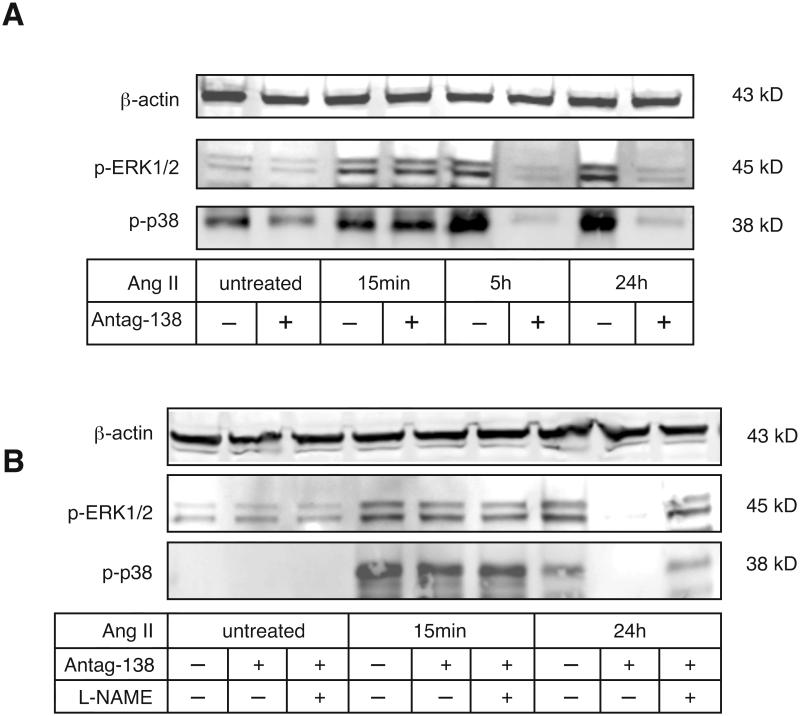
We next investigated the effects of miR-138 inhibition on cytokine signal transduction. As might be expected from the timecourse of miR-138 induction, activation of the immediate, receptor proximal, signal transduction cascades are unimpeded by the inhibition of miR-138. Thus the MAPKs p38 and ERK1/2 are both rapidly and strongly activated by treatment of HMVECs with Ang II (Figure 4A, see Figure S12 for quantification). However, continued activation of MAPKs by Ang II at later timepoints (i.e. post 5h) was strongly dependent on miR-138, as treatment with the antagomir-138 completely prevented phosphorylation of p38 and ERK1/2 at 5h and 24h. Moreover, incubation of HMVECs with a miR-138 mimic for 24h also led to MAPK activation, even in the absence of cytokine treatment (Figure S13). The reduction of this late MAPK activation by miR-138 inhibition was due to the restoration of eNOS activity, since incubation with the NOS-inhibitor L-NAME increased the phosphorylation of both p-p38 and p-ERK, even in the presence of the miR-138 inhibitor (Figure 4B, see Figure S14 for quantification).
Figure 4. Inhibition of miR-138 prevents chronic activation of cytokine-induced MAPK phosphorylation.
A) Primary HMVECs were treated with Ang II ± antagomir-138 for times indicated and the phosphorylation state of the MAPK p38 and ERK1/2 were assessed by Immunoblot analysis. Representative images are shown. Experiment was repeated twice more, for quantification see Figure S12. B) Primary HMVECs were treated with Ang II ± antagomir-138 ± L-NAME (NOS inhibitor) for times indicated and the phosphorylation state of the MAPK p38 and ERK1/2 were assessed by Immunoblot analysis. Representative image is shown. Experiment was repeated twice more, for quantification see Figure S14.
4. Discussion
Chronic vascular inflammation is recognized as significantly predisposing to the development of cardiovascular disease. This condition correlates with reduced bioavailability of cardioprotective NO and consequent diminished endothelium-dependent dilation leading to a chronic pro-coagulative and vaso-constrictive state. The most salient finding of our work here is the identification of a signaling cascade (Figure 5) whereby vasoactive or pro-inflammatory cytokine signaling increases stability of HIF1-α, induces miR-138 expression that leads to loss of S100A1 and consequent inactivation of eNOS. This signaling axis induces and maintains the reduced bioavailability of NO, characteristic of the dysfunction observed in ECs chronically exposed to vasoactive cytokines, such as Ang II, Et-1 or TNF [29-35].
