Abstract
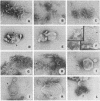
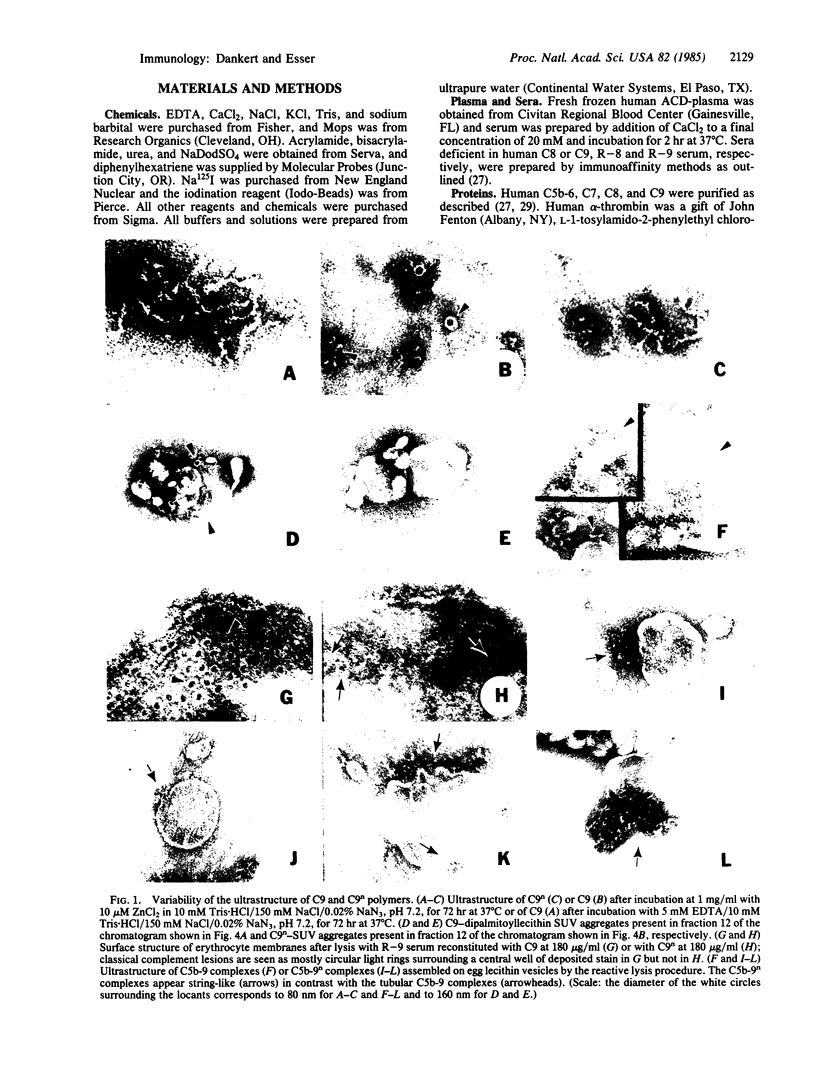
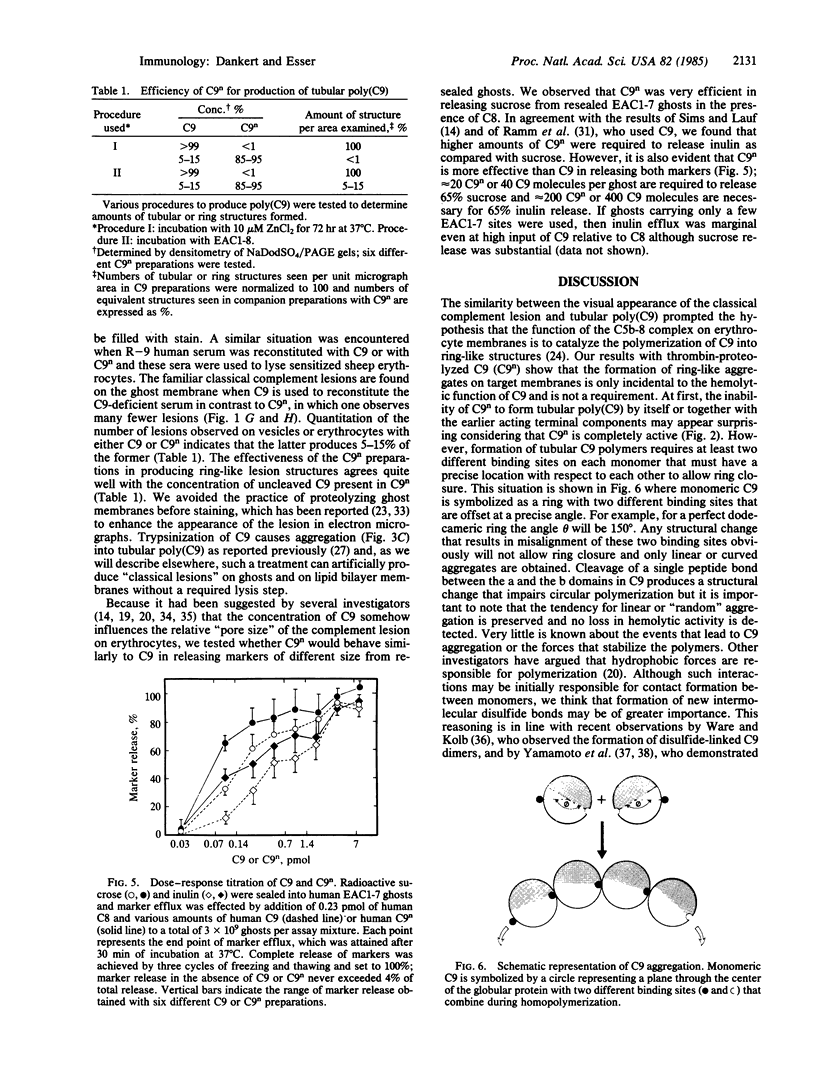
We have compared the ability of thrombin-cleaved C9 (C9n) with that of native C9 to produce tubular or ring-like poly(C9) and to express the classical complement lesion on target membranes. Three procedures were used to produce poly(C9): (i) limited proteolysis with trypsin, (ii) interaction with small unilamellar lipid vesicles, and (iii) incubation with a 2- to 4-fold molar excess of ZnCl2. In contrast to C9, which could be converted to tubular poly(C9), C9n was converted to smaller peptides by the first procedure and was aggregated into string-like poly(C9) by the other two methods. C9-depleted human serum (R-9 serum) was reconstituted with either C9 or C9n and these sera were then used to lyse sensitized sheep erythrocytes. Numerous classical complement lesions could be detected on ghost membranes obtained from cells lysed by C9-reconstituted R-9 serum but only a few on ghost membranes produced by C9n-reconstituted R-9 serum. C9n was shown to be hemolytically as active as C9 even when tested under "single-hit" conditions and it was about twice as efficient when compared with C9 in releasing sucrose and inulin from resealed ghosts. These results are interpreted to indicate that formation of the classical complement lesion is only incidental to lysis and not an obligatory event and that enlargement of the "functional pore size" of the complement lesion is not linked to formation of a circular membrane attack complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORSOS T., DOURMASHKIN R. R., HUMPHREY J. H. LESIONS IN ERYTHROCYTE MEMBRANES CAUSED BY IMMUNE HAEMOLYSIS. Nature. 1964 Apr 18;202:251–252. doi: 10.1038/202251a0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. On the cause and nature of C9-related heterogeneity of terminal complement complexes generated on target erythrocytes through the action of whole serum. J Immunol. 1984 Sep;133(3):1453–1463. [PubMed] [Google Scholar]
- Boyle M. D., Borsos T. The terminal stages of immune hemolysis--a brief review. Mol Immunol. 1980 Mar;17(3):425–432. doi: 10.1016/0161-5890(80)90064-4. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Gee A. P., Borsos T. Studies on the terminal stages of immune hemolysis. VI. Osmotic blockers of differing Stokes' radii detect complement-induced transmembrane channels of differing size. J Immunol. 1979 Jul;123(1):77–82. [PubMed] [Google Scholar]
- Boyle M. D., Langone J. J., Borsos T. Studies on the terminal stages of immune hemolysis. III. Distinction between the insertion of C9 and the formation of a transmembrane channel. J Immunol. 1978 May;120(5):1721–1725. [PubMed] [Google Scholar]
