Abstract
Biodegradable tissue engineering scaffolds are commonly fabricated from poly(lactide-co-glycolide) (PLGA) or similar polyesters that degrade by hydrolysis. PLGA hydrolysis generates acidic breakdown products that trigger an accelerated, autocatalytic degradation mechanism that can create mismatched rates of biomaterial breakdown and tissue formation. Reactive oxygen species (ROS) are key mediators of cell function in both health and disease, especially at sites of inflammation and tissue healing, and induction of inflammation and ROS are natural components of the in vivo response to biomaterial implantation. Thus, polymeric biomaterials that are selectively degraded by cell-generated ROS may have potential for creating tissue engineering scaffolds with better matched rates of tissue in-growth and cell-mediated scaffold biodegradation. To explore this approach, a series of poly(thioketal) (PTK) urethane (PTK-UR) biomaterial scaffolds were synthesized that degrade specifically by an ROS-dependent mechanism. PTK-UR scaffolds had significantly higher compressive moduli than analogous poly(ester urethane) (PEUR) scaffolds formed from hydrolytically-degradable ester-based diols (p < 0.05). Unlike PEUR scaffolds, the PTK-UR scaffolds were stable under aqueous conditions out to 25 weeks but were selectively degraded by ROS, indicating that their biodegradation would be exclusively cell-mediated. The in vitro oxidative degradation rates of the PTK-URs followed first-order degradation kinetics, were significantly dependent on PTK composition (p < 0.05), and correlated to ROS concentration. In subcutaneous rat wounds, PTK-UR scaffolds supported cellular infiltration and granulation tissue formation, followed first-order degradation kinetics over 7 weeks, and produced significantly greater stenting of subcutaneous wounds compared to PEUR scaffolds. These combined results indicate that ROS-degradable PTK-UR tissue engineering scaffolds have significant advantages over analogous polyester-based biomaterials and provide a robust, cell-degradable substrate for guiding new tissue formation.
1. Introduction
Biodegradable scaffolds made from synthetic polymers have been extensively investigated for use in tissue engineering and regenerative medicine. Examples include poly(lactic-co-glycolic acid) (PLGA) [1, 2], poly(ε-caprolactone) (PCL) [3, 4], polyanhydrides (PAA) [5, 6], and polyurethanes [7, 8], all of which have a history of use in products approved by the FDA [9–12]. These materials are applicable for a diverse range of regenerative applications because they offer a high degree of tunability, generate a minimal host inflammatory response, and degrade into non-cytotoxic components [13, 14] that are resorbed and cleared from the body [15, 16].
In situ curing, injectable scaffolds such as poly(ester urethanes) (PEURs) that support cellular infiltration and degrade into non-toxic breakdown products represent a particularly promising class of biomaterials [17]. Porous PEUR scaffolds are formed by mixing hydroxyl-functionalized polyols (e.g., 900 g mol−1 triols comprised of caprolactone, glycolide, and D,L-lactide) [13] with isocyanate-functional precursors to form a crosslinked network. Water can be added as a blowing agent to create an inter-connected pore structure, and the mechanical, chemical, and degradation properties of the scaffold can be modified through the selection of the polyol and isocyanate precursors [18, 19]. Unlike many other techniques used for fabrication of porous scaffolds, this approach does not require a porogen leaching step. This in situ foaming method, combined with the relatively short working time of the reactive liquid mixture [20], renders PEURs useful as injectable and settable scaffolds suitable for minimally invasive procedures in the clinic.
PEUR scaffolds are primarily degraded by acid-catalyzed hydrolysis of ester bonds in the amorphous soft segment, resulting in chain scission and formation of hydroxyl and carboxylic acid end groups. Residual carboxylic acids in the polymer reduce the local pH at later stages of degradation [21, 22], thereby catalyzing accelerated hydrolysis of the polymer [23]. As the polymers degrade, low molecular weight and soluble α-hydroxy acids diffuse from the scaffold into the medium, resulting in mass loss. Although α-hydroxy acids are non-toxic and can be cleared from the body [13, 24], autocatalytic degradation of the PEUR network driven by residual carboxylic acid groups can result in a mismatch in the rates of scaffold degradation and tissue in-growth that leads to resorption gaps and compromised tissue regeneration [25].
Environmentally-responsive polymers have been heavily investigated for the development of smart materials that respond to specific biological stimuli [26]. In particular, biomaterials that degrade by cell-mediated mechanisms, such as materials with protease-cleavable peptides, have been successfully utilized to synthesize environmentally-sensitive nanoparticles [27, 28], hydrogels [29, 30], and polymeric scaffolds [31, 32]. However, it is difficult to establish this approach as a generalizable tissue engineering platform because these peptide sequences are cleaved by specific enzymes that are upregulated in specific pathological environments [33] and feature highly variable levels across patient populations [34]. Also, manufacturing peptides on the scale necessary to fabricate large tissue scaffolds is both expensive and time-consuming with current technology [35]. Development of degradable polymers that can be affordably synthesized in large scales, similar to polyesters, but that target a ubiquitous cell-mediated signal for scaffold degradation may provide a more generalizable and better-performing biomaterial. Scaffolds degraded by cell-generated reactive oxygen species (ROS) are a promising candidate because ROS serve as important biological mediators in many normal biological processes [36], and elevated ROS, or “oxidative stress”, is a hallmark of inflammation and the pathogenesis of myriad diseases [37]. Polymeric biomaterial implants have also been shown to elicit a stable three-fold increase in ROS production at surgery sites over a four week timeframe [38], further highlighting the potential utility of this cell-generated signal as a trigger for material degradation. This has motivated the recent emergence of new classes of ROS-responsive polymer-based nanoparticles [39–44] and development of salt-leached, porous scaffolds composed of a combination of the polyester PCL and ROS-sensitive, proline-based peptides [45].
Here we sought to develop a generalizable, cell-degradable polyurethane scaffold formulated from polyols exhibiting ROS-dependent degradation. To do so, we synthesized a class of polyols based on ROS-degradable poly(thioketals). Poly(thioketals) (PTKs) were recently applied for development of orally-delivered nanoparticles that remain stable in transit through the stomach and specifically release their cargo “on demand” at sites of ulcerative colitis [40]. To date, however, this unique polymer chemistry has solely been utilized in targeted nanoparticle drug delivery applications [40, 44]. Herein, we report the development and testing of PTK macrodiols amenable to synthesis of injectable, porous poly(thioketal)-urethane (PTK-UR) tissue engineering scaffolds that are selectively degraded by cell-generated ROS. These fully synthetic, non-peptide based scaffolds have been developed to further explore utilization of an ROS-dependent degradation mechanism in order to yield scaffolds with better matched rates of cellular infiltration and degradation.
2. Methods
2.1. Materials
All chemicals were purchased from Sigma-Aldrich (Milwaukee, WI) except the following. 2-mercaptoethyl ether (MEE), glutaraldehyde, and cobalt chloride were purchased from Fisher Scientific (Pittsburgh, PA), and the tertiary amine catalyst (TEGOAMIN33) was obtained from Goldschmidt (Hopewell, VA). Glycolide and D,L-lactide were obtained from Polysciences (Warrington, PA). Coscat83, an organobismuth urethane catalyst, was supplied by ChasChem, Inc. (Rutherford, NJ). Hexamethylene diisocyanate trimer (HDIt, Desmodur N3300A) was received as a gift from Bayer Material Science (Pittsburgh, PA). Cell culture reagents, including Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were supplied by Gibco Cell Culture (Carlsbad, CA). All materials were used as received unless otherwise indicated.
2.2. PTK dithiol synthesis
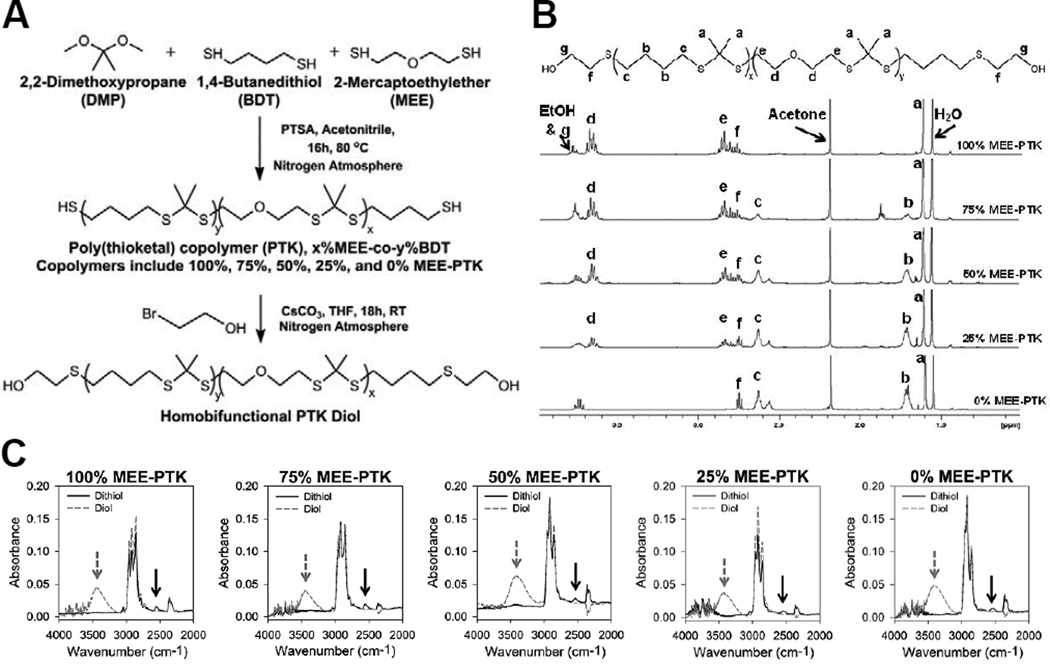
The condensation polymerization protocol for PTK prepolymer synthesis was adapted from Wilson et al. [40]. Briefly, p-toluenesulphonic acid monohydrate (PTSA) was added to a tri-necked boiling flask equipped with an attached addition funnel. The vessels were placed under vacuum for 15 min before being purged with nitrogen. The boiling flask was charged with anhydrous acetonitrile and batch-specific amounts of MEE (x molar eq) and 1,4 butanedithiol (BDT) (1-x molar eq) where x = 1, 0.75, 0.5, 0.25, and 0 for the synthesized PTKs, respectively. The addition funnel was also charged with anhydrous acetonitrile and 2,2-dimethoxypropane (DMP) (0.83 molar eq). A molar excess of dithiol monomers was utilized relative to DMP to ensure the formation of polymers with free terminal thiols. Both the addition funnel and boiling flask’s solutions were purged with flowing nitrogen for 30 min before submerging the boiling flask into an oil bath at 80°C. After 15 min of temperature equilibration, the addition funnel stopcock was set so that the acetonitrile-DMP solution was added drop-wise into the continuously stirring boiling flask over a period of 16 h. Post synthesis, the acetonitrile was removed by rotary evaporation and the resultant PTKs were isolated by precipitation into cold ethanol and dried under vacuum. To evaluate polymer compositions, samples of the respective PTKs were dissolved in deuterated chloroform (CDCl3) and analyzed with 1H nuclear magnetic resonance spectroscopy (NMR, Bruker 400 MHz Spectrometer). 1H NMR chemical shifts were reported as δ values in ppm relative to the deuterated CDCl3 (δ = 7.26). Multiplicities are reported as follows: s (singlet), d (doublet), t (triplet), and m (multiplet). The number of protons (n) for a given resonance is indicated as nH and is based on integration values. 1H NMR (400 MHz, CDCl3): δ = 3.67-3.61 (m, 4H), δ = 2.83 (t, 4H), δ = 2.63 (t, 4H), δ = 1.72 (t, 4H), δ = 1.60 (s, 6H).
