Abstract
Rift Valley Fever is a zoonotic, arthropod-borne disease that affects livestock and humans. The etiologic agent, Rift Valley fever virus (RVFV; Bunyaviridae, Phlebovirus) is primarily transmitted through mosquito bites, but can also be transmitted by exposure to infectious aerosols. There are presently no licensed vaccines or therapeutics to prevent or treat severe RVFV infection in humans. We have previously reported on the activity of favipiravir (T-705) against the MP-12 vaccine strain of RVFV and other bunyaviruses in cell culture. In addition, efficacy has also been documented in mouse and hamster models of infection with the related Punta Toro virus. Here, we challenged hamsters with the highly pathogenic ZH501 strain of RVFV to evaluate the activity of favipiravir against lethal infection. Subcutaneous RVFV challenge resulted in substantial serum and tissue viral loads and caused severe disease and mortality within 2–3 days after infection. Oral favipiravir (200 mg/kg/day) prevented mortality in 60% or greater in hamsters challenged with RVFV when administered within 1 or 6 h post-exposure and reduced RVFV titers in serum and tissues relative to the time of treatment initiation. In contrast, although ribavirin (75 mg/kg/day) was effective at protecting animals from the peracute RVFV disease, most ultimately succumbed from a delayed-onset neurologic disease associated with high RVFV burden in the brain observed in moribund animals. When combined, T-705 and ribavirin treatment started 24 h post-infection significantly improved survival outcome and reduced serum and tissue virus titers compared to monotherapy. Our findings demonstrate significant post-RVFV exposure efficacy with favipiravir against both peracute disease and delayed-onset neuroinvasion, and suggest added benefit when combined with ribavirin.
1. Introduction
Rift Valley fever virus (RVFV), genus Phlebovirus, family Bunyaviridae, is a significant human and veterinary pathogen. RVF is a zoonotic, arthropod-borne disease which causes acute febrile illness in both humans and livestock species. Significant morbidity and mortality associated with the more severe manifestations of disease, coupled with the current absence of licensed therapeutics or vaccines to prevent or treat severe RVFV infection in humans, underline the need to develop antiviral therapies.
Ribavirin is a broad spectrum antiviral that acts as a purine nucleoside analog with activity against several bunyaviral infections including RVFV (Ergonul, 2008; Peters et al., 1986). The principal mechanism of action is not clear, but evidence suggests both direct (polymerase inhibition, RNA capping interference and lethal mutagenesis) and indirect (inosine monophosphate dehydrogenase inhibition and immunomodulatory effects) mechanisms may have roles in ribavirin’s antiviral effects (Graci and Cameron, 2006; Leyssen et al., 2008). However, due to concerns with dose-related hemolytic anemia and possible teratogenic effects, ribavirin is only approved for compassionate use under investigational new drug protocols (Borio et al., 2002; Snell, 2001).
Favipiravir (T-705; 6-flouro-3-hydroxy-2-pyrazinecarboxamine) is a promising pyrazine derivative that has demonstrated potent antiviral activity against multiple RNA viruses (Furuta et al., 2013). Intracellular host enzymes act upon T-705 converting it to its active form, T-705-4-ribofuranosyl-5-triphosphate (T-705RTP) (Naesens et al., 2013). T-705RTP functions as a purine nucleotide analog that selectively inhibits RNA-dependent RNA polymerase (RdRp) of the influenza virus (Furuta et al., 2005; Jin et al., 2013; Sangawa et al., 2013). T-705 has demonstrated a 150-fold weaker inhibition of inosine monophosphate dehydrogenase than ribavirin, and unlike ribavirin it does not interfere with DNA or RNA synthesis. The specificity of T-705 likely contributes to its low toxicity. The compound is presently in clinical development as an influenza inhibitor in Japan (New Drug Application filed) and the United States (Phase 3 clinical trial).
T-705 has demonstrated robust activity against the MP-12 vaccine strain of RVFV in cell culture (Gowen et al., 2007; Gowen et al., 2010). Additionally, the antiviral activity of T-705 in vitro against several other related bunyaviruses (several hantaviruses, La Crosse virus, and Punta Toro and sandfly fever phleboviruses) have been reported (Buys et al., 2011; Gowen et al., 2007; Safronetz et al., 2013). Punta Toro virus (PTV), a more accessible and less biohazardous agent (biosafety level 2; BSL-2) belonging to the same genus as RVFV, has been used to model severe RVFV infection in different animal models (Anderson et al., 1990; Fisher et al., 2003; Pifat and Smith, 1987). Consequently, initial studies evaluated the efficacy of T-705 in PTV infection models (Gowen et al., 2007; Gowen et al., 2010). Based on promising results demonstrating T-705 inhibition of PTV infection in established rodent models and in vitro activity of the compound against the MP-12 strain of RVFV, the current study investigated the efficacy of T-705 against pathogenic ZH501 RVFV infection in golden Syrian hamsters.