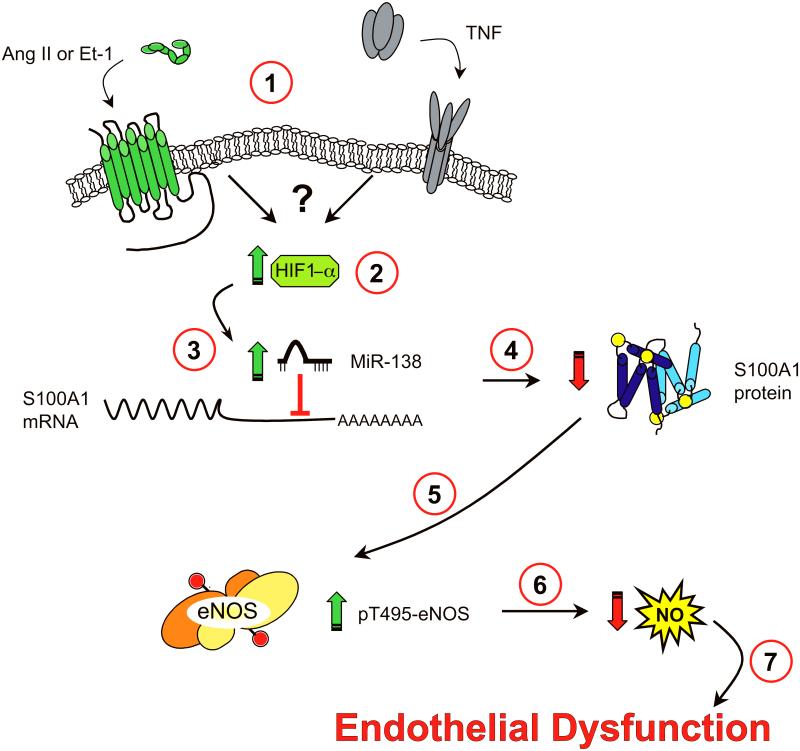
Figure 5. Proposed Model of cytokine induced, miR-138-mediated, EC dysfunction.
(1) Engagement of vasocative or pro-inflammatory cytokine receptors by their respective ligands leads to stabilization of HIF1-α in ECs through as yet incompletely defined mechanisms (2). Stabilization of HIF1-α increases miR-138 levels (3), which destabilize S100A1 mRNA or suppress its translation, leading to rapid loss of S100A1 protein in ECs (4). This leads to increased phospho-Thr495 eNOS levels (5) with consequent reduction of NO generation (6). This loss of endothelial NO generation engenders endothelial dysfunction (7).
A large number of regulatory factors have been identified that control the activity of eNOS (see ref [24] for a recent review). Aside from the required cofactors Heme, NADPH, FAD, FMN, and BH4 [36-40], Ca2+/calmodulin (CaM) serves to enhance the catalytic activity of eNOS in response to Ca2+ oscillations [41]. S100A1, belonging to the same EF-hand Ca2+ binding protein family as CaM, also directly interacts with eNOS and serves as a required cofactor, as we have recently shown [11]. Not surprisingly, S100A1 deficient mice display reduced stimulus-induced endothelium-dependent vasorelaxation and are hypertensive [10,42], similar to eNOS deficient mice [43,44].
Activity of eNOS is enhanced by increased Ser-1177 phosphorylation by Akt [45], and possibly other kinases [24] and decreased by phosphorylation of Thr-495 [28]. Pointing towards a mechanism revealing how S100A1 may control eNOS activity, we have shown that siRNA knockdown-induced loss of S100A1 in ECs leads to increased PKC activity and phosphorylation of eNOS on the inhibitory Thr-495 site, with resultant loss of stimulus-induced NO generation [11].