2.3. Polyester polyol synthesis
Trifunctional or bifunctional polyester polyols were synthesized as previously documented [13]. To synthesize the trifunctional polyol, glycerol was vacuum dried for 48 h at 80°C and then added to a 100 mL three neck flask. By molar amount, 60% ε-caprolactone, 30% glycolide, and 10% D,L-lactide were added to the glycerol starter along with a stannous octoate catalyst to yield a 900 g mol−1 triol, a 1000 g mol−1 diol, and a 1500 g mol−1 triol.
2.4. PTK hydroxyl functionalization
The hydroxyl-functionalization of the PTK dithiols was completed [46] in order to generate polyols compatible with standard polyurethane synthesis. Briefly, PTK dithiol polymers were transferred to a boiling flask, placed under vacuum, and then exposed to a nitrogen atmosphere. The flask was charged with anhydrous dichloromethane (DCM) before adding a 10x molar excess of β-mercaptoethanol to the solution. This solution was stirred continuously at room temperature to reduce any disulfide bonds and recover the reactive thiol end groups. After 3 h of stirring, the DCM was evaporated and the residue was washed three times in cold ethanol to remove residual β-mercaptoethanol. The reduced PTK polymers were dissolved in anhydrous tetrahydrofuran (THF) before adding a 10x molar excess of cesium carbonate (CsCO3) under nitrogen and stirring for 30 min at room temperature. A 5x molar excess of 2-bromoethanol was next added to the solution and stirred for 18 h under nitrogen at room temperature. After stirring, the solution was added to a separation funnel with an excess of deionized water to effectively separate the PTK-solubilizing THF layer from the water-soluble CsCO3 catalyst. The hydroxyl-functionalized PTKs were extracted in THF before removing the solvent by rotary evaporation, followed by precipitation three times in cold ethanol before vacuum drying for 24 h. Molecular weights and polydispersities of the five synthesized PTK diols were analyzed by gel permeation chromatography (GPC, Agilent Technologies, Santa Clara, CA) using a mobile phase of N,N-dimethylformamide (DMF) with 100mM LiBr. Polymer molecular weights were quantified using a calibration curve generated from poly(ethylene glycol) (PEG) standards (400 – 4000 g mol−1). Hydroxyl-functionalization was confirmed by 1H NMR (400 MHz, CDCl3): δ = 2.74 (t, 4H) and attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR; Bruker Tensor 27 FTIR, Billerica, MA). For ATR-FTIR, thiol-terminated and hydroxyl-terminated PTK polymers were placed in contact with a ZnSe ATR crystal to quantify absorbance at 2550 cm−1 and 3400 cm−1, which correspond to absorbance peaks of free thiol and free hydroxyl groups, respectively. The hydroxyl (OH) numbers of the different PTK diols were determined by titration (Metrohm 798 MPT Titrino, Herisan, Switzerland) according to ASTM E1899 – 08 [47]. Eq (1) was used to relate the molecular weight to the hydroxyl number of each titrated PTK:
| (1) |
where 56,100 represents the molecular weight of KOH in mg/mol,f represents the hydroxyl functionality of the PTK (assumed to be 2 for the linear homobifunctional polymers in this study), and Mn represents the number-average molecular weight of the polymer.
2.5. PTK-UR and PEUR synthesis
The PTK-UR and PEUR scaffolds were prepared using two-component reactive liquid molding of: (a) hexamethylene diisocyanate trimer (HDIt), and (b) a hardener component comprising the PTK diol, 0.5–1.5 parts per hundred parts polyol (pphp) water, 10.0 pphp TEGOAMTN33 catalyst, 0.5 – 3.0 pphp sulfated castor oil stabilizer, and 4.0 pphp calcium stearate pore opener [13]. The makeup of the hardener components for the different respective PTK diols was individually optimized to yield scaffolds with mechanical integrity and an intact porous structure. PEUR scaffolds were respectively designated by their polyester precursor as 900t-PEUR, 1000d-PEUR, and 1500t-PEUR and served as hydrolytically-degradable controls. The hardener component elements were first mixed for 30 s at 3300 rpm in a Hauschild DAC 150 FVZ-K SpeedMixer (FlackTek, Inc., Landrum, SC) before adding the HDIt and mixing for an additional 30 s. This reactive liquid mixture was allowed to rise freely for 10–20 min for complete setting and hardening. The targeted index (ratio of NCO to OH equivalents times 100) was 115, where the number of OH equivalents is calculated from the experimentally measured OH number for the relevant PTK diol.
2.6. Characterization of scaffold physical properties
The core densities of PTK-UR and PEUR scaffolds were determined by measuring the mass and volume of cylindrical porous scaffold core samples, with the core porosities being subsequently calculated from these density values [13]. The porous morphologies of the different PTK-UR scaffolds were qualitatively assessed by scanning electron microscopy (Hitachi S-4200 SEM, Finchampstead, UK). The amount of unreacted components (sol fraction) in the cross-linked network was measured from the mass loss of dried scaffold cylinders (25 mm × 12 mm) previously incubated in DCM for 24 h. To measure the molecular weight between crosslinks (Mc), scaffold samples (n = 3) were weighed dry and then incubated in DCM for 24 h. After incubation, samples were gently blotted to remove excess DCM and then the samples’ swollen mass was measured. These values, along with the solvent parameters, were used in the Flory-Rhener equation to determine Mc. For measuring scaffold hydrophilicity, PTK-UR films of 100%, 50%, and 0% MEE-PTK diols were synthesized using an index of 105 and the gelling catalyst Coscat83 at 1000 ppm. After mixing the catalyst and PTK diol for 30 s at 3300 rpm, HDIt was added and mixed for an additional 30 s. The mixtures were cast into Teflon compression molds and allowed to cure for 18 h at 60°C. The contact angle of water on these PTK-UR films was measured using a Rame-Hart (Mountain Lakes, NJ) Model A-100 contact angle goniometer. A 4 µL water drop was added to the film surface, and after 10 min, an equilibrium contact angle was measured to account for molecular surface reorganization which increased the hydrophilicity at the contact site [48].
2.7. Thermal and mechanical properties
Thermal transitions were measured by a TA Instruments (New Castle, DE) Q200 DSC and Q800 DMA. For DSC analysis, samples ranging in mass from 10–15 mg were heated from −80° C to 200° C at a rate of 10° C min−1, cooled to −80° C at a rate of −20° C min−1, and heated a second time to 200° C at a rate of 10° C min−1. All transitions were obtained from the second heating run. For dynamic mechanical analysis (DMA, Q800 DMA, TA Instruments, New Castle, DE), cylindrical samples (6 × 6 mm) were analyzed from −80° to 55° C at a ramp rate of 1° C min−1. Scaffolds were compressed at a frequency of 1 Hz with 1% strain during the thermal treatment. Glass transitions were obtained at the peak of tan δ.
The mechanical properties of the different PTK-UR and PEUR scaffold formulations were measured in compression at 37°C in a submersion compression clamp using the Q800 DMA. Cylindrical 6 × 6 mm scaffold samples were tested after incubation in phosphate buffered saline (PBS) for 7 days at 37°C. Using a preload force of 0.1 N, samples were compressed along the longitudinal axis at a strain rate of 10% per min until 60% compressive strain was achieved. The Young’s modulus for each sample was calculated from the slope of the initial linear region of each respective stress-strain curve after toe-in.