- Dourmashkin R. R. The structural events associated with the attachment of complement components to cell membranes in reactive lysis. Immunology. 1978 Aug;35(2):205–212. [PMC free article] [PubMed] [Google Scholar]
- Esser A. F., Bartholomew R. M., Jensen F. C., Müller-Eberhard H. J. Disassembly of viral membranes by complement independent of channel formation. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5843–5847. doi: 10.1073/pnas.76.11.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser A. F., Kolb W. P., Podack E. R., Müller-Eberhard H. J. Molecular reorganization of lipid bilayers by complement: a possible mechanism for membranolysis. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1410–1414. doi: 10.1073/pnas.76.3.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. Membrane-cytoskeleton interaction. Biochim Biophys Acta. 1983 Aug 11;737(3-4):305–341. doi: 10.1016/0304-4157(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Dourmashkin R. R., Payne S. N., Humphrey J. H., Lachmann P. J. Lesions due to complement in lipid membranes. Nature. 1971 Oct 29;233(5322):620–623. doi: 10.1038/233620a0. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H., Dourmashkin R. R. The lesions in cell membranes caused by complement. Adv Immunol. 1969;11:75–115. doi: 10.1016/s0065-2776(08)60478-2. [DOI] [PubMed] [Google Scholar]
- Ishida B., Wisnieski B. J., Lavine C. H., Esser A. F. Photolabeling of a hydrophobic domain of the ninth component of human complement. J Biol Chem. 1982 Sep 25;257(18):10551–10553. [PubMed] [Google Scholar]
- Kinsky S. C. Antibody-complement interaction with lipid model membranes. Biochim Biophys Acta. 1972 Feb 14;265(1):1–23. doi: 10.1016/0304-4157(72)90017-2. [DOI] [PubMed] [Google Scholar]
- Lachmann P. J., Munn E. A., Weissmanng Complement-mediated lysis of liposomes produced by the reactive lysis procedure. Immunology. 1970 Dec;19(6):983–986. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Thompson R. A. Reactive lysis: the complement-mediated lysis of unsensitized cells. II. The characterization of activated reactor as C56 and the participation of C8 and C9. J Exp Med. 1970 Apr 1;131(4):643–657. doi: 10.1084/jem.131.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K. Immunological and physiological characteristics of the rapid immune hemolysis of neuraminidase-treated sheep red cells produced by fresh guinea pig serum. J Exp Med. 1975 Oct 1;142(4):974–988. doi: 10.1084/jem.142.4.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. M. Complement. Historical perspectives and some current issues. Complement. 1984;1(1):2–26. [PubMed] [Google Scholar]
