Abstract
Gout is a common crystal-induced arthritis, in which monosodium urate (MSU) crystals precipitate within joints and soft tissues and elicit an inflammatory response. The causes of elevated serum urate and the inflammatory pathways activated by MSU crystals have been well studied, but less is known about the processes leading to crystal formation and growth. Uric acid, the final product of purine metabolism, is a weak acid that circulates as the deprotonated urate anion under physiologic conditions, and combines with sodium ions to form MSU. MSU crystals are known to have a triclinic structure, in which stacked sheets of purine rings form the needle-shaped crystals that are observed microscopically. Exposed, charged crystal surfaces are thought to allow for interaction with phospholipid membranes and serum factors, playing a role in the crystal-mediated inflammatory response. While hyperuricemia is a clear risk factor for gout, local factors have been hypothesized to play a role in crystal formation, such as temperature, pH, mechanical stress, cartilage components, and other synovial and serum factors. Interestingly, several studies suggest that MSU crystals may drive the generation of crystal-specific antibodies that facilitate future MSU crystallization. Here, we review MSU crystal biology, including a discussion of crystal structure, effector function, and factors thought to play a role in crystal formation. We also briefly compare MSU biology to that of uric acid stones causing nephrolithasis, and consider the potential treatment implications of MSU crystal biology.
Keywords: Monosodium urate, Crystallization, Gout, Synovial fluid, Cartilage, Immunomodulation, Kidney stones
Introduction
Gout is the most common arthropathy associated with crystal formation, and the most common inflammatory arthritis overall [1, 2]. In gout, deposition of monosodium urate (MSU) crystals within joints and connective tissue engenders highly inflammatory but localized responses. The susceptibility to form MSU crystals is a consequence of excessive blood levels of soluble urate, one of the final products of the metabolic breakdown of purine nucleotides [3]. Hyperuricemia is typically defined as occurring above the saturation point of MSU, at which point the risk of crystallization increases. Using this definition, hyperuricemia occurs at serum urate levels >6.8 mg/dL [4].
The causes of hyperuricemia have been extensively studied, as have the mechanisms by which crystals initiate inflammation. The baseline risk factor for hyperuricemia, universal to humans as well as some other primates, is a series of mutational inactivations of the gene for the enzyme uricase, which in other mammals degrades urate to the more soluble molecule allantoin [5–7]. However, additional factors are required to push the individual over the threshold into hyperuricemia, including: renal underexcretion of urate; conditions of excessive cell and purine turnover (e.g., leukemias, hemolytic anemias, etc.) [3]; high purine dietary intake [8]; and/or genetic factors that result in primary urate overproduction [9]. Once formed, MSU crystals activate resident tissue macrophages, which secrete inflammatory cytokines including IL-1β [10, 11]. These mediators, along with complement directly activated at MSU crystal surfaces, initiate a neutrophilic influx that is the classic pathophysiologic feature of acute gout [12]. Upon infiltration, neutrophils are further activated by the crystals they encounter, producing additional pro-inflammatory mediators such as the arachidonic acid products PGE2 and LTB4 [3]. Interestingly, MSU crystals can persist in the joint fluid between attacks, suggesting that the inflammatory potential of MSU crystals may be modulated by synovial fluid elements [13].
Less is known about the critical intermediate step between hyperuricemia and the inflammatory Response—the process of MSU crystal formation. Clearly, physicochemical factors play an important role, but other, less well-established factors must also be operative. Although the presence of hyperuricemia is essential for the formation of crystals, only a fraction of hyperuricemic patients develop gout—ranging from 2 to 36 % of patients in studies with approximately 5–10 years of follow-up—suggesting that not all hyperuricemics undergo MSU crystal formation [14, 15]. Conversely, patients are sometimes observed to have a normal serum urate (≤6.0 mg/dL) at the time of an acute gout attack, indicating that the relationship between serum urate level and acute MSU crystallization is complex [16]. Thus, local and/or systemic biological environments are likely to modulate MSU solubility, precipitation, and/or stability.