2.8. In vitro degradation of PTK-UR and PEUR scaffolds
Long-term hydrolytic stability of PTK-UR and PEUR scaffolds was determined by incubating 10 mg samples in PBS at 37°C on a shaker and measuring the mass loss at each time point (n = 3). Before beginning the experiment, scaffolds were soaked in an excess of DCM for 24 h to remove any unreacted components before vacuum drying for 24 h. Scaffold samples were removed from the buffer at each time point, rinsed in deionized water, vacuum dried for 48 h, and weighed. The buffer medium was not changed between time points. Short term oxidative degradation rates of PTK-UR scaffolds were similarly assessed using an oxidative degradation medium that simulates in vivo oxidative degradation at an accelerated rate [49, 50]. This oxidative medium comprised 20 wt% hydrogen peroxide (H2O2) in 0.1 M cobalt chloride (CoCl2), with the H2O2 and cobalt ion reacting to stimulate oxidative radical formation [49]. As with the long-term study, triplicate samples were pre-soaked in DCM for 24 h before vacuum drying and incubated at 37°C in the oxidative medium on a shaker. At specified time points over 10 days, samples were removed, rinsed with deionized water, vacuum dried, and weighed. The oxidative medium was replaced every 3 days, and the morphology of both PBS-incubated and H2O2-incubated scaffolds was qualitatively assessed with SEM.
The effect of radical concentration on PTK-UR scaffold degradation kinetics was also explored. The original 20% H2O2 in 0.1 M CoCl2 degradation medium was diluted ten and one hundred fold to yield a 2% H2O2 in 0.01 M CoCl2 solution and a 0.2% H2O2 in 0.001 M CoCl2 solution. These three degradation media were used to incubate 100%, 50%, and 0% MEE-PTK-UR scaffolds along with 900t-PEUR control samples, with material preparation steps and incubation conditions being the same as previously described.
2.9. Mathematical modeling of PTK-UR oxidative degradation
The degradation behavior of the PTK-UR scaffold formulations were fit to first-order decay kinetics equation to create a mathematical model of scaffold degradation with respect to H2O2 concentration. The first-order degradation model is given in Eq 2.
| (2) |
In this equation, Mt is the scaffold mass remaining at time t, M0 is the initial scaffold mass, and k is the degradation rate constant. Non-linear regression was used to fit this first-order degradation model to the experimentally determined degradation data. This method was used to determine the degradation rate constant k for the scaffolds incubated in the different media.
2.10. In vitro culture of macrophages on PTK-UR scaffolds
RAW 264.7 macrophages were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. 100% and 0% MEE-PTK-UR scaffolds were cut into 6.5 × 1-mm discs, sterilized by UV-radiation for 1 h (30 min per side), placed into 96-well plates, and incubated with culture medium for 30 min. Macrophages were seeded onto the scaffolds at a density of 2.5 × 105 cells/scaffold. The cells were allowed to adhere to the scaffolds for 3 h, at which point the media were removed and the cells were treated with either fresh culture media or activation media containing 5 µg mL−1 lipopolysaccharide (LPS) and 1000 U mL−1 interferon gamma (IFN-γ). Cells were incubated on the scaffolds for 3 days with fresh culture media being applied daily. After the 3 day incubation, the scaffolds were fixed in 5% glutaraldehyde for 2 h followed by 2% osmium tetroxide for 1 h. These fixed scaffolds were dehydrated in ascending grades of ethanol before being vacuum dried, sputter-coated, and imaged with SEM to evaluate surface pitting.
2.11. Cytotoxicity of PTK-UR and PEUR scaffolds
NIH 3T3 mouse fibroblasts stably transfected with a firefly luciferase reporter gene were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. 100% MEE-PTK-UR, 0% MEE-PTK-UR, and 900t-PEUR scaffolds were cut into 6.5 × 1-mm discs, sterilized by UV-radiation for 1 h (30 min per side), placed into a black-walled 96-well plate, and incubated with culture medium for 30 min. Fibroblasts were seeded at a density of 5.0 × 104 cells/scaffold on n=3 scaffolds and allowed to grow for 0, 1, and 3 days in 200 µL of culture media per well (changed every two days). At the endpoint, the cell-seeded scaffolds were treated with culture media containing a luciferin substrate. After 10 min, the scaffolds were imaged with an IVIS 200 (Xenogen, Alameda, CA) bioluminescence imaging system with an exposure time of 2 min to quantify the luciferase-based bioluminescence signal from each scaffold’s viable cell population. All readings were normalized to day 0 bioluminescence values.
2.12. In vivo degradation of PTK-UR scaffolds implanted subcutaneously in rats
All surgical procedures were reviewed and approved by Vanderbilt University’s Institutional Animal Care and Use Committee. 100% MEE-PTK-UR, 0% MEE-PTK-UR, and 900t-PEUR scaffolds were cut into 10 × 2.5 mm discs, sterilized with ethylene oxide, and implanted into ventral subcutaneous sites in adult male Sprague-Dawley rats. Scaffolds were excised from euthanized animals at weeks 1, 3, 5 and 7 to evaluate new granulation tissue formation in the implants. The excised tissues were fixed in formalin, processed, sectioned, and stained with hematoxylin & eosin. Histological sections were evaluated with Metamorph Imaging Software (Molecular Devices Inc., Sunnyvale, CA) to assess wound dimensions and scaffold degradation. The wound area was defined as the cross-sectional area occupied by the scaffold and new tissue growth. Values for the percentage of scaffold area occupying the wound area were normalized to week 1 values to eliminate the effect of scaffold compression over time, and 100% MEE-PTK-UR degradation was fit to the first-order degradation kinetics model seen in Eq. 2.
2.13. Statistical Analysis
All data are reported as the mean and standard error of the mean unless otherwise indicated. Statistical analysis was performed using single factor analysis of variance (ANOVA) and Tukey post-hoc comparison tests, with p-values less than 0.05 considered statistically significant.
3. Results
3.1. PTK polymer synthesis and characterization
Thiol-terminated PTK polymers were successfully synthesized from the condensation polymerization of MEE, BDT, and DMP monomers using PTSA as a catalyst (Figure 1A). Five copolymers were synthesized with varying percent molar composition of MEE and BDT, and each polymer is designated by its relative mol% MEE. 1H-NMR spectra confirmed that the composition of the synthesized polymers closely matched the monomer ratios in the feed (Figure 1B, Table 1), and gel permeation chromatography (GPC) analysis showed that the polymers had Mn values of around 1000 g mol−1 with polydispersity index (PDI) values near 1.35 (Figure S1, Table 1).
Figure 1.
Synthesis and characterization of a family of PTK diols. (A) Scheme for the condensation polymerization of thiol-terminated PTKs and their conversion into PTK diols. (B) 1H-NMR spectra of the PTK copolymer diols. Peaks associated with MEE and BDT monomers closely matched the molar composition used in the polymer feed. (C) ATR-FTIR spectra of thiol- and hydroxyl-terminated PTKs. The thiol absorbance peak is seen at 2550 cm−1 (black arrow) and the hydroxyl absorbance peak is seen at 3400 cm−1 (grey arrow). These spectra demonstrate efficient conversion of PTK terminal thiols into hydroxyls.
Table 1.
Characterization of PTK diols.
| Copolymer (PTK diol) |
Feed MEE% |
Actual MEE%a |
GPC Mnb |
PDIb | Titration Mnc |
|---|---|---|---|---|---|
| 100% MEE-PTK | 100% | 100% | 1027 | 1.38 | 825 |
| 75% MEE-PTK | 75% | 76% | 1005 | 1.34 | 850 |
| 50% MEE-PTK | 50% | 52% | 947 | 1.35 | 810 |
| 25% MEE-PTK | 25% | 26% | 1053 | 1.36 | 745 |
| 0% MEE-PTK | 0% | 0% | 807 | 1.32 | 680 |
Calculated from NMR peaks at δ=1.72 and δ=3.64 ppm.
Calculated from GPC standards.
Calculated from measured titration OH numbers.
Efficient conversion of terminal thiols to hydroxyls was demonstrated by ATR-FTIR. The thiol absorbance peak at 2550 cm−1 was apparent in the thiol-terminated, parent PTKs but did not appear with the hydroxyl-terminated polymers, which generated a characteristic ATR-FTIR hydroxyl peak at 3400 cm−1 (Figure 1C). OH numbers experimentally measured with titration were utilized to calculate a titration Mn (Table 1) that was used to balance the hydroxyl-isocyanate reaction used to form PTK-URs. Consistent with previous findings, the experimental OH numbers trended higher than theoretical values [18].
3.2. PTK-UR scaffold formation and physical properties
PTK-UR scaffolds were successfully synthesized from the PTK diols and HDIt, yielding porous, mechanically robust 3D scaffolds (SEM images shown in Figure S2). PEUR control scaffolds were also successfully formed from HDIt and the three different polyester prepolymers (1000d, 1500t, and 900t). The resulting PTK-UR and PEUR scaffolds possessed similar sol fraction and porosity, as seen in Table 2. The average molecular weight between crosslinks (Mc) for 1000d- and 1500t-PEUR was statistically equal to all of the PTK-UR scaffolds, while the 900t-PEURs had a significantly lower Mc (p < 0.05) relative to all other formulations except for the 100% and 0% MEE-PTK-UR scaffolds (Table 2). The relative surface hydrophilicity of the PTK-UR materials was assessed using contact angle measurements on films, with 100%, 50%, and 0% MEE-PTK-URs having contact angle values of 66°, 77°, and 80°, respectively.
Table 2.