- Mayer M. M. Mechanism of cytolysis by complement. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2954–2958. doi: 10.1073/pnas.69.10.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Daw R. A., Siddle K., Luzio J. P., Campbell A. K. Immunoaffinity purification of human complement component C9 using monoclonal antibodies. J Immunol Methods. 1983 Nov 25;64(3):269–281. doi: 10.1016/0022-1759(83)90434-9. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. The membrane attack complex. Springer Semin Immunopathol. 1984;7(2-3):93–141. doi: 10.1007/BF01893017. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Konigsberg P. J. Cytolytic T cell granules. Isolation, structural, biochemical, and functional characterization. J Exp Med. 1984 Sep 1;160(3):695–710. doi: 10.1084/jem.160.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podack E. R. Molecular composition of the tubular structure of the membrane attack complex of complement. J Biol Chem. 1984 Jul 10;259(13):8641–8647. [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Membrane attack by complement. Mol Immunol. 1984 Jul;21(7):589–603. doi: 10.1016/0161-5890(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Tschopp J. Polymerization of the ninth component of complement (C9): formation of poly(C9) with a tubular ultrastructure resembling the membrane attack complex of complement. Proc Natl Acad Sci U S A. 1982 Jan;79(2):574–578. doi: 10.1073/pnas.79.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Mayer M. M. Transmembrane channel formation by complement: functional analysis of the number of C5b6, C7, C8, and C9 molecules required for a single channel. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4751–4755. doi: 10.1073/pnas.79.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J. Complement pores in erythrocyte membranes. Analysis of C8/C9 binding required for functional membrane damage. Biochim Biophys Acta. 1983 Aug 10;732(3):541–552. doi: 10.1016/0005-2736(83)90230-4. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Analysis of solute diffusion across the C5b-9 membrane lesion of complement: evidence that individual C5b-9 complexes do not function as discrete, uniform pores. J Immunol. 1980 Dec;125(6):2617–2625. [PubMed] [Google Scholar]