2. Materials and Methods
2.1 Virus and cells
RVFV, strain ZH501, was obtained from Dr. Stuart Nichol (CDC, Atlanta, GA). The virus stock (1 passage in BSRT7 cells and 3 passages in Vero E6 cells) was derived from a clarified cell culture lysate and titrated to be at a concentration of 1.1 × 108 plaque-forming units (PFU)/ml. The African green monkey kidney cell line, Vero 76, was purchased from the American Type Culture Collection (ATCC) (Manassas, VA) and maintained in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific HyClone, Logan, UT). For in vitro antiviral assays, the serum was reduced to 2% FBS and gentamicin (Sigma-Aldrich, St. Louis, MO) was added to the medium to a final concentration of 50 µg/ml.
2.2 Compounds
T-705 was provided by the Toyama Chemical Company, Ltd. (Toyama, Japan). Ribavirin was from ICN Pharmaceuticals, Inc. (Costa Mesa, CA). For in vivo studies, both compounds were dissolved in 0.4% carboxymethylcellulose (CMC) (Sigma-Aldrich, St. Louis, MO) prior to administration. For cell culture testing, T-705 and ribavirin were dissolved in MEM containing 50 µg/mL gentamicin.
2.3 In vitro antiviral testing
RVFV was titrated on Vero 76 cells (~80% confluent) plated in 96-well microplates in culture medium containing 2% FBS to a cell culture infectious dose (CCID) that produced maximal cytopathic effects (CPE) in 3 days. Half-log dilutions of T-705 and ribavirin were added in triplicate to test wells at the time of infection with the highest test compound concentrations starting at 1000 µM. For toxicity determinations, drugs were added in the absence of virus infection. Plates were incubated at 37°C and 5% CO2 until virus-infected control wells were observed to have > 90% CPE (day 3). The neutral red (NR) assay to measure cell viability was performed as previously described (Kumaki et al., 2011). Briefly, the supernatants were removed for virus yield reduction (VYR) assays and infected cells and controls were subsequently stained with 0.011% NR solution for 2 h at 37°C and 5% CO2. After incubation, the NR solution was removed, the wells were rinsed with phosphate-buffered saline (PBS), and the incorporated dye extracted using ethanol buffered with Sorenson’s citrate buffer. The plates were read at 405 (primary) and 540 (reference) nanometer wavelengths using a Opsys MR™ microplate reader (Dynex Technologies, Chantilly, VA) to quantitate the extracted NR. The absorbance values were expressed as percentages of untreated, uninfected controls, which took up maximal dye. The values obtained were converted to percentages of untreated, uninfected controls. The 50% cell cytotoxic dose (CC50) and 50% virus effective dose (EC50), representing the concentration at which 50% of the monolayers would show compound cytotoxicity or viral CPE, respectively, were estimated by regression analysis. The selectivity index (SI) was calculated using the formula: SI = CC50/EC50. For the VYR assays, viral titers were determined by endpoint dilution (Reed and Muench, 1938). The VYR data are presented as the concentration of drug that reduced the virus yield by 1 log10 unit (EC90) based on linear regression analysis, with SI values determined as the CC50/EC90.
2.4 Animals and ethics regulation
Female 90–115 g golden Syrian hamsters (The Charles River Laboratory, Willimantic, CT) were quarantined for 7 days prior to challenge and fed standard Harlan lab block and tap water ad libitum. All animal procedures complied with USDA guidelines and were conducted at the AAALAC-accredited Laboratory Animal Research Center at Utah State University under protocol 1502, approved by the Utah State University Animal Care and Use Committee.
2.5 T-705 Dosing optimization for RVFV treatment
To determine the most appropriate dose for subsequent efficacy studies, hamsters (n=15/group) were challenged by subcutaneous (s.c.) injection (ventral, right side of the abdomen) with a 0.1 ml inoculum containing 30 PFU of RVFV and varying doses of oral (p.o.) T-705 were evaluated for efficacy. Treatments, including 75 mg/kg/day of ribavirin (positive control) or 0.4% CMC placebo, were initiated 1 h post-infection (hpi) and administered twice daily for 10 days. Five animals from each treatment group were designated for sacrifice on day 3 of infection for analysis of serum, liver and spleen viral titers. The remaining animals were observed 28 days for morbidity and mortality. Sham-infected normal animals were included as baseline controls for morbidity and mortality (n=3), and virus titer assays (n=3). Serum, brain, liver and spleen samples were collected from two moribund animals with late-onset encephalitic disease for viral titer determination and histopathology.
2.6 Extended post RVFV exposure T-705 efficacy study
Since hamsters begin to succumb to s.c. RVFV challenge within 48 h, we performed a follow-up experiment wherein treatments with the effective dose of T-705 were initiated 1, 6, and 24 hpi. Hamsters (n=14 each for treatment and placebo groups) challenged with 30 PFU of RVFV were dosed orally, twice daily for 14 days, with T-705 (200 mg/kg/day), ribavirin (75 mg/kg/day), or placebo starting 1, 6 or 24 hpi. Four animals from each treatment group were sacrificed on day 2 of infection for analysis of viral titers. The remaining animals were observed 28 days for morbidity and mortality. Sham-infected normal animals were included as baseline controls for survival (n=3) and virus titers (n=3). As done in the first study, serum, brain, liver and spleen samples were collected from several moribund animals with late-onset encephalitic disease for viral titer determination and histopathology.