Physical properties of PTK-UR and PEUR scaffolds.
| Scaffold | Sol Fraction (%) |
Core Porosity (vol. %) |
Mc (kg mol−1) |
|---|---|---|---|
| 100% MEE PTK-UR | 6.9%±1.6% | 90.9%±0.4% | 7.6±4.2 |
| 75% MEE PTK-UR | 8.4%±1.4% | 89.0%±1.2% | 10.1 ±4.9 |
| 50% MEE PTK-UR | 9.7%±6.1% | 86.9%±1.4% | 13.8±6.5 |
| 25% MEE PTK-UR | 9.1 %±2.7% | 90.6%±1.5% | 9.0±5.0 |
| 0% MEE PTK-UR | 8.3%±3.2% | 88.8%±1.4% | 9.0±5.8 |
| 900t PEUR | 4.1%±1.6% | 89.8%±1.2% | 2.5±1.6 |
| 1500t PEUR | 4.7%±0.1% | 91.3%±0.2% | 13.2±5.4 |
| 1000d PEUR | 7.7%±0.1% | 92.7%±0.7% | 7.7±2.8 |
All values presented as mean ± standard deviation
3.3. Thermal and mechanical analysis of PTK-UR scaffolds
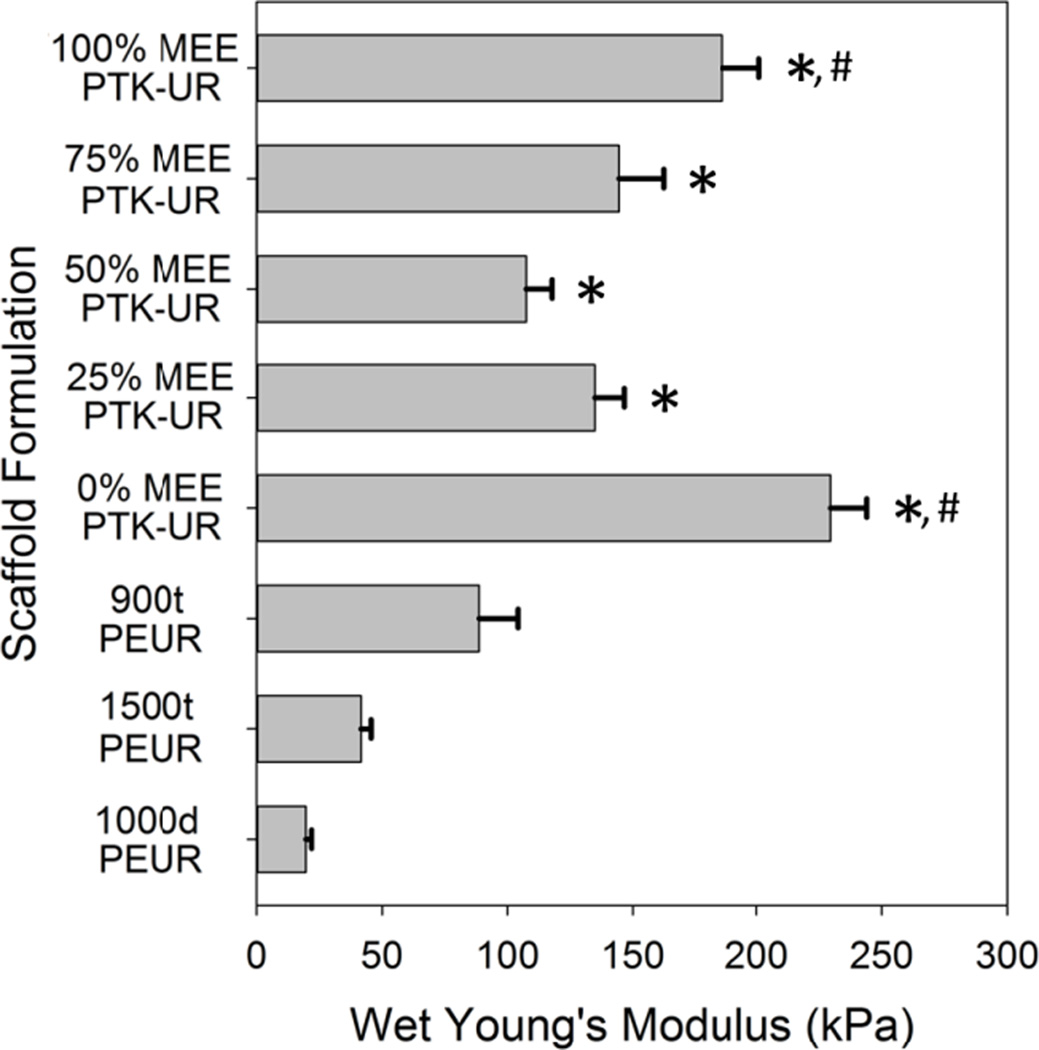
The glass-transition temperature (Tg) of PTK polyols was determined by differential scanning calorimetry (DSC), and the Tg of the PTK-UR scaffolds was measured by DSC and dynamic mechanical analysis (DMA) (Table S1). The wet compressive moduli of the PTK-UR scaffolds ranged from 100 – 250 kPa, and the PEUR moduli ranged from 20 – 100 kPa (Figure 2). All the PTK-UR formulations had significantly higher modulus values than the 1500t-PEUR and 1000d-PEUR materials, while the lower Mc 900t-PEUR scaffolds possessed stiffness values closer to the PTK-UR samples. However, even this more tightly crosslinked formulation was significantly less stiff than the 100% and 0% MEE-PTK-UR materials.
Figure 2.
PTK-UR and PEUR scaffold mechanical properties. The compressive moduli of porous scaffolds were determined under aqueous conditions at 37°C. * p < 0.05 compared to 1500t- and 1000d-PEUR. #p < 0.05 compared to 900t-PEUR.
3.4. In vitro degradation of PTK-UR scaffolds under aqueous and oxidative conditions
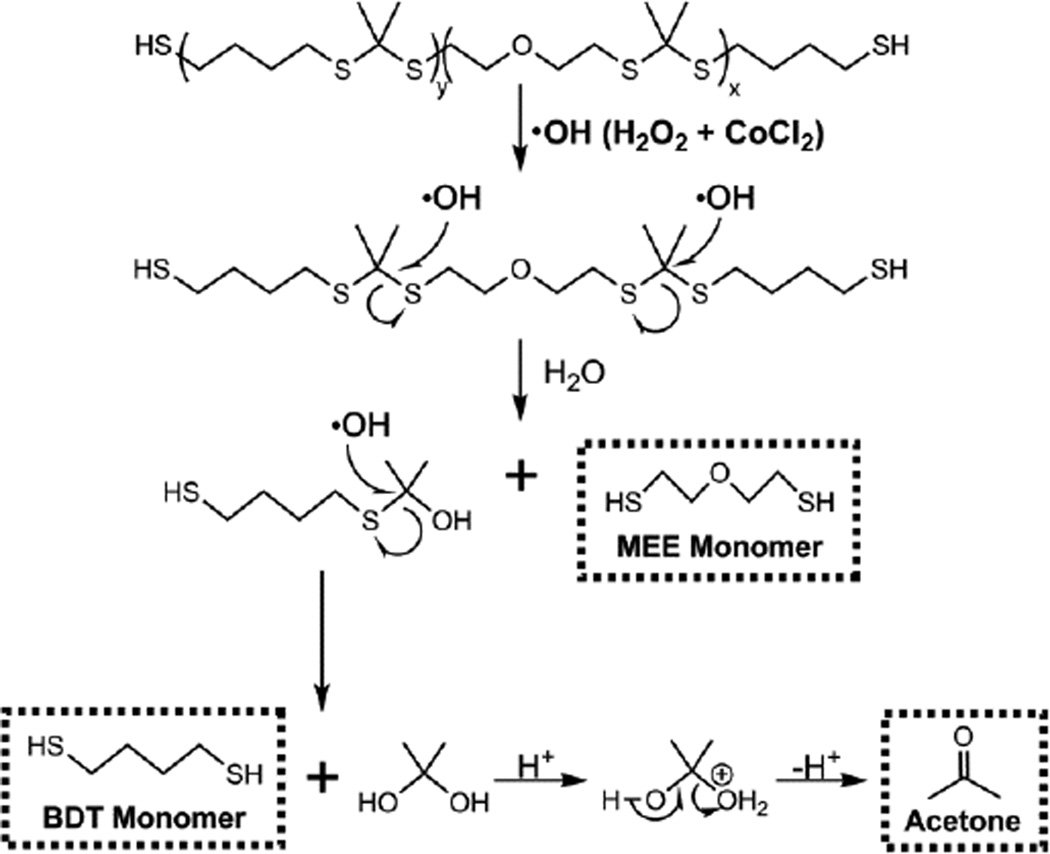
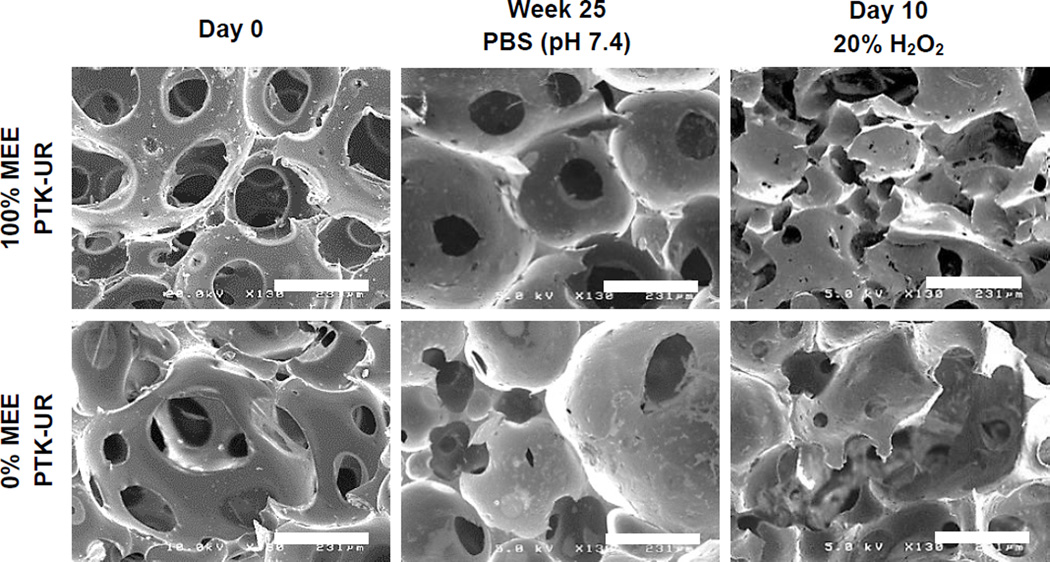
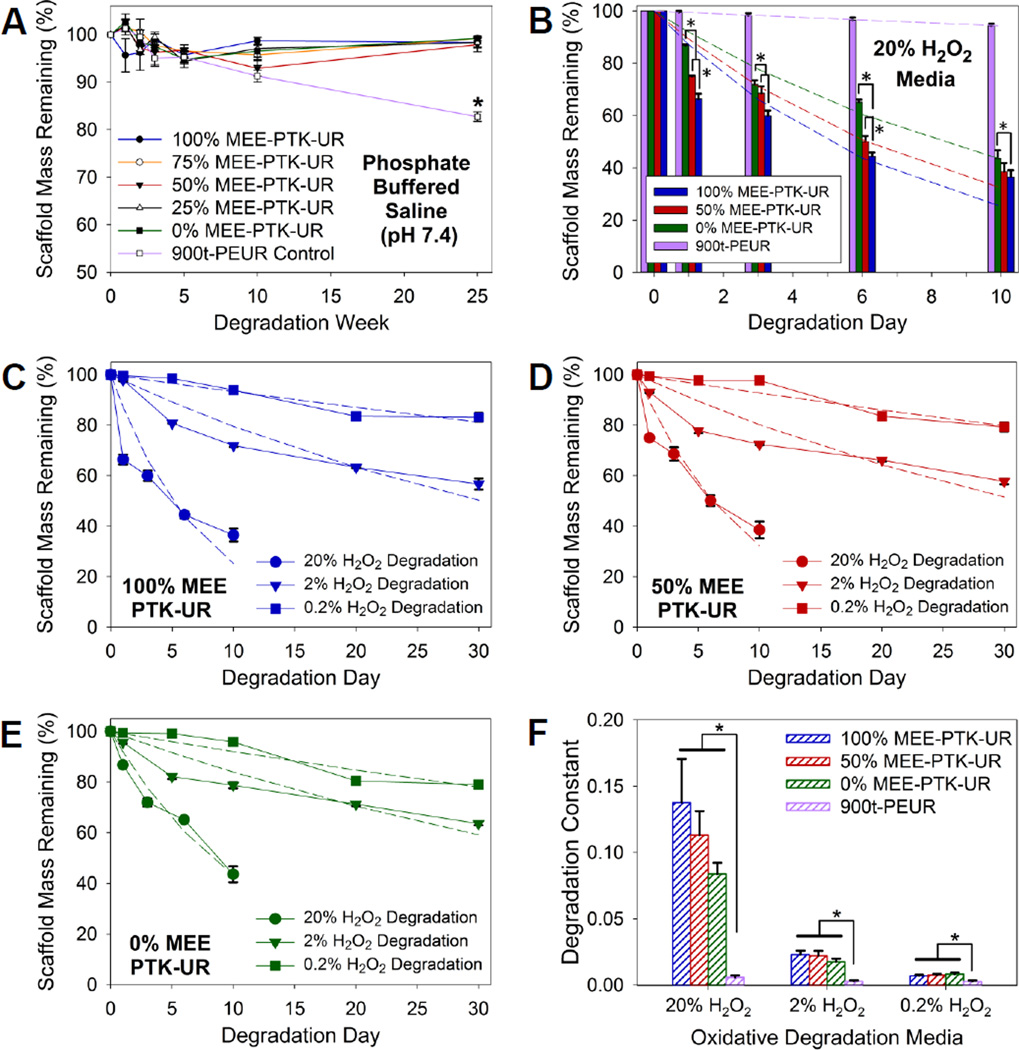
The hypothesized oxidative degradation mechanism of PTK copolymers is seen in Figure 3. Qualitative PTK-UR degradation was demonstrated by SEM as scaffolds incubated for 10 days in oxidative media illustrated loss of porous architecture and surface pitting, while these morphological changes in scaffold architecture were not apparent following PTK-UR scaffold incubation in PBS for 25 weeks (Figure 4 and S2). The PTK-UR scaffolds were stable over a long-term, 25-week study in PBS at 37°C, while the 900t-PEUR scaffolds underwent significant hydrolytic degradation over this time period (Figure 5A). Conversely, the PTK-URs rapidly degraded under accelerated oxidative conditions (20% H2O2 in 0.1 M CoCl2) as seen in Figure 5B. The comprehensive temporal degradation data for all PTK-UR formulations tested in the 20% H2O2 media are displayed in Figure S3.
Figure 3.
Proposed mechanism for hydroxyl radical degradation of PTK polymers.
Figure 4.
Representative SEM images of in vitro PTK-UR degradation. Freshly made scaffolds (left column), scaffolds incubated in PBS for 25 weeks (middle column), and scaffolds incubated in 20% H2O2 media for 10 days (right column). Scale bars = 231 µm. The ROS-degraded PTK-URs feature more irregular pore morphology and surface pitting while PBS-incubated scaffolds appear unchanged relative to freshly made scaffolds.
Figure 5.
In vitro degradation kinetics of PTK-UR scaffolds. Data are presented as mean ± standard error with n = 3. (A) Long-term stability of PTK-UR scaffolds incubated in PBS. (B) Percent degradation of PTK-UR scaffolds incubated in oxidative medium (20% H2O2 in 0.1M CoCl2) as a function of PTK composition. Dashed lines represent model-generated curves for first-order degradation kinetics, *p < 0.05. Percent mass remaining of (C) 100% MEE-PTK-UR, (D) 50% MEE-PTK-UR, and (E) 0% MEE-PTK-UR scaffolds incubated in oxidative media containing 20%, 2%, and 0.2% H2O2. (F) Degradation constants used to generate the best-fit curves in (B-E), as determined by non-linear regression analysis. The PTK-UR but not the PEUR scaffolds exhibited H2O2 dose-dependent degradation.