Here, we review the known biology of MSU crystal formation and the factors that modulate the process of urate crystallization, with a brief discussion of how serum urate crystallization differs from uric acid kidney stone formation. We also briefly consider possible treatment implications arising from the growing understanding of crystal biology.
Uric Acid in its Soluble and Crystal Forms: Structure, Morphology, and Effector Function
Uric acid is a weak, hydrogenated organic acid with dual pKas of 5.75 and 10.3 [4]. Under normal physiologic conditions (i.e., pH 7.4 and 37°C), urate circulates in plasma and synovial fluid in a mono-deprotonated ionic form. In the crystalline state, urate is observed in either its fully protonated acid form (i.e., uric acid, as in the case of renal stones, discussed below) or as a variety of salts formed by deprotonated or partially deprotonated urate. Monosodium urate (MSU) monohydrate (NaC5H3N4O3·H2O), in which a urate molecule is bonded to one sodium and one water molecule, is one of the most common forms of crystallized urate and comprises the primary deposits seen in gouty arthritis (Fig. 1) [17], although urate precipitation with other mineral phases is also possible [18].
Figure 1.
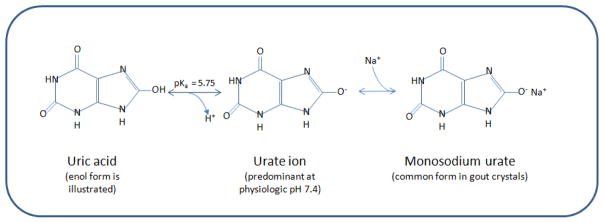
Physical chemistry of uric acid and monosodium urate formation. At physiologic pH of 7.4 and 37 °C, urate predominates. Urate can combine with sodium present in solution to form the less-soluble salt form, monosodium urate
The crystallographic features of MSU crystals were first reported by Mandel and Mandel in 1976 [17], with further characterization of crystal structure and growth patterns under model physiologic conditions established by Perrin et al. in 2011 [19]. MSU crystals have a triclinic structure. (A triclinic crystal has faces along three unequal axes, none of which is perpendicular to the others.) Macroscopically, this structure makes up the well-known needle-shaped crystals of MSU. In MSU crystals, urate anions are hydrogen-bonded together and intimately aligned along their edges, forming sheets of purine rings. Water molecules are held in the structure by coordinating to sodium ions and by bonding to the purine ring of the urate molecule; sodium ion coordination to the urate molecules leads to a rippling of the sheets [17]. The sheets themselves are stacked, generating the long axis of the crystal (Fig. 2). Exposed crystal surfaces contain hydrogen atoms and are rich in charged oxygen atoms and sodium ions [17].
Figure 2.
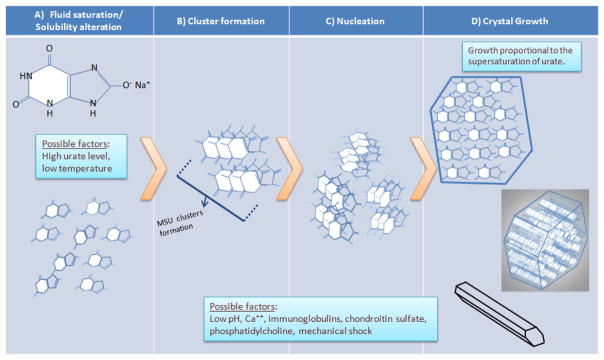
Hypothetical model of the initiation and propagation of monosodium urate crystallization. A) Monosodium urate molecules remain fully in solution until an event that changes their solubility (e.g., increased concentration and decreased temperature, as discussed in the text). B) Soluble monosodium urate molecules begin to cluster while still in solution. C) Clusters aggregate into crystal nuclei, the basis for additional crystal formation and growth. D) Formation of fully-aggregated monosodium urate crystals. Top, monosodium urate in the crystals are arranged in flat sheets as seen in cross-section. Middle, the surface of these flat sheets forms the major growth faces of the crystal, with the flat sheets stacking along the crystal’s long axis. Bottom, fully formed (but potentially still growing) crystal, whose three axes are non-perpendicular (i.e., triclinic structure). Local factors may promote nucleation and/or crystal growth (e.g., pH and others; see text)
It is hypothesized that the ionic character of the MSU crystal surfaces allows for interaction with biological phospholipid membranes (such as those of neutrophil plasma membranes and phagocytic vacuoles), as well as serum factors, including both anionic and cationic protein species. Of note, MSU crystals isolated from sites of gouty inflammation are typically coated with immunoglobulins, mainly of the IgG class, whose surface concentrations decline as inflammation resolves (inversely correlating with a rise in apolipoprotein B surface coating), suggesting a role for IgG in promoting the inflammation of an acute attack [20, 21]. To at least some extent, these antibodies are likely to be specifically directed to the crystal surface (see below). The configuration of the antibodies bound to urate