2.7 Combined post-exposure treatment of RVFV Infection with T-705 and ribavirin
The combined antiviral effects of T-705 and ribavirin were evaluated based on differences in their protective effects observed in the monotherapy studies. Hamsters (n=14 for treatment groups, n=24 for placebo group) were challenged s.c. with 30 PFU of RVFV and T-705, ribavirin and placebo were dosed separately or in combination, as described in Table 1, starting 24 hpi. Four animals from each treatment group were sacrificed on day 2 of infection for analysis of viral titers. The remaining animals were observed 28 days for morbidity and mortality. Sham-infected controls were included for comparison.
Table 1.
Post-exposure T-705 plus ribavirin combination treatment regimens.
| Group | Compounds | High/Low Dosea |
Dosage (mg/kg/day)b | ||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Days 3–10 | |||
| G1 | T-705 | H | 200 | 200 | 200 |
| Ribavirin | H (LD) | 75 | 37.5 | - | |
| G2 | T-705 | H | 200 | 200 | 200 |
| Ribavirin | L (LD) | 40 | 20 | - | |
| G3 | T-705 | L | 100 | 100 | 100 |
| Ribavirin | H (LD) | 75 | 37.5 | - | |
| G4 | T-705 | - | - | - | - |
| Ribavirin | L | 40 | 40 | 40 | |
| G5 | T-705 | H (LD) | 400 | 200 | 200 |
| Ribavirin | - | - | - | - | |
| G6 | Placebo | - | - | - | - |
| Placebo | - | - | - | - | |
H = high, L = low, LD = loading dose.
Hamsters were treated p.o., twice per day, beginning 24 h post-infection.
2.8 Serum, liver, spleen and brain virus titers
Virus titers were assayed using an infectious cell culture assay as previously described (Gowen et al., 2013). Briefly, a specific volume of tissue homogenate or serum was serially diluted and added to triplicate wells of Vero 76 cell monolayers in 96-well microtiter plates. The viral CPE was determined 7 days after plating and the 50% endpoints were calculated as described (Reed and Muench, 1938). The lower limits of detection for virus titers were 1.49 log10 50% cell culture infectious doses (CCID50)/ml serum and 1.97 log10 CCID50/g of tissue, respectively.
2.9 Histopathology
Several moribund animals were discovered between days 7–14 of the dose optimization and post-exposure T-705 efficacy study. These animals were sacrificed for determination of serum, liver, spleen and brain virus titers. In parallel, sections from these tissues preserved in 10% neutral buffered formalin were sent to the Utah Veterinary Diagnostic Laboratory (Logan, UT) for blinded histopathology examination and analysis by a board certified veterinary pathologist.
2.10 Statistical analysis
The Mantel-Cox log-rank test was used for analysis of Kaplan-Meier survival curves. A one-way analysis of variance (ANOVA) with a Newman-Keuls posttest was performed to compare differences in viral loads. All statistical evaluations were done using Prism (GraphPad Software, La Jolla, CA).
3. Results
3.1 In vitro antiviral activity of T-705 against the ZH501 strain of RVFV
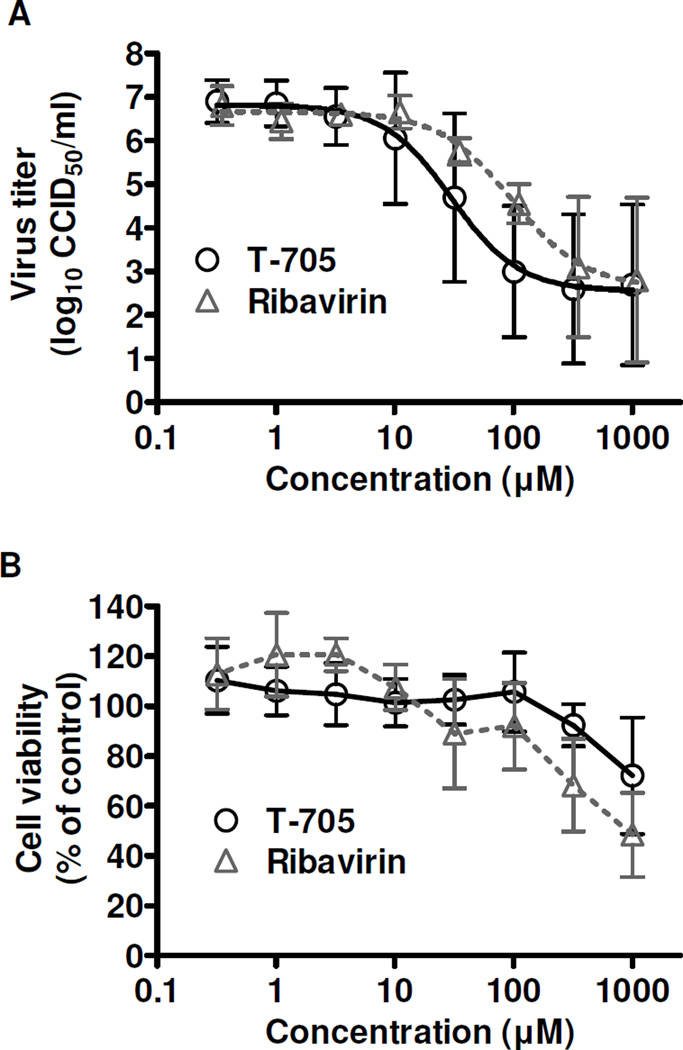
The antiviral activity of T-705 was first evaluated in cell culture against the highly virulent ZH501 strain of RVFV, and included ribavirin for comparison. The CC50 was >1000 µM for both compounds. The inhibitory activity (EC50) against RVFV was 31 µM ± 18 for T-705 and 53 µM ± 22 for ribavirin by NR CPE reduction assay, with SI values of >32 and >19, respectively. The antiviral activity of T-705 was confirmed by measuring reduction in virus yield (Figure 1) by endpoint titration of the day 3 post-infection culture supernatants. The EC90 of T-705 was 11 ± 27 µM and ribavirin’s was 12 ± 9 µM (SI >91 and >83, respectively), consistent with the activity detected by the NR uptake assay. The data is consistent with the previously observed T-705 activity against the MP-12 strain of RVFV (Gowen et al., 2007; Gowen et al., 2010).