3.5. Mathematical model of ROS-dependent PTK-UR scaffold degradation
To further elucidate the relationship between ROS concentration and the degradation rates of the different PTK-UR scaffold formulations, degradation was measured in oxidative media comprising 20%, 2%, and 0.2% H2O2 and 0.1, 0.01, and 0.001 M CoCl2, respectively. The degradation rates of PTK-UR scaffolds were dependent on the concentration of H2O2 (Figure 5C–F). The mass loss profiles of the PTK-UR scaffolds were fit to first-order degradation kinetics (Eq. 2) to mathematically model the process of scaffold degradation with respect to H2O2 concentration. The model-generated degradation profiles are concurrently shown with the respective experimental data as dotted lines in Figure 5B–E, with the derived degradation rate constants being shown in Figure 5F. The 900t-PEUR samples incubated in these same oxidative media did not display significant degradation over the same timeframe (Figure 5F and S4).
3.6. In vitro cell-mediated degradation and cytocompatibility of PTK-UR scaffolds
100% and 0% MEE-PTK-UR scaffolds were seeded with murine-derived RAW 267.4 macrophages. Seeded cells were treated with either control culture media or macrophage-activating media containing LPS and IFN-γ. Qualitative SEM imaging of scaffolds after 3 days illustrated potential surface pitting by activated macrophages, but cell-mediated scaffold degradation was not as apparent for the scaffolds seeded with non-activated cells (Figure S5A).
NIH 3T3 mouse fibroblasts stably transduced to express luciferase were seeded onto 100% MEE-PTK-UR, 0% MEE-PTK-UR, and 900t-PEUR scaffolds, and relative cell number was measured based on luciferase activity over 3 days of culture (Figure S5B). Cell-generated bioluminescent signal was constant over the culture period, and there were no significant differences between the scaffold compositions tested.
3.7. In vivo degradation of PTK-UR scaffolds in a rat subcutaneous wound model
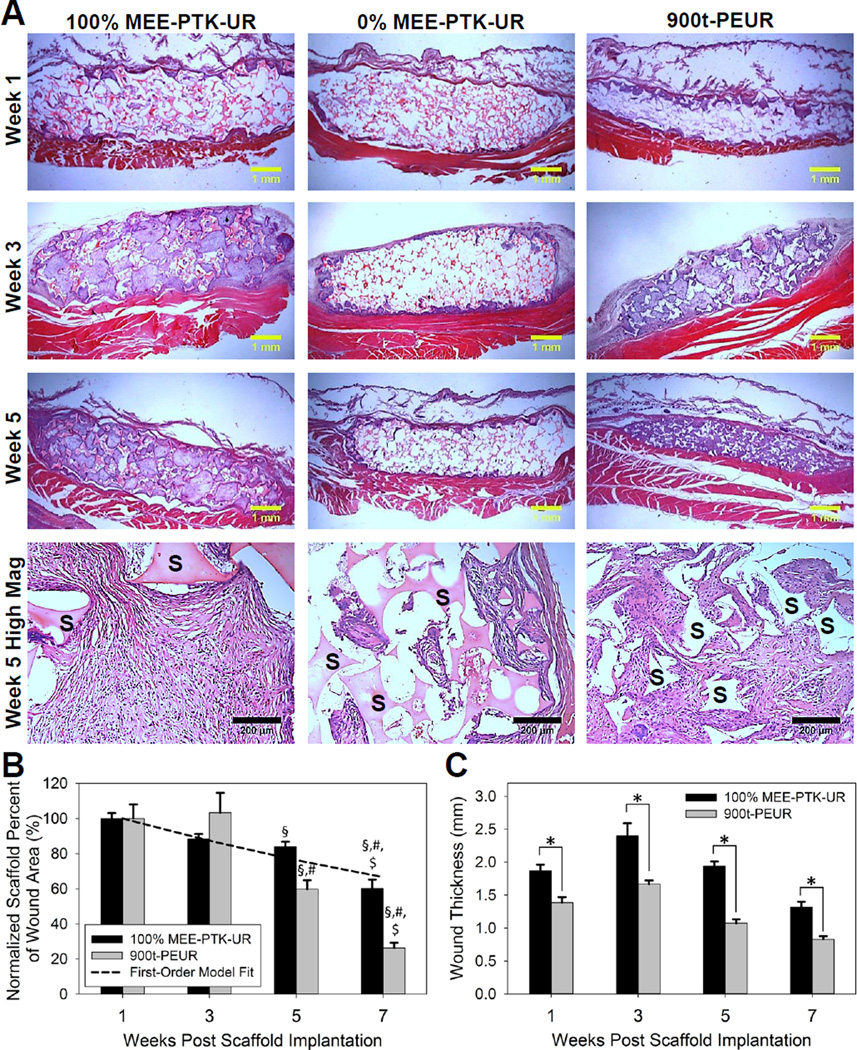
100% MEE-PTK-UR and 900t-PEUR scaffolds subcutaneously implanted into male Sprague-Dawley rats demonstrated robust cellular infiltration, a minimal inflammatory response, and granulation tissue formation by 3 weeks post implantation (Figure 6A). The 0% MEE-PTK-UR materials also provoked a minimal inflammatory response from the native tissue but supported visibly less tissue in-growth and were thus not quantitatively analyzed. Both the 100% MEE-PTK-UR and 900t-PEUR materials displayed significant degradation over 7 weeks (Figure 6B). The 100% MEE-PTK-UR implants followed first-order degradation kinetics (dashed line, Figure 6B) and degraded at a consistent rate up to 40% degradation over 7 weeks. Conversely, the 900t-PEUR scaffolds were 75% degraded at 7 weeks. The PEUR scaffold degradation was slower initially, followed by a more rapid degradation phase between weeks 3–7. The 900t-PEUR scaffolds were also significantly more compressed than the PTK-UR materials, which stented the implant site significantly more than the PEUR scaffolds (Figure 6C). Wound lengths were relatively consistent between PTK-UR and PEUR implant sites over time, while wound area measurements followed trends similar to the scaffold thickness values (Figures S6 and S7).