Overall expression levels of S100A1 are down-regulated in several pathologies linked to tissue malperfusion. Patients in heart failure [9], as well as those with critical limb ischemia [11] show reduced myocardial and gastrocnemius muscle S100A1 levels, respectively. The ECs of these tissues appear especially susceptible to hypoxia-induced loss of S100A1 [11]. We recently identified miR-138 as the hypoxia-induced negative regulator of S100A1 in microvascular ECs [18]. In this cell type, the rapid induction of miR-138 by hypoxia critically depends on the stabilization of HIF1-α [18]. Although most prominently regulated by the action of oxygen-sensing prolylhydroxylases (PHDs), stability of the limiting α-subunit of HIF1 can also occur under normoxic conditions (see [46] for review), and has been demonstrated in response to pro-inflammatory cytokines, including angiotensin II and TNF in different cell types [14,26,27] and by endothelin-1 in lymphatic ECs [25]. Here we find that at least 3 different vasoactive pro-inflammatory cytokines (Ang II, Et-1 and TNF) are able to increase stability of HIF1-α in primary human microvascular ECs with consequent induction of miR-138, followed by down-regulation of S100A1 and resultant loss of eNOS activity.
We found that even basal NO generation in HMVECs is strongly reduced upon cytokine treatment, with complete loss of stimulus-induced NO generation (Fig. 1B), while inhibition of miR-138 restores normal S100A1 expression to HMVECs (Fig. 3B) and, in turn, is capable of restoring both basal and VEGF-induced levels of NO generation to HMVECs (Fig. 3C). This occurs even in the continued presence of cytokines, and coincides with reduced phosphorylation of the inhibitory Thr-495 site of eNOS (Fig. 3B), an event we have shown to correlate with restored S100A1 function in ECs [18].
Finally our study demonstrates the importance of miR-138 induced loss of S100A1, and subsequent eNOS inhibition, to the maintenance of cytokine-induced pro-inflammatory signal transduction in ECs. While, as expected, the immediate angiotensin II-induced activation of the MAPKs p38 and ERK1/2 is not impacted by inhibition of either miR-138 (via antagomir-138) or eNOS (via L-NAME), the long term (post 5h) activation of these signaling intermediates is dependent upon the inactivation of eNOS brought on by the miR-138 induced loss of S100A1, as phosphorylation of ERK1/2 or p38 is abrogated at these later timepoints upon miR-138 inhibition but restored when eNOS is inhibited (Fig. 4B). These studies may provide an explanation for the deleterious effects of chronic, rather than acute, cytokine exposure in ECs.
Taken together our study provides a compelling rationale to further investigate the therapeutic potential of selective miR-138 inhibition for the treatment of cardiovascular pathologies linked to endothelial dysfunction.
Supplementary Material
Highlights.
Pro-inflammatory cytokines activate HIF1-α in endothelial cells.
Activation of HIF1-α induces microRNA-138 and consequent loss of S100A1.
Loss of S100A1 inactivates eNOS to cause EC dysfunction.
Inhibition of miR-138 restores S100A1 expression and reverses EC dysfunction.
Funding sources
This work was funded by National Institutes of Health grants R01 HL 072842 (KP), R01 HL 092130-01 and HL 092130-02S1 (PM and KP), and grants from the Deutsche Forschungsgemeinschaft (562, PM) and the German Cardiovascular Research Center (DZHK (German Center for Cardiovascular Research), Partner site Heidelberg/Mannheim, Heidelberg UniversityHospital, Heidelberg University, Germany (PM).