Figure 1. In vitro activity of T-705 against the ZH501 strain of RVFV.
Vero 76 cell cultures were infected with RVFV, treated with various concentrations of T-705 or ribavirin, and A) the inhibition of viral replication was determined by endpoint titration of the culture supernatants. B) Cytotoxicity of the compounds was determined by neutral red dye uptake to measure cell viability in cultures of uninfected cells treated in parallel. Cytotoxicity data represent the percent cell viability after a 3-day incubation compared to untreated controls. The data are representative of 3 independent experiments and reflect the mean and standard deviations from triplicate samples.
3.2 In vivo dose optimization of T-705
We next evaluated the antiviral activity of T-705 in hamsters challenged with the ZH501 strain of RVFV. In the initial study, we assessed oral treatments of 200, 60, and 20 mg/kg/day of T-705 administered starting 1 h post s.c. RVFV infection, dosing twice-daily for a duration of 10 days. Ribavirin, previously shown to have activity against RVFV infection (Peters et al., 1986), was included for comparison. As shown in Figure 2A, treatment with 200 mg/kg/day T-705 was the most efficacious regimen, protecting 80% of the challenged animals from mortality. This dose of T-705 was significantly better than the ribavirin treatment (75 mg/kg/day), which only resulted in 20% survival. All the animals in the placebo and 20 mg/kg/day T-705 treatment groups succumbed to infection by day 3. A slight protective effect was observed at a dose of 60 mg/kg/day of T-705, with 20% survival and a slight delay in the mean day of death by approximately 1 day.
Figure 2. T-705 post-RVFV exposure treatment protects hamsters from lethal disease.
Hamsters challenged s.c. with 30 PFU of RVFV-ZH501 received the indicated doses of T-705, ribavirin, or placebo (p.o., twice daily) beginning 1 h post-infection. A) Survival outcome and day 3 B) serum, C) liver, and D) spleen virus titers from animals infected and treated in parallel are shown. All animals from the 20 mg/kg/day T-705 and placebo treatment groups, and one hamster from the 60 mg/kg/day T-705 treatment group, succumbed prior to sacrifice. Unique symbols in each treatment group represent values for the same animal in B-D. For percent survival, **P < 0.01 and ***P < 0.001 compared to placebo; bP < 0.01 compared to animals treated with ribavirin. For viral titers, *P < 0.05 and **P < 0.01 compared to animals treated with 60 mg/kg/day T-705.
The effect of drug treatments on reducing viral titers was evaluated on day 3 in hamsters infected and treated in parallel to those observed for mortality. Due to the peracute lethality of the RVFV challenge in hamsters, one animal in the 60 mg/kg/day T-705 group, and all animals in the low-dose T-705 and placebo-treated groups, expired prior to the time of sacrifice. Viral titers in the sera, livers, and spleens of hamsters treated with 200 mg/kg/day T-705 were significantly reduced when compared to titers in hamsters treated with 60 mg/kg/day T-705 (P < 0.05) (Figure 2B-D). Ribavirin had a similar effect on viral titers as the 200 mg/kg/day T-705 treatment. Although no virus was detected in the spleens of any RVFV-challenged hamsters treated with ribavirin, the difference was not significant compared to the high-dose T-705 group (Figure 2D).
In addition to the day 3 virus titer data, two animals in the ribavirin-treated group were found in a moribund state on day 9 post-infection and sacrificed for analysis of serum, liver, spleen, and brain virus titers, and histopathology. Remarkably, there was no virus present in the serum, liver, or spleen; however, approximately 8.6 log10 CCID50/g of tissue was present in the brains of both animals (Figure 3A), indicating that these animals were deteriorating due to virus replication in the brain and the associated late-onset encephalitis. This was corroborated by histopathologic analysis which revealed neutrophilic and lymphocytic meningoencephalitis of variable severity in the brains of both animals (Figure 3B, C).