Figure 6.
In vivo response of subcutaneous PTK-UR scaffolds in Sprague-Dawley rats. (A) Histological illustration of cellular infiltration into PTK-UR and control PEUR scaffolds (residual scaffold material is designated with ‘S’ in the week 5 high magnification panels). The 100% MEE-PTK-URs supported robust tissue in-growth, but the 0% MEE-PTK-UR formulation did not. (B) In vivo scaffold degradation normalized to week 1. Though initially 90% porous, the PEURs were compressed post-implantation and experienced more accelerated degradation after 3 weeks, while the PTK-URs degraded more slowly and with more temporally-constant first-order degradation kinetics (dashed line represents model-generated curve). §p < 0.05 compared to week 1, #p < 0.05 compared to week 3, $p < 0.05 compared to week 5. (C) Compression of PTK-UR vs. PEUR scaffolds in vivo. The PTK-UR scaffolds maintained their mechanical integrity/thickness and provided a greater stenting effect relative to the PEUR implants, *p < 0.05.
4. Discussion
Most currently utilized tissue engineering scaffolds feature hydrolytically degradable ester bonds that nonspecifically degrade in the presence of water. Cleavage of ester bonds produces free carboxylic acids which can acidify the local microenvironment and cause autocatalytic degradation [23], with this accelerated scaffold breakdown leading to reduced tissue regeneration [25]. Here, a PTK-based scaffold technology is presented that is specifically degraded by cell-generated ROS while remaining insensitive to hydrolysis (Figure 5A) [40]. Because these PTK-UR materials selectively degrade by cell-mediated activity, they avoid autocatalytic degradation and are anticipated to yield better matched rates of cellular infiltration and scaffold degradation. To this end, PTK copolymers were successfully synthesized with varying chain compositions but similar Mn and PDI values (Figure 1, Table 1). The Mn and PDI values observed here are both lower than for PTK polymers reported by Wilson et al. In this previous study, the authors utilized an excess of the DMP monomer with respect to the dithiol monomers to achieve a higher degree of polymerization [40]. In the current work, an excess of the dithiol monomer was used to ensure that the resulting polymers possessed telechelic dithiol chain ends that could be subsequently converted to hydroxyls and utilized for polyurethane formation. This modification potentially limited the step-wise growth of the PTK chain, leading to lower Mn. As part of the polymer purification in the current studies, the PTK polymers were washed with ethanol which presumably preferentially removed both unreacted monomer and lower molecular weight PTKs, leading to isolation of a larger molecular weight population of PTKs with lower polydispersity relative to the crude product. The resulting dithiol-terminated MEE-PTK polymers were converted into diols to generate telechelic end groups compatible with standard polyurethane synthesis and to provide PTK polyols amenable to direct comparison with polyesters used in PEUR scaffold formation.
The PTK-UR scaffolds were fabricated using HDIt and compared to PEUR scaffolds made from 900t, 1000d, and 1500t polyester-based PEUR scaffolds. The 900t-PEUR represented a biological control that has been successfully used for in vivo applications [19, 51, 52] while the 1000d-PEUR and 1500t-PEUR were synthesized for a more direct material comparison to the PTK-URs because they yield PEUR scaffolds with crosslink densities that are more similar to the PTK-UR scaffolds. The PTK-UR scaffolds produced from the PTK macrodiols were approximately 90% porous and were morphologically similar to more conventional PEUR 3D porous scaffolds. This level of porosity is optimal for promoting cellular in-growth, nutrient exchange, and neo-vascularization in tissue engineering applications [53–55]. The PTK-URs also featured relatively low sol fraction values, indicating that the isocyanates and diols were well matched and efficiently reacted during scaffold formation. As expected, the scaffolds’ relative hydrophilicity was influenced by the composition of the PTK polyol, and the contact angle was inversely correlated with the mol% of the more hydrophilic MEE monomer in the PTK copolymer. These data suggest that the 100% MEE-PTK-UR with a contact angle of 66° may be optimal for cellular adhesion and tissue formation in vivo, since more hydrophobic surfaces with contact angles > 76° (such as the 50% and 0% MEE-PTK-UR formulations) preferentially adsorb hydrophobic serum proteins such as albumin over cellular adhesion proteins like fibronectin and vitronectin [56, 57].
Thermal analysis of PTK-UR and PEUR scaffolds, along with their polymeric precursors, indicated that the scaffolds are phase-mixed materials since the 3D materials all possessed a Tg exceeding that of the polyol precursor soft segment [18]. The scaffold Tg values determined by DMA also exceeded those measured by DSC by 30 – 50°C, as has been previously reported for similar 3D PEUR materials [13]. Wet compression testing of these materials indicated that although the 1500t-PEUR, 1000d-PEUR, and PTK-UR scaffolds had similar Mc values (Table 2), all of the PTK-UR formulations had significantly higher modulus values than the 1500t-PEUR and 1000d-PEUR materials (Figure 2). However, there was no consistent trend between PTK-UR scaffold composition and modulus. Due to its higher crosslink density, the 900t-PEUR achieved stiffness values closer to the PTK-UR samples, though even this formulation was significantly less stiff than the 100% and 0% MEE-PTK-UR materials.
Previous work has demonstrated the selective, ROS-mediated degradation of poly(thioketal) nanoparticles [40]. The PTK-UR scaffolds were formulated with HDIt because it is more oxidatively stable relative to lysine-derived isocyanates [17, 19, 51], allowing more specific study of the degradation behavior of the polyol component. Degradation of PTK-UR and 900t-PEUR scaffolds was tested in an oxidative degradation medium comprising H2O2 and CoCl2 that produces hydroxyl radicals [49]. These radicals destabilize the thioketal bond, leading to chain scission and breakdown into the original constitutive monomers (MEE and BDT) and acetone (Figure 3). It is predicted that these small byproducts would be rapidly cleared and would not cause cytotoxicity in an in vivo environment. This is supported by previous work showing that when incorporated into a similar polyurethane system, MEE monomers cause limited in vitro cytotoxicity [58] and a minimal host inflammatory response in vivo [59].
The long-term stability of PTK-UR scaffolds over 25 weeks in PBS (Figure 5A) was significantly different than these materials’ behavior under accelerated oxidative conditions as seen in Figure 5B, highlighting the ROS-specific degradation mechanism of the PTK-UR scaffolds. Furthermore, there was a relationship between the PTK composition and degradation rate, as the scaffolds with higher MEE content in the PTK polyol degraded faster (Figure 5B). It has been previously reported that ethers are stable in aqueous media but that oxidative radicals can degrade them in vitro and in vivo [49]. Thus, it is hypothesized that the faster ROS-dependent degradation seen in both the 100% and 50% MEE-PTK-UR materials may result from a combination of oxidative degradation of both thioketals and ethers, while the 0% MEE-PTK-UR scaffolds are degraded solely by thioketal scission. These results indicate that ROS-dependent scaffold degradation rates can be tuned by the composition of the PTK polyol.
For all PTK-UR compositions tested, the degradation rate was dependent on ROS concentrations (Figure 5C–E). This dose-dependent relationship between ROS levels and degradation rate coupled with the agreement between the model and experimental data confirm that the PTK-UR scaffolds degrade by first-order kinetics with respect to ROS concentration. The degradation rate constants derived from the non-linear regression fitting of the experimental data gathered in 20% H2O2 media (Figure 5F) also illustrate the relationship between degradation rate and the %MEE-PTK polyol used in PTK-UR scaffold fabrication, though this trend was decreased under lower H2O2 concentrations. In contrast, the 900t-PEUR samples incubated in these same oxidative media did not display H2O2 dose-dependent degradation (Figure 5F and S4), highlighting the unique degradation mechanism of the PTK-UR relative to PEUR scaffolds. These collective data confirm that PTK-based polyols are selectively cleaved by ROS and that their rate of degradation is first-order with respect to the concentration of radical species in the local environment.