with respect to the crystal surface has been studied in in vitro models of synthetic MSU crystals, where it was determined that the cationic Fab portions bind the crystal, while the Fc portions are pointed away and exposed [22]. The availability of “away-pointing” Fc portions are thought to play a role both in the ability of the crystal to activate complement, and in the ability of Fc-receptor-bearing cells to phagocytose crystals and undergo cell activation [20]. Complement activation products such as fragments of C1 have also been identified on MSU crystal surfaces, with evidence suggesting that MSU crystals can activate complement in whole plasma independent of IgG [23].
Physicochemical Processes of MSU Crystallization
The general physicochemical process of MSU crystallization bears resemblance to the formation of other crystals, and is thought to depend on both urate concentration and other factors (Fig. 2). As noted earlier, plasma is considered to be saturated with urate when its level reaches the solubility limit of approximately 6.8 mg/dl (405 μmol/L) [4]. Beyond this concentration, the solution is considered supersaturated. In the supersaturation range, MSU crystal formation begins with a further alteration of the solubility of urate or other inciting event, and crystallization further propagates depending on local conditions.
Nucleation, or the process of new microcrystal precipitation, is the rate-limiting step of MSU crystal genesis. As a general principle, during nucleation the dispersed molecules in solution first gather into clusters, overcoming the dispersion forces of the solvent; these clusters then aggregate further to form crystal nuclei [24]. Nucleation can occur in a homogeneous (formation in the absence of a foreign surface or other crystal) or heterogeneous (when a foreign surface serves as the nidus for crystallization) fashion. If additional crystals form in the presence of pre-existing crystals, the process is referred to as secondary nucleation; along with heterogenous nucleation, this process can occur at lower supersaturation levels than during homogeneous crystal formation.
Once the urate crystal nucleus reaches a critical size and its primitive structure is stabilized, crystal growth occurs most rapidly at the crystal’s longitudinal ends. It is this predisposition to longitudinal over latitudinal growth (a property not shared universally by other crystals) that gives the MSU crystal its characteristic long narrow shape. While mechanisms of in vivo crystal growth remain unresolved, urate growth under model conditions has been studied, with recent evidence, suggesting that the spontaneous appearance of islands on the crystal surface may play a role in crystal growth [19]. As in the case of nucleation (where supersaturation levels are a factor in crystal initiation), model systems have shown that crystal propagation increases with increasing urate concentration [25]. This is consistent with epidemiological data indicating that gout incidence increases with greater serum urate levels [14]. Interestingly, the risk of gout flares also increases early after initiation of urate lowering therapy [26], which has been postulated to result from microcrystal liberation from tophi or microtophi (urate “sloughing” or “stripping”); it is possible these sloughed crystals may not only be proinflammatory but may also serve as nuclei for further crystal formation.
Temperature is another environmental factor that appears to play a role in MSU crystal formation through effects on urate solubility [27–29]. In vitro studies performed in aqueous solutions suggest that a reduction of even 2 °C, from 37 to 35 °C, is sufficient to lower the solubility point of urate from 6.8 to 6.0 mg/dL [28]. This response to temperature may explain in part why the first metatarsophalangeal joint—an area of both relatively reduced perfusion (suggesting reduced heat delivery from the body core), and relatively increased surface-to-volume ratio (promoting heat radiation and loss)—is the most common site for first gouty attacks. Conversely, the heat engendered by the inflammatory gouty attack (owing to increased perfusion and tissue metabolism of the affected joint) may contribute to the subsequent dissolution of crystals, and to the observation that gout attacks are typically self-limiting.