Figure 3. Sub-acute central nervous system RVFV infection in ribavirin-treated animals that survive the acute disease.
Hamsters were treated as described in Figure 1. Two animals in the ribavirin-treated group were found to be moribund on day 9 post-infection and were sacrificed for analysis of serum, liver, spleen, and brain virus titers, and histopathology. A) Analysis of viral titers in moribund RVFV-infected hamsters treated with ribavirin. Histopathologic findings in the cerebrum display B) multifocal neuronal necrosis and neuronophagia and C) neuropil necrosis. H&E staining, bars = 30 µm.
3.3 Extended T-705 post-exposure efficacy
Because most hamsters succumb to RVFV challenge within 48 hpi, we next evaluated the efficacy of the twice-daily 200 mg/kg/day T-705 treatments when delayed until 1, 6, and 24 h post s.c. RVFV infection. As in the initial experiment, animals receiving high-dose T-705 treatments initiated 1 hpi fared significantly better compared to the ribavirin-treated animals (P < 0.001; 70% vs. 10% survival), which survived the acute infection but ultimately succumbed to a late-onset neurologic disease (Figure 4A). T-705 treatment initiated at 6 hpi also demonstrated significant protection from mortality (60% overall survival), with 3 of the 10 animals succumbing from acute systemic infection, and a 4th from late-onset encephalitic disease. Interestingly, ribavirin treatment delayed until 24 hpi performed similarly to treatments initiated at 1 or 6 hpi, suggesting combination therapy with T-705 starting as late as one day after challenge may be an effective strategy for the peracute RVFV hamster infection.
Figure 4. T-705 intervention is effective out to 6 h post-RVFV exposure.
Hamsters were treated with T-705 (200 mg/kg/day), ribavirin (75 mg/kg/day), or placebo, twice-daily p.o. for 14 days beginning 1, 6 or 24 h post-infection (hpi). A) Percent survival and day 2 B) serum, C) liver, and D) spleen virus titers are shown. Five animals from varying placebo groups and one animal from the T-705 24 hpi group succumbed prior to sacrifice. Unique symbols in each treatment group represent values for the same animal in B-D. *P < 0.05 and ***P < 0.001 compared to respective placebo groups; bP < 0.01 and cP < 0.001 compared to animals treated with ribavirin.
The effect of treatments on reducing viral titers on day 2 post-infection is shown in Figure 4B-D; however, many of the placebo animals ultimately expired prior to sacrifice, thereby limiting statistical comparison. T-705 administered at 1 hpi was very effective at limiting viral replication as demonstrated by low or undetectable serum, liver, and spleen viral titers. Most of the animals in the 6 hpi T-705 group had titers considerably higher than the 1 hpi T-705-treated animals, which was remarkable considering 6 out of 10 animals in the observational group survived the challenge, and suggests even a slight reduction in titers or a delay in viral replication may be an important factor for a favorable outcome. In general, a trend of diminished viral titers was observed in the 6 and 24 hpi T-705-treated animals compared to the few placebo animals that could be included in the analysis. Consistent with survival data where ribavirin prevented death due to acute infection, ribavirin-treated animals had the lowest titers at the 6 and 24 hpi treatment groups (Figure 4B-D).
In addition to the day 2 virus titer data, four animals from the ribavirin-treated groups and one animal in the T-705 24 hpi group were found in a moribund state after the first week of the study (day 7–14). These animals were sacrificed for determination of serum, liver, spleen, and brain virus titers, and histopathology. Consistent with the previous experiment and the transition from a systemic hemorrhagic disease to a viral encephalitis disease in mice (Smith et al., 2010), there was no virus present in the serum or spleen and only one animal from the T-705 24 hpi group had detectable virus in the liver (5.97 log10 CCID50/g; data not shown), whereas analysis of brain tissue revealed viral loads between 7.72–9.47 log10 CCID50/g in all moribund animals (Figure 5A).
Figure 5. Analysis of brain viral titers in moribund RVFV-infected hamsters treated with T-705 or ribavirin.
Hamsters were treated as described in Figure 4. Five animals were discovered moribund and euthanized on the indicated day (d) post-infection for analysis of virus titers and histopathology. A) Brain viral titers in moribund RVFV-infected hamsters treated with T-705 (blue symbol) or ribavirin (red symbols). Histopathologic analysis of cerebrums displayed B) perivascular mixed inflammatory cell infiltration, C) neutrophilic choroiditis and ventriculitis, D) neutrophilic meningitis and E) neuropil vasculitis and hemorrhage. H&E staining, bars = 20 µm.
Similar to the observations of the previous study, histopathologic analysis of tissues from the moribund animals suggests that the deterioration of the animals was likely due to a neutrophilic and lymphocytic meningoencephalitis of variable severity (Figure 5B-E). Minimal to no lesions of hepatitis were present in most animals (data not shown). Collectively, the histopathology data is consistent with the notion that death of animals beyond day 7 is primarily due to RVFV replication in the brain. Central nervous system (CNS) infection and associated disease was likely the cause of death in the 29 ribavirin-treated animals that succumbed to the RVFV challenge.