PTK-UR scaffolds were shown to display low levels of in vitro cytotoxicity with both RAW 267.4 macrophages and NIH 3T3 fibroblasts. Seeded macrophages were treated with either control culture media or media containing LPS and IFN-γ to activate the macrophages through the classical pathway [60, 61], which is known to lead to ROS production [19, 45]. Scaffolds with activated macrophages displayed low levels of surface pitting while cell-mediated remodeling of the scaffold surface was less evident for the control cells (Figure S5A), potentially indicating that the PTK-UR scaffolds were degraded by physiologically relevant concentrations of ROS. Further highlighting these materials’ limited cytotoxicity, luciferase-expressing fibroblasts seeded on PTK-UR and PEUR scaffolds steadily maintained their bioluminescent signal over the culture period (Figure S5B), similar to cell growth profiles seen in other non-cytotoxic 3D scaffolds [62, 63]. Furthermore, none of the scaffold formulations displayed a significant difference in bioluminescence over time or relative to each other, indicating that PTK-UR scaffolds had cytotoxicity profiles similar to analogous PEUR scaffolds that have been successfully utilized in vivo [19].
The PTK-UR scaffolds’ lack of cytotoxicity and compatibility with cellular infiltration were confirmed in vivo by histological analysis of subcutaneous implants. These studies showed that neither the 100% nor 0% MEE-PTK-UR formulations elicited an inflammatory response from the native tissue that was obviously different from the conventional PEUR scaffolds (Figure 6A). However, the 0% MEE-PTK-UR scaffolds supported much less robust tissue infiltration into the scaffold interior relative to the 100% MEE-PTK-UR or 900t-PEUR scaffolds. One possible explanation for this result is that the relative hydrophobicity of the 0% MEE-PTK-UR scaffolds (80° contact angle) did not allow cells to properly adhere and migrate into the scaffold interior. As a result, only the 100% MEE-PTK-UR and 900t-PEUR histology samples were quantitatively analyzed. Both of these formulations supported new tissue growth into the scaffold interior 3 weeks after implantation and displayed significant biodegradation over 7 weeks (Figure 6B). The 900t-PEURs experienced accelerated degradation after 3 weeks as expected from previous work with these materials [19], while the 100% MEE-PTK-UR scaffolds displayed first-order degradation over time. This finding confirms the initial hypothesis that PTK-UR scaffolds degrade by a cell-mediated mechanism compared to the hydrolytic degradation of the more conventional PEUR materials, which have been recently shown to undergo autocatalytic degradation in vivo resulting in a reduced wound healing response [25]. Furthermore, the PTK-UR samples were more mechanically resilient and were more effective in maintaining implant geometry as seen in Figure 6A and C. Though all scaffolds initially possessed 90% porosity and were cut to the same dimensions preimplantation, the PEUR materials were significantly more compressed than the PTK-UR scaffolds by week 1. As the wound length was relatively consistent between PTK-UR and PEUR scaffolds (Figure S6), the total wound area values closely mirrored the trends seen in the scaffold thickness measurements (Figure S7). This in vivo compression of PEUR scaffolds can be potentially attributed to both the significantly higher modulus of the 100% MEE-PTK-UR samples relative to the 900t-PEUR formulation (Figure 2) and also to the 900t-PEUR Tg value (34.4 °C) which is close to body temperature (Table S1). This relatively high Tg predicts that the PEUR scaffolds would be less mechanically resilient at body temperature because they would be in their glass transition viscoelastic region. The stenting effect seen in these PTK-UR scaffolds is advantageous because it ensures that the scaffold pores remain open, maximizing cell infiltration and new tissue formation and potentially decreasing scarring in clinical applications [64].
5. Conclusions
ROS are key mediators of cell function in both health and disease, especially at sites of inflammation and tissue healing. Utilizing these cell-generated species as triggers for selective polymer degradation represents a promising methodology for creating tissue engineering scaffolds with well-matched rates of tissue in-growth and cell-mediated scaffold degradation. Here, poly(thioketal) polymers featuring tunable chain compositions and ROS-mediated degradation rates have been developed towards this end. These PTK polymers were successfully incorporated into 3D porous tissue engineering scaffolds, generating materials with more robust mechanical properties than similar constructs fabricated from standard polyesters. These PTK-UR scaffolds were selectively degraded by ROS but were stable under aqueous conditions, indicating that their biodegradation was exclusively cell-mediated, as opposed to PEURs that hydrolytically degrade independent of cellular activity. Moreover, the in vitro oxidative degradation rates of the PTK-URs followed first-order degradation kinetics and displayed dose-dependent degradation with respect to ROS concentration. The PTK-UR scaffolds also supported cell infiltration and granulation tissue formation in vivo, and their superior mechanical properties produced significantly greater stenting of subcutaneous implants compared to more standard PEUR scaffolds. Furthermore, the PTK-URs experienced controlled, first-order kinetics of biodegradation in vivo, in contrast to the PEUR scaffolds which experienced accelerated rates of degradation over time. These collective data indicate that PTK-URs represent a useful class of biomaterials that provide a robust, cell-degradable substrate for guiding new tissue formation.
Supplementary Material
Acknowledgements
Funding was provided by the Vanderbilt University School of Engineering, the NIH through grants R21EB012750 and R01AR056138, and the NSF through grant DMR-1006558. The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for these efforts that could have influenced their outcome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whang K, Thomas CH, Healy KE, Nuber G. A novel method to fabricate bioabsorbable scaffolds. Polymer. 1995;36(4):837–842. [Google Scholar]
- 2.Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, Mikos AG. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19(15):1405–1412. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 3.Lowry KJ, Hamson KR, Bear L, Peng YB, Calaluce R, Evans ML, et al. Polycaprolactone/glass bioabsorbable implant in a rabbit humerus fracture model. J Biomed Mater Res. 1997;36(4):536–541. doi: 10.1002/(sici)1097-4636(19970915)36:4<536::aid-jbm12>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Ciapetti G, Ambrosio L, Savarino L, Granchi D, Cenni E, Baldini N, et al. Osteoblast growth and function in porous poly ε-caprolactone matrices for bone repair: a preliminary study. Biomaterials. 2003;24(21):3815–3824. doi: 10.1016/s0142-9612(03)00263-1. [DOI] [PubMed] [Google Scholar]
- 5.Leong KW, Simonte V, Langer RS. Synthesis of polyanhydrides: melt-polycondensation, dehydrochlorination, and dehydrative coupling. Macromolecules. 1987;20(4):705–712. [Google Scholar]
- 6.Ibim SEM, Uhrich KE, Attawia M, Shastri VR, El-Amin SF, Bronson R, et al. Preliminary in vivo report on the osteocompatibility of poly(anhydride-co-imides) evaluated in a tibial model. J Biomed Mater Res. 1998;43(4):374–379. doi: 10.1002/(sici)1097-4636(199824)43:4<374::aid-jbm5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg-AM. 2005;30(2):380–389. doi: 10.1016/j.jhsa.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Gogolewski S, Gorna K, Turner AS. Regeneration of bicortical defects in the iliac crest of estrogen-deficient sheep, using new biodegradable polyurethane bone graft substitutes. J Biomed Mater Res A. 2006;77A(4):802–810. doi: 10.1002/jbm.a.30669. [DOI] [PubMed] [Google Scholar]
- 9.Lü J-M, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, et al. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev Mol Diagn. 2009;9(4):325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezwada RS, Jamiolkowski DD, Lee I-Y, Agarwal V, Persivale J, Trenka-Benthin S, et al. Monocryl suture, a new ultra-pliable absorbable monofilament suture. Biomaterials. 1995;16(15):1141–1148. doi: 10.1016/0142-9612(95)93577-z. [DOI] [PubMed] [Google Scholar]
- 11.Dang W, Daviau T, Brem H. Morphological characterization of polyanhydride biodegradable implant Gliadel during in vitro and in vivo erosion using scanning electron microscopy. Pharm Res. 1996;13(5):683–691. doi: 10.1023/a:1016035229961. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Xu J, Wang A, Zheng M. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devic. 2009;6(1):61–73. doi: 10.1586/17434440.6.1.61. [DOI] [PubMed] [Google Scholar]
- 13.Hafeman AE, Li B, Yoshii T, Zienkiewicz K, Davidson JM, Guelcher SA. Injectable biodegradable polyurethane scaffolds with release of platelet-derived growth factor for tissue repair and regeneration. Pharm Res. 2008;25(10):2387–2399. doi: 10.1007/s11095-008-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ignatius AA, Claes LE. In vitro biocompatibility of bioresorbable polymers: poly(L, DL-lactide) and poly(L-lactide-co-glycolide) Biomaterials. 1996;17(8):831–839. doi: 10.1016/0142-9612(96)81421-9. [DOI] [PubMed] [Google Scholar]
- 15.Hua N, Sun J. Body distribution of poly(D,L-lactide-co-glycolide) copolymer degradation products in rats. J Mater Sci: Mater Med. 2008;19(10):3243–3248. doi: 10.1007/s10856-008-3460-z. [DOI] [PubMed] [Google Scholar]
- 16.Visscher GE, Robison RL, Maulding HV, Fong JW, Pearson JE, Argentieri GJ. Biodegradation of and tissue reaction to 50:50 poly(DL-lactide-co-glycolide) microcapsules. J Biomed Mater Res. 1985;19(3):349–365. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- 17.Guelcher SA. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng Pt B-Rev. 2008;14(1):3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 18.Guelcher SA, Srinivasan A, Hafeman A, Gallagher KM, Doctor JS, Khetan S, et al. Synthesis, in vitro degradation, and mechanical properties of two-component poly(ester urethane)urea scaffolds: effects of water and polyol composition. Tissue Eng. 2007;13(9):2321–2333. doi: 10.1089/ten.2006.0395. [DOI] [PubMed] [Google Scholar]