- Sims P. J., Lauf P. K. Steady-state analysis of tracer exchange across the C5b-9 complement lesion in a biological membrane. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5669–5673. doi: 10.1073/pnas.75.11.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P. J. Permeability characteristics of complement-damaged membranes: evaluation of the membrane leak generated by the complement proteins C5b-9. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1838–1842. doi: 10.1073/pnas.78.3.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. L., Monahan J. B., Brickner A., Sodetz J. M. Measurement of the ratio of the eighth and ninth components of human complement on complement-lysed membranes. Biochemistry. 1984 Aug 28;23(18):4016–4022. doi: 10.1021/bi00313a002. [DOI] [PubMed] [Google Scholar]
- Stolfi R. L. Immune lytic transformation: a state of irreversible damage generated as a result of the reaction of the eighth component in the guinea pig complement system. J Immunol. 1968 Jan;100(1):46–54. [PubMed] [Google Scholar]
- Tranum-Jensen J., Bhakdi S., Bhakdi-Lehnen B., Bjerrum O. J., Speth V. Complement lysis: the ultrastructure and orientation of the C5b-9 complex on target sheep erythrocyte membranes. Scand J Immunol. 1978;7(1):45–46. doi: 10.1111/j.1365-3083.1978.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Tschopp J. Circular polymerization of the membranolytic ninth component of complement. Dependence on metal ions. J Biol Chem. 1984 Aug 25;259(16):10569–10573. [PubMed] [Google Scholar]
- Tschopp J., Engel A., Podack E. R. Molecular weight of poly(C9). 12 to 18 C9 molecules form the transmembrane channel of complement. J Biol Chem. 1984 Feb 10;259(3):1922–1928. [PubMed] [Google Scholar]
- Tschopp J., Müller-Eberhard H. J., Podack E. R. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982 Aug 5;298(5874):534–538. doi: 10.1038/298534a0. [DOI] [PubMed] [Google Scholar]
- Tschopp J. Ultrastructure of the membrane attack complex of complement. Heterogeneity of the complex caused by different degree of C9 polymerization. J Biol Chem. 1984 Jun 25;259(12):7857–7863. [PubMed] [Google Scholar]
- Ware C. F., Kolb W. P. Assembly of the functional membrane attack complex of human complement: formation of disulfide-linked C9 dimers. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6426–6430. doi: 10.1073/pnas.78.10.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow M. B., Ramm L. E., Mayer M. M. Penetration of C8 and C9 in the C5b-9 complex across the erythrocyte membrane into the cytoplasmic space. J Biol Chem. 1985 Jan 25;260(2):998–1005. [PubMed] [Google Scholar]
- Yamamoto K., Kawashima T., Migita S. Glutathione-catalyzed disulfide-linking of C9 in the membrane attack complex of complement. J Biol Chem. 1982 Aug 10;257(15):8573–8576. [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Mechanisms for the spontaneous formation of covalently linked polymers of the terminal membranolytic complement protein (C9). J Biol Chem. 1983 Jul 10;258(13):7887–7889. [PubMed] [Google Scholar]