3.4 T-705 and ribavirin combination treatment of RVFV infection
Although ribavirin effectively protected hamsters from the acute disease when initiated 24 hpi, most succumbed from late-developing brain infection and disease. Considering ribavirin’s capacity to provide protection against lethality due to acute disease even when treatment is delayed until 24 hpi, and the encouraging efficacy of T-705 when given early during infection, we evaluated the two compounds as a combination therapy in an effort to provide optimal protection in hamsters challenged with RVFV. Several T-705 and ribavirin regimens (Table 1) were tested with treatment initiated at 24 hpi. Because ribavirin treatment courses commonly employ a “loading dose” strategy (Borio et al., 2002; McCormick et al., 1986), we designed the combination study with this consideration in mind. In addition, a lower dose of ribavirin was also evaluated to address concerns with toxicity (Russmann et al., 2006), since effectively reducing the dose requirement would greatly benefit patients.
As shown in Figure 6A, all combination treatments resulted in significant benefit compared to either drug alone. Treatments for G2 and G3 were the most effective, providing a 40% protection, whereas the treatment for G1 resulted in only 10% survival. Although G1 received the highest effective concentration of both compounds, the G2 and G3 treatments (Table 1) appeared to be superior in that there were ultimately more survivors. Notably, the combination treatment groups were the only ones to have any survivors, as all but one animal (which ultimately succumbed on day 23) from the individual drug treatment groups expired by day 11. Overall, the G2 and G3 combination therapy groups trended towards hamsters surviving longer than the ribavirin 40 mg/kg/day monotherapy.
Figure 6. Combined T-705 and ribavirin therapies significantly improve survival outcome and reduce viral burden when starting treatment 24 h post-RVFV challenge.
Hamsters were treated p.o. with T-705, ribavirin, or a combination of both compounds starting 24 h post-infection (see Table 1 for detailed description of the treatment regimens). A) Percent survival and day 2 B) serum, C) liver, and D) spleen virus titers are shown. One animal each in the T-705 monotherapy and placebo-treated group succumbed prior to sacrifice on day 2. Unique symbols in each treatment group represent values for the same animal for B-D. *P < 0.05, **P < 0.01, and ***P < 0.001 compared to placebo-treated animals; aP < 0.05, bP < 0.01, cP < 0.001 compared to animals receiving T-705 monotherapy; xP < 0.05, zP < 0.001 compared to hamsters treated only with ribavirin. H (high dose), L (low dose), LD (loading dose).
The effect of the drug combinations on reducing viral titers on day 2 post-infection is shown in Figure 6B-D. Consistent with the survival data, a general trend of diminished viral titers was observed in all of the T-705 and ribavirin combination treatment regimens. The G2 combination treatment appeared to be the most effective at reducing viral titers in the serum, liver and spleen when compared to the T-705 monotherapy and placebo treated groups, but it was not significantly better than the ribavirin monotherapy. Collectively, the data indicates that combined oral treatment of T-705 and ribavirin significantly enhance post-exposure efficacy compared to monotherapy with either drug, but the challenge of preventing late-onset encephalitis is still problematic when treatment is postponed until 24 h after RVFV challenge.
4. Discussion
The present study demonstrates for the first time the antiviral activity of T-705 against pathogenic RVFV infection. Despite similar inhibitory concentrations in the cell culture experiments, T-705 was superior to ribavirin in terms of overall survival and preventing late-onset CNS infection in RVFV-infected hamsters. Although a detailed characterization of the hamster model is lacking, similar pathological changes to those described in mice have been reported. The liver is the principal target with severe hepatic disease the likely cause of death during the acute infection with a delayed-onset encephalitic disease developing after the first week (Niklasson et al., 1984; Smith et al., 2010). Hamsters generally succumb within 2–3 days from acute disease, whereas mortality in mice occurs during the first 3–6 days (Ikegami and Makino, 2011). While both both species are highly susceptible to RVFV, neither reproduces the hemorrhagic fever or ocular disease observed in human cases. The rapid progression of disease in hamsters presents challenges in terms of the abbreviated therapeutic window; however, the model is very useful for evaluating experimental therapies in the context of post-exposure intervention and to demonstrate proof-of-concept in a robust small animal model.
It is possible that doses higher than 200 mg/kg/day of T-705 may have achieved complete protection against lethal RVFV challenge in hamsters. The present dose of 200 mg/kg/day is equivalent to a human dose of 27 mg/kg/day based on body surface area conversion (Reagan-Shaw et al., 2008). In the Phase 2 clinical trial evaluating T-705 as an anti-influenza drug, the high dose arm consisted of 2400 mg on day 1 and 1600 mg on days 2–5. Assuming the average weight of the participants was 60 kg, the dosages received were in the range of 40 and 27 mg/kg/day, respectively. Although a 400 mg/kg/day loading dose with subsequent transition to 200 mg/kg/day was evaluated in the 3rd study, the therapeutic window for T-705 in the hamster model appears not to extend much further than beyond 6 h. Considering the excellent tolerability of T-705 in hamsters (LD50 >1500 mg/kg/day) (Gowen et al., 2007), and the severity of the RVFV infection, it may be worthwhile to explore higher daily doses administered three times per day to obtain more consistent therapeutic levels systemically and in tissues.