- 19.Hafeman AE, Zienkiewicz KJ, Zachman AL, Sung H-J, Nanney LB, Davidson JM, et al. Characterization of the degradation mechanisms of lysine-derived aliphatic poly(ester urethane) scaffolds. Biomaterials. 2011;32(2):419–429. doi: 10.1016/j.biomaterials.2010.08.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumas JE, BrownBaer PB, Prieto EM, Guda T, Hale RG, Wenke JC, et al. Injectable reactive biocomposites for bone healing in critical-size rabbit calvarial defects. Biomed Mater. 2012;7(2):024112. doi: 10.1088/1748-6041/7/2/024112. [DOI] [PubMed] [Google Scholar]
- 21.Fu K, Pack DW, Klibanov AM, Langer RS. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm Res. 2000;17(1):100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Peter SJD, Lyman M, Lai H-L, Leite SM, Tamada JA, et al. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21(18):1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 23.Antheunis H, van der Meer J-C, de Geus M, Heise A, Koning CE. Autocatalytic equation describing the change in molecular weight during hydrolytic degradation of aliphatic polyesters. Biomacromolecules. 2010;11(4):1118–1124. doi: 10.1021/bm100125b. [DOI] [PubMed] [Google Scholar]
- 24.An YH, Woolf SK, Friedman RJ. Pre-clinical in vivo evaluation of orthopaedic bioabsorbable devices. Biomaterials. 2000;21(24):2635–2652. doi: 10.1016/s0142-9612(00)00132-0. [DOI] [PubMed] [Google Scholar]
- 25.Dumas JE, Prieto EM, Zienkiewicz KJ, Guda T, Wenke JC, Bible JE, et al. Balancing the rates of new bone formation and polymer degradation enhances healing of weight-bearing allograft/polyurethane composites in rabbit femoral defects. Tissue Eng Pt A. 2014;20(1–2):115–129. doi: 10.1089/ten.tea.2012.0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roy D, Cambre JN, Sumerlin BS. Future perspectives and recent advances in stimuli-responsive materials. Prog Polym Sci. 2010;35(1–2):278–301. [Google Scholar]
- 27.Li H, Yu SS, Miteva M, Nelson CE, Werfel T, Giorgio TD, et al. Matrix metalloproteinase responsive, proximity-activated polymeric nanoparticles for siRNA delivery. Adv Funct Mater. 2013;23(24):3040–3052. doi: 10.1002/adfm.201202215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ku T-H, Chien M-P, Thompson MP, Sinkovits RS, Olson NH, Baker TS, et al. Controlling and switching the morphology of micellar nanoparticles with enzymes. J Am Chem Soc. 2011;133(22):8392–8395. doi: 10.1021/ja2004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.West JL, Hubbell JA. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1998;32(1):241–244. [Google Scholar]
- 30.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21(5):513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 31.Dey J, Xu H, Shen J, Thevenot P, Gondi SR, Nguyen KT, et al. Development of biodegradable crosslinked urethane-doped polyester elastomers. Biomaterials. 2008;29(35):4637–4649. doi: 10.1016/j.biomaterials.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stakleff KS, Lin F, Smith Callahan LA, Wade MB, Esterle A, Miller J, et al. Resorbable, amino acid-based poly(ester urea)s crosslinked with osteogenic growth peptide with enhanced mechanical properties and bioactivity. Acta Biomater. 2013;9(2):5132–5142. doi: 10.1016/j.actbio.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31(30):7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 34.Parrott MC, Luft JC, Byrne JD, Fain JH, Napier ME, DeSimone JM. Tunable bifunctional silyl ether cross-linkers for the design of acid-sensitive biomaterials. J Am Chem Soc. 2010;132(50):17928–17932. doi: 10.1021/ja108568g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thayer AM. Improving peptides. Chem Eng News. 2011;89(22):13–20. [Google Scholar]
- 36.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radical Bio Med. 2000;28(10):1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 37.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiolo Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu WF, Ma M, Bratlie KM, Dang TT, Langer RS, Anderson DG. Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials. 2011;32(7):1796–1801. doi: 10.1016/j.biomaterials.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nat Mater. 2004;3(3):183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 40.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9(11):923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, et al. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J Am Chem Soc. 2012;134(38):15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta MK, Meyer TA, Nelson CE, Duvall CL. Poly(PS-b-DMA) micelles for reactive oxygen species triggered drug release. J Control Release. 2012;162(3):591–598. doi: 10.1016/j.jconrel.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broaders KE, Grandhe S, Fréchet JMJ. A biocompatible oxidation-triggered carrier polymer with potential in therapeutics. J Am Chem Soc. 2010;133(4):756–758. doi: 10.1021/ja110468v. [DOI] [PubMed] [Google Scholar]
- 44.Shim MS, Xia Y. A reactive oxygen species (ROS)-responsive polymer for safe, efficient, and targeted gene delivery in cancer cells. Angew Chem Int Edit. 2013;52(27):6926–6929. doi: 10.1002/anie.201209633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu SS, Koblin RL, Zachman AL, Perrien DS, Hofmeister LH, Giorgio TD, et al. Physiologically relevant oxidative degradation of oligo(proline) cross-linked polymeric scaffolds. Biomacromolecules. 2011;12(12):4357–4366. doi: 10.1021/bm201328k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salvatore RN, Smith RA, Nischwitz AK, Gavin T. A mild and highly convenient chemoselective alkylation of thiols using Cs2CO3-TBAI. Tetrahedron Lett. 2005;46(51):8931–8935. [Google Scholar]
- 47.ASTM-International. E1899 - 08. Standard test method for hydroxyl groups using reaction with p-toluenesulfonyl isocyanate (TSI) and potentiometric titration with tetrabutylammonium hydroxide. 2008 [Google Scholar]
- 48.Pike JK, Ho T, Wynne KJ. Water-induced surface rearrangements of poly(dimethylsiloxane-urea-urethane) segmented block copolymers. Chem Mater. 1996;8(4):856–860. [Google Scholar]
- 49.Schubert MA, Wiggins MJ, Anderson JM, Hiltner A. Role of oxygen in biodegradation of poly(etherurethane urea) elastomers. J Biomed Mater Res. 1997;34(4):519–530. doi: 10.1002/(sici)1097-4636(19970315)34:4<519::aid-jbm12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 50.Christenson EM, Anderson JM, Hiltner A. Oxidative mechanisms of poly(carbonate urethane) and poly(ether urethane) biodegradation: in vivo and in vitro correlations. J Biomed Mater Res A. 2004;70A(2):245–255. doi: 10.1002/jbm.a.30067. [DOI] [PubMed] [Google Scholar]
- 51.Adolph EJ, Hafeman AE, Davidson JM, Nanney LB, Guelcher SA. Injectable polyurethane composite scaffolds delay wound contraction and support cellular infiltration and remodeling in rat excisional wounds. J Biomed Mater Res A. 2012;100A(2):450–461. doi: 10.1002/jbm.a.33266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page JM, Prieto EM, Dumas JE, Zienkiewicz KJ, Wenke JC, Brown-Baer P, et al. Biocompatibility and chemical reaction kinetics of injectable, settable polyurethane/allograft bone biocomposites. Acta Biomater. 2012;8(12):4405–4416. doi: 10.1016/j.actbio.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y, Grainger DW, Winn SR, Hollinger JO. Fabrication of poly(α-hydroxy acid) foam scaffolds using multiple solvent systems. J Biomed Mater Res. 2002;59(3):563–572. doi: 10.1002/jbm.1269. [DOI] [PubMed] [Google Scholar]
- 54.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Mikos AG, Temenoff JS. Formation of highly porous biodegradable scaffolds for tissue engineering. Electron J Biotechn. 2000;3(2):114–119. [Google Scholar]
- 56.Harbers GM, Grainger DW. Cell-material interactions: fundamental design issues for tissue engineering and clinical considerations. In: Guelcher SA, Hollinger JO, editors. An introduction to biomaterials. Boca Raton, FL: CRC Press/Taylor & Francis Group; 2006. [Google Scholar]
- 57.Arima Y, Iwata H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials. 2007;28(20):3074–3082. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Eglin D, Griffon S, Alini M. Thiol-Containing Degradable poly(thiourethane-urethane)s for tissue engineering. J Biomat Sci-Polym E. 2010;21(4):477–491. doi: 10.1163/156856209X424404. [DOI] [PubMed] [Google Scholar]
- 59.Laschke MW, Strohe A, Scheuer C, Eglin D, Verrier S, Alini M, et al. In vivo biocompatibility and vascularization of biodegradable porous polyurethane scaffolds for tissue engineering. Acta Biomater. 2009;5(6):1991–2001. doi: 10.1016/j.actbio.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. The J Immunol. 1998;160(12):5936–5944. [PubMed] [Google Scholar]
- 61.Jayakumar A, Widenmaier R, Ma X, McDowell MA. Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages. J Parasitol. 2008;94(1):84–93. doi: 10.1645/GE-1153.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh SH, Ward CL, Atala A, Yoo JJ, Harrison BS. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials. 2009;30(5):757–762. doi: 10.1016/j.biomaterials.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 63.Rockwood DN, Akins RE, Jr, Parrag IC, Woodhouse KA, Rabolt JF. Culture on electrospun polyurethane scaffolds decreases atrial natriuretic peptide expression by cardiomyocytes in vitro . Biomaterials. 2008;29(36):4783–4791. doi: 10.1016/j.biomaterials.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SY, Oh JH, Kim JC, Kim YH, Kim SH, Choi JW. In vivo conjunctival reconstruction using modified PLGA grafts for decreased scar formation and contraction. Biomaterials. 2003;24(27):5049–5059. doi: 10.1016/s0142-9612(03)00411-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.