Abbreviations
- Ang II
Angiotensin II
- Akt
v-Akt murine thymoma viral oncogene homolog
- BH4
(6R-)5,6,7,8-tetrahydro-L-biopterin
- CaM
calmodulin
- CLI
critical limb ischemia
- EC
endothelial cell
- Et-1
Endothelin-1
- eNOS
endothelial nitric oxide synthase
- FAD
flavin adenine dinucleotide
- FMN
flavin mononucleotide
- GM
gastrocnemius muscle
- HIF1-α
hypoxia inducible factor 1 alpha
- HMVEC
human microvascular endothelial cell
- NADPH
nicotinamide-adenine-dinucleotide phosphate
- PKC
protein kinase C
- SKO
S100A1 knockout
- TNF
tumor necrosis factor-alpha
- VEGF
vascular endothelial growth factor
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–75. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 5.Rudic RD, Sessa WC. Nitric oxide in endothelial dysfunction and vascular remodeling: clinical correlates and experimental links. Am J Hum Genet. 1999;64:673–7. doi: 10.1086/302304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imanishi T, et al. Addition of eplerenone to an angiotensin-converting enzyme inhibitor effectively improves nitric oxide bioavailability. Hypertension. 2008;51:734–41. doi: 10.1161/HYPERTENSIONAHA.107.104299. [DOI] [PubMed] [Google Scholar]
- 7.Rohde D, Ritterhoff J, Voelkers M, Katus HA, Parker TG, Most P. S100A1: a multifaceted therapeutic target in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:525–37. doi: 10.1007/s12265-010-9211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraus C, et al. S100A1 in cardiovascular health and disease: closing the gap between basic science and clinical therapy. J Mol Cell Cardiol. 2009;47:445–55. doi: 10.1016/j.yjmcc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Most P, Remppis A, Pleger ST, Katus HA, Koch WJ. S100A1: a novel inotropic regulator of cardiac performance. Transition from molecular physiology to pathophysiological relevance. Am J Physiol Regul Integr Comp Physiol. 2007;293:R568–77. doi: 10.1152/ajpregu.00075.2007. [DOI] [PubMed] [Google Scholar]
- 10.Pleger ST, et al. Endothelial S100A1 modulates vascular function via nitric oxide. Circ Res. 2008;102:786–94. doi: 10.1161/CIRCRESAHA.108.172031. [DOI] [PubMed] [Google Scholar]
- 11.Most P, et al. S100A1 deficiency impairs postischemic angiogenesis via compromised proangiogenic endothelial cell function and nitric oxide synthase regulation. Circ Res. 2013;112:66–78. doi: 10.1161/CIRCRESAHA.112.275156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17:5085–94. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- 14.Haddad JJ, Land SC. A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1alpha. FEBS Lett. 2001;505:269–74. doi: 10.1016/s0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- 15.van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412:477–84. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–61. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 17.Blouin CC, Page EL, Soucy GM, Richard DE. Hypoxic gene activation by lipopolysaccharide in macrophages: implication of hypoxia-inducible factor 1alpha. Blood. 2004;103:1124–30. doi: 10.1182/blood-2003-07-2427. [DOI] [PubMed] [Google Scholar]
- 18.Sen A, Ren S, Lerchenmüller C, Sun J, Weiss N, Most P, Peppel K. MicroRNA-138 Regulates Hypoxia-Induced Endothelial Cell Dysfunction By Targeting S100A1. PLoS One. 2013;8:e78684. doi: 10.1371/journal.pone.0078684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 20.Drexler H, Hornig B. Endothelial dysfunction in human disease. J Mol Cell Cardiol. 1999;31:51–60. doi: 10.1006/jmcc.1998.0843. [DOI] [PubMed] [Google Scholar]
- 21.Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–4. doi: 10.1161/01.CIR.0000153349.77489.CF. [DOI] [PubMed] [Google Scholar]
- 22.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–47. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 23.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 24.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spinella F, Garrafa E, Di Castro V, Rosano L, Nicotra MR, Caruso A, Natali PG, Bagnato A. Endothelin-1 stimulates lymphatic endothelial cells and lymphatic vessels to grow and invade. Cancer Res. 2009;69:2669–76. doi: 10.1158/0008-5472.CAN-08-1879. [DOI] [PubMed] [Google Scholar]