One of the more remarkable findings from our studies was that although both ribavirin and T-705 had dramatic antiviral effects on RVFV-infected hamsters, their effects on survival were very distinct. T-705 was more effective in terms of protecting the animals from both acute and late-onset CNS disease, but the window for successful post-exposure intervention was limited to approximately 6 h. In contrast, ribavirin was highly effective at protecting all animals from the rapidly overwhelming effects of the acute infection, even when delaying treatment until 24 hpi, whereas only a slight beneficial effect could be observed with T-705 treatments. However, almost all ribavirin-treated animals ultimately succumbed to CNS infection and associated disease in the absence of detectable levels of virus in serum or liver and spleen samples. These findings are consistent with a well characterized mouse RVFV infection model wherein late-onset neuroinvasion and encephalitis was described in animals succumbing during the latter part of the infection (Smith et al., 2010). This secondary disease observed in hamsters could serve as a model for human cases of delayed-onset encephalitis that occur weeks to months after acute RVFV infection (Ikegami and Makino, 2011).
It is unclear why ribavirin is so effective at controlling viral replication and abrogating disease when initiating treatment at an advanced time point (24 hpi) in the peracute infection. Ribavirin may achieve higher concentrations and activation in the primary lymphatic tissues where the virus is likely to initially replicate before seeding the blood and secondary target organs including the liver. We can only speculate as to why T-705 is able to prevent CNS infection, but has a narrower window for intervention. The complexities with the biodistribution of the parent T-705 compound, the efficiency of its intracellular conversion to the active ribofuranosyl triphosphate in various cell types and tissues, and the rate of elimination all probably influence the observed efficacy and the shorter window for effective intervention. T-705 is likely to specifically target the RVFV polymerase directly affecting the virus life cycle by inhibiting viral transcription and replication, whereas ribavirin has multiple modes of action and may prevent RVFV lethality through later acting cumulative effects from depletion of ribonucleotide pools (Leyssen et al., 2008).
Our findings evaluating T-705 and ribavirin as monotherapies provided the rationale for combining the two antivirals with the goal of integrating the beneficial aspects of each independent treatment to extend the therapeutic window to 24 hpi. The results of the drug combination study indicated oral treatment with T-705 and ribavirin significantly enhanced post-exposure efficacy suggestive of synergy, as demonstrated by protection (up to 40% survival) against both the acute hepatic and late-onset encephalitic disease that would otherwise result in death in animals treated with either drug alone. Despite this success, the challenge of preventing late-onset encephalitis when treatment is postponed until 24 h after RVFV infection in hamsters is still largely unmet. Interestingly, our findings hint that the highest dose combination of T-705 and ribavirin was not necessarily optimal (only 10% survival), as treatments where the dose of one of the drugs was lowered resulted in improved (40%) survival outcomes. A comprehensive drug combination matrix would be needed to clearly demonstrate possible antagonism at higher doses, as well as synergy with suboptimal dosing regimens of T-705 and ribavirin.
In summary, post-RVFV exposure treatment of hamsters with T-705 within 6 hpi, or T-705 + ribavirin combination therapy within 24 hpi, significantly improved survival outcome and reduced viral loads in serum and tissues. In fatal cases of RVF, severe hemorrhagic disease manifestations typically lead to death within 3–6 days of the onset of clinical signs of illness (Ikegami and Makino, 2011). Because hamsters succumb to the acute phase of the infection within a day of displaying initial clinical signs (lethargy, hunched posture, and ruffled fur), it is difficult to extrapolate to the human disease. However, considering the rapid progression and lethality of RVFV infection and disease modeled in hamsters, the data presented are certainly encouraging. In view of the failure of ribavirin to prevent CNS infection in mice challenged through the upper respiratory tract (Reed et al., 2013), future studies investigating the efficacy of T-705 against aerosol exposure in rodents is warranted. Ultimately, investigational new drug-enabling efficacy studies in a nonhuman primate model of RVF will be required for advancing the compound towards clinical evaluation.
Highlights.
The efficacy of T-705 was evaluated against RVFV challenge in a hamster model
T-705 treatments significantly reduced mortality and RVFV burden
Ribavirin protects against acute disease but not late-onset neurologic complication
Combined T-705 and ribavirin therapy may offer added protection against infection
ACKNOWLEDGMENTS
We are grateful to Luci Wandersee for excellent technical support.