Like cold temperature, the presence of an acidic environment also appears to facilitate MSU crystallization. Wilcox et al. demonstrated that reduction of pH promoted MSU nucleation in an in vitro system [30]. In this model, they found that the pH directly affected the nucleation of crystals by a mechanism not well understood, but apparently independent of the MSU solubility level. In addition, pH exerted an indirect effect on MSU crystallization by increasing calcium ion concentrations that consequently reduced MSU solubility and promoted nucleation [30]. The authors therefore proposed that increased levels of free Ca++ ions in the setting of lower pH (resulting from displacement of plasma protein-bound Ca++ into the liquid phase) may explain the relationship between acidic environments and MSU nucleation. They also speculate that the initial nucleus of an MSU crystal may be a calcium urate molecule, or that, because of their nearly identical atomic radii, calcium ions may substitute for sodium in the urate crystal lattice [30]. Acidosis occurs in conditions such as strenuous exercise, respiratory insufficiency, and ethanol consumption, all of which are associated with the development of gout attacks. (Since systemic acidosis also promotes decreased renal urate excretion, the resulting increases in systemic hyperuricemia may also contribute to the risk for crystallization in conditions of acidosis.) Interestingly, the metabolic activity of neutrophils during phagocytosis of existing crystals may result in lactic acid generation, thus lowering the synovial fluid pH and promoting additional local impetus for crystal formation. Consistent with this model, increasing lactic acid and declining pH have been observed in the synovial fluid of acute gout (incubated in vitro at 38 °C) [31]. It has been postulated that the increased incidence of acute gouty attacks that occurs during sleep may be due in part to the mild respiratory acidosis that ensues when breathing rates decline [32].
In addition to the above-mentioned chemical factors, mechanical effects have also been recognized as a potentially promoting MSU crystallization. Physical perturbations may promote MSU crystal nucleation: in vitro, “snapping” a slide containing a supersaturated urate solution can directly induce the formation of MSU crystals [30]. These observations may be consistent with the predilection for gout to occur in joints (rather than other tissues), since joints undergo repeated daily mechanical shocks.
Synovial Fluid and Cartilage Factors Modulating Crystallization
Joint fluid and cartilage elements have been speculated to play a role in urate crystal formation. The addition of gout patients’ synovial fluid to supersaturated solutions in vitro has been shown to promote MSU crystallization, whereas synovial fluid from patients with rheumatoid or degenerative arthritis did not have this effect [30, 33, 34]. These observations were independent of the urate concentration in the synovial fluid, and testify to the possibility that the contents of the gouty joint may themselves provide a hospitable environment for MSU crystal formation.
A number of studies have explored the possibility that proteins and other organic macromolecules, whether sloughed off, enzymatically-degraded, or otherwise originating from cartilage or synovium, may play roles in MSU crystallization. Burt et al. analyzed the kinetics of MSU crystal growth and observed that both chondroitin sulfate and phosphatidylcholine (but not phosphotidylserine) increased MSU crystal formation in an in vitro system, possibly by promoting nucleation or increasing the growth rate of crystals [35]. The chondroitin findings are supported by one prior study [36], but contradicted by another [34]. Burt’s studies found no significant effect on crystal growth from proteoglycan monomers/aggregates nor, consistent with the findings of other investigators, from hyaluronic acid [34]. While these studies do not explain how cartilage components might affect MSU crystallization, they provide some evidence that the presence of cartilage damage, whether occurring through mechanical trauma or as a consequence of osteoarthritis or other joint-damaging processes, may contribute to the risk for localized gouty attacks. More recently, there has been speculation that collagen fibers or fragments may promote MSU crystallization in linear bands, based on ultrasound images of linear MSU formation in joints and the appearance of crystals from synovial fluid as seen by light microscopy [37].