- 26.Xia L, et al. The TNF-alpha/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis. Carcinogenesis. 2012;33:2250–9. doi: 10.1093/carcin/bgs249. [DOI] [PubMed] [Google Scholar]
- 27.Kosaka T, Miyajima A, Shirotake S, Kikuchi E, Hasegawa M, Mikami S, Oya M. Ets-1 and hypoxia inducible factor-1alpha inhibition by angiotensin II type-1 receptor blockade in hormone-refractory prostate cancer. Prostate. 2010;70:162–9. doi: 10.1002/pros.21049. [DOI] [PubMed] [Google Scholar]
- 28.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr(495) regulates Ca(2+)/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 29.Wimalasundera R, Fexby S, Regan L, Thom SA, Hughes AD. Effect of tumour necrosis factor-alpha and interleukin 1beta on endothelium-dependent relaxation in rat mesenteric resistance arteries in vitro. Br J Pharmacol. 2003;138:1285–94. doi: 10.1038/sj.bjp.0705168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vila E, Salaices M. Cytokines and vascular reactivity in resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1016–21. doi: 10.1152/ajpheart.00779.2004. [DOI] [PubMed] [Google Scholar]
- 31.Shatanawi A, Romero MJ, Iddings JA, Chandra S, Umapathy NS, Verin AD, Caldwell RB, Caldwell RW. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am J Physiol Cell Physiol. 2011;300:C1181–92. doi: 10.1152/ajpcell.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo RW, Yang LX, Li MQ, Liu B, Wang XM. Angiotensin II induces NF-kappa B activation in HUVEC via the p38MAPK pathway. Peptides. 2006;27:3269–75. doi: 10.1016/j.peptides.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima H, Suzuki H, Ohtsu H, Chao JY, Utsunomiya H, Frank GD, Eguchi S. Angiotensin II regulates vascular and endothelial dysfunction: recent topics of Angiotensin II type-1 receptor signaling in the vasculature. Curr Vasc Pharmacol. 2006;4:67–78. doi: 10.2174/157016106775203126. [DOI] [PubMed] [Google Scholar]
- 34.Pernow J, Shemyakin A, Bohm F. New perspectives on endothelin-1 in atherosclerosis and diabetes mellitus. Life Sci. 2012;91:507–16. doi: 10.1016/j.lfs.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 35.Imanishi T, et al. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52:563–72. doi: 10.1161/HYPERTENSIONAHA.108.111120. [DOI] [PubMed] [Google Scholar]
- 36.Bec N, Gorren AFC, Mayer B, Schmidt PP, Andersson KK, Lange R. The role of tetrahydrobiopterin in the activation of oxygen by nitric-oxide synthase. J Inorg Biochem. 2000;81:207–11. doi: 10.1016/s0162-0134(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 37.Brunner K, Tortschanoff A, Hemmens B, Andrew PJ, Mayer B, Kungl AJ. Sensitivity of flavin fluorescence dynamics in neuronal nitric oxide synthase to cofactor-induced conformational changes and dimerization. Biochemistry. 1998;37:17545–53. doi: 10.1021/bi981138l. [DOI] [PubMed] [Google Scholar]
- 38.Klatt P, et al. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J Biol Chem. 1996;271:7336–42. doi: 10.1074/jbc.271.13.7336. [DOI] [PubMed] [Google Scholar]
- 39.Kotsonis P, Frohlich LG, Shutenko ZV, Horejsi R, Pfleiderer W, Schmidt HH. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J. 2000;346:767–76. Pt 3. [PMC free article] [PubMed] [Google Scholar]
- 40.List BM, et al. Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem J. 1997;323:159–65. doi: 10.1042/bj3230159. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abu-Soud HM, Stuehr DJ. Nitric oxide synthases reveal a role for calmodulin in controlling electron transfer. Proc Natl Acad Sci U S A. 1993;90:10769–72. doi: 10.1073/pnas.90.22.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desjardins JF, Pourdjabbar A, Quan A, Leong-Poi H, Teichert-Kuliszewska K, Verma S, Parker TG. Lack of S100A1 in mice confers a gender-dependent hypertensive phenotype and increased mortality after myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1457–65. doi: 10.1152/ajpheart.00088.2008. [DOI] [PubMed] [Google Scholar]
- 43.Shesely EG, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–81. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 45.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuschel A, Simon P, Tug S. Functional regulation of HIF-1alpha under normoxia--is there more than post-translational regulation? J Cell Physiol. 2012;227:514–24. doi: 10.1002/jcp.22798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.