This work was supported by NIH contract HSN272201000039I. Partial support was provided by NIH grant U54 AI-065357 (Rocky Mountain RCE; J. Belisle, PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GW, Jr, Slayter MV, Hall W, Peters CJ. Pathogenesis of a phleboviral infection (Punta Toro virus) in golden Syrian hamsters. Arch. Virol. 1990;114:203–212. doi: 10.1007/BF01310749. [DOI] [PubMed] [Google Scholar]
- Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O'Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Jr, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- Buys KK, Jung KH, Smee DF, Furuta Y, Gowen BB. Maporal virus as a surrogate for pathogenic New World hantaviruses and its inhibition by favipiravir. Antivir. Chem. Chemother. 2011;21:193–200. doi: 10.3851/IMP1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergonul O. Treatment of Crimean-Congo hemorrhagic fever. Antiviral Res. 2008 doi: 10.1016/j.antiviral.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Fisher AF, Tesh RB, Tonry J, Guzman H, Liu D, Xiao SY. Induction of severe disease in hamsters by two sandfly fever group viruses, Punta toro and Gabek Forest (Phlebovirus, Bunyaviridae), similar to that caused by Rift Valley fever virus. Am. J. Trop. Med. Hyg. 2003;69:269–276. [PubMed] [Google Scholar]
- Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, Nomura N, Egawa H, Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49:981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Bailey KW, Scharton D, Vest Z, Westover JB, Skirpstunas R, Ikegami T. Post-exposure vaccination with MP-12 lacking NSs protects mice against lethal Rift Valley fever virus challenge. Antiviral Res. 2013;98:135–143. doi: 10.1016/j.antiviral.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwell RW. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob. Agents Chemother. 2007;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Smee DF, Morrey JD, Furuta Y. Efficacy of favipiravir (T-705) and T-1106 pyrazine derivatives in phlebovirus disease models. Antiviral Res. 2010;86:121–127. doi: 10.1016/j.antiviral.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T, Makino S. The Pathogenesis of Rift Valley Fever. Viruses. 2011;3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5'-triphosphate towards influenza A virus polymerase. PLoS One. 2013;8:e68347. doi: 10.1371/journal.pone.0068347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki Y, Wandersee MK, Smith AJ, Zhou Y, Simmons G, Nelson NM, Bailey KW, Vest ZG, Li JK, Chan PK, Smee DF, Barnard DL. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antiviral Res. 2011;90:22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyssen P, De Clercq E, Neyts J. Molecular strategies to inhibit the replication of RNA viruses. Antiviral Res. 2008;78:9–25. doi: 10.1016/j.antiviral.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, Belmont-Williams R. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- Naesens L, Guddat LW, Keough DT, van Kuilenburg AB, Meijer J, Vande Voorde J, Balzarini J. Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir) Mol. Pharmacol. 2013;84:615–629. doi: 10.1124/mol.113.087247. [DOI] [PubMed] [Google Scholar]
- Niklasson BS, Meadors GF, Peters CJ. Active and passive immunization against Rift Valley fever virus infection in Syrian hamsters. Acta Pathol. Microbiol. Immunol. Scand. [C] 1984;92:197–200. doi: 10.1111/j.1699-0463.1984.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Peters CJ, Reynolds JA, Slone TW, Jones DE, Stephen EL. Prophylaxis of Rift Valley fever with antiviral drugs, immune serum, an interferon inducer, and a macrophage activator. Antiviral Res. 1986;6:285–297. doi: 10.1016/0166-3542(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Pifat DY, Smith JF. Punta Toro virus infection of C57BL/6J mice: a model for phlebovirus-induced disease. Microb. Pathog. 1987;3:409–422. doi: 10.1016/0882-4010(87)90011-8. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Reed C, Lin K, Wilhelmsen C, Friedrich B, Nalca A, Keeney A, Donnelly G, Shamblin J, Hensley LE, Olinger G, Smith DR. Aerosol exposure to Rift Valley fever virus causes earlier and more severe neuropathology in the murine model, which has important implications for therapeutic development. PLoS Negl Trop Dis. 2013;7:e2156. doi: 10.1371/journal.pntd.0002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Russmann S, Grattagliano I, Portincasa P, Palmieri VO, Palasciano G. Ribavirin-induced anemia: mechanisms, risk factors and related targets for future research. Curr. Med. Chem. 2006;13:3351–3357. doi: 10.2174/092986706778773059. [DOI] [PubMed] [Google Scholar]
- Safronetz D, Falzarano D, Scott DP, Furuta Y, Feldmann H, Gowen BB. Antiviral Efficacy of Favipiravir against Two Prominent Etiological Agents of Hantavirus Pulmonary Syndrome. Antimicrob. Agents Chemother. 2013;57:4673–4680. doi: 10.1128/AAC.00886-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangawa H, Komeno T, Nishikawa H, Yoshida A, Takahashi K, Nomura N, Furuta Y. Mechanism of Action of T-705 Ribosyl Triphosphate against Influenza Virus RNA Polymerase. Antimicrob. Agents Chemother. 2013;57:5202–5208. doi: 10.1128/AAC.00649-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, Steele KE, Shamblin J, Honko A, Johnson J, Reed C, Kennedy M, Chapman JL, Hensley LE. The pathogenesis of Rift Valley fever virus in the mouse model. Virology. 2010;407:256–267. doi: 10.1016/j.virol.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Snell NJ. Ribavirin--current status of a broad spectrum antiviral agent. Expert Op. Pharmacother. 2001;2:1317–1324. doi: 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]