A role for albumin in the crystallization process is even more controversial. Investigators have variously suggested that albumin has no effect on MSU crystallization [38], or promotes or strongly inhibits MSU crystallization in vitro. Possible explanations for an inhibitory role of albumin in MSU crystallization could relate to albumin blocking crystal active growth sites, increasing urate solubility, or altering the free/bound Ca++ [35, 39]. In contrast to these reports, Perl-Treves et al. have reported that albumin considerably accelerates the kinetics of MSU crystallization under different in vitro conditions, mainly by favoring the processes of clustering and nucleation; however, this was seen only at lower albumin concentrations and varied with pH [40]. In this study, structural analysis of synthetic crystals raised in growth media, including human serum albumin, revealed that carboxylate groups on albumin may actually incorporate into the MSU crystal structure to stabilize the crystal nuclei, thus catalyzing crystallization [40]. Further studies are clearly needed to understand the role of albumin in urate crystallization within the joint fluid.
Humoral Immunomodulation of MSU Crystallization
As discussed above, it has long been appreciated that immunoglobulins can adhere to mature MSU crystals, and in so doing promote crystal-induced inflammation through several mechanisms. More controversially, some investigators have provided evidence that the presence of antibodies capable of interacting with MSU may actually contribute to the formation of MSU crystals. This raises the question of whether humoral immune responses may partially explain why some patients with hyperuricemia develop crystals (and therefore gout), while others do not. The specificity of antibodies to the crystal surface has also been a subject of investigation.
Kaneko et al. analyzed MSU crystal formation rates in the presence or absence of -globulin in vitro, and found that the addition of γ-globulin to a supersaturated solution of urate accelerated MSU crystal formation in a reaction whose extent was directly proportional to time and the urate dose [41]. Whether these effects reflected immunoglobulin specificities for urate, or non-specific effects of the proteins themselves, was not evaluated.
However, other studies suggest that in gout patients, antibodies may be generated that are specifically directed against MSU, and that promote MSU crystallization by stabilizing intermediate crystal structures. Kam et al. purified IgG immunoglobulins from the synovial fluid of patients with gout, rheumatoid arthritis, pseudogout, or osteoarthritis [42]. Using an in vitro system, they observed that IgG from gouty synovial fluid, but not the synovial fluid of non-gout patients, promoted MSU crystal formation. The authors speculated that IgG may act as a catalytic agent for nucleation, reducing the activation energy required to start the formation of crystals. To explore whether antibodies functioning in this manner represent an immunologic response to MSU crystals, these same authors “immunized” rabbits with serial injections of MSU crystals [42]. IgG isolated from rabbit sera post-immunization provoked an increase in in vitro MSU nucleation, whereas pre-immunization IgG, or IgG from rabbits immunized with control (allopurinol) crystals did not. This finding led the authors to hypothesize that serum antibodies can specifically recognize MSU crystal surfaces to promote MSU crystal formation, perhaps explaining why many patients with longstanding gout continue to have attacks even after their serum urate levels appear to have been adequately lowered. In a subsequent study, the same authors used a similar rabbit model to show that immunization with magnesium urate octahydrate and allopurinol crystals could generate IgG antibodies that would in turn promote nucleation of their respective crystal types in vitro, supporting the idea of specificity of antibodies to crystals [43].
Kanevets et al. found further evidence to support a specific humoral immune response in gout [44]. These authors immunized mice with crystallized MSU biweekly for 2 months. At the end of the study period, immunized mice demonstrated up to a ten-fold increased presence of IgM immunoglobulins that could directly bind MSU (but not xanthine) crystals in vitro, relative to unimmunized mice. B cell-deficient mouse strains (i.e., unable to make immunoglobulins) failed to demonstrate this response despite immunization. The authors further confirmed that the IgM immuoglobulins promoted MSU crystallization in vitro, in a dose-dependent manner. They went on to suggest that the immunologic promotion of MSU crystallization may be important not only in gout but also in endogenous danger signal mediation, a cellular immune response in which MSU precipitation has been implicated: compared with the wild-type, mice deficient in B cells had reduced cytolytic T cell function in response to urate exposure. One significant difference between Kanevet’s studies in mice, and those of Kam et al. in rabbits, is that Kam’s studies identified the MSU-binding antibodies as being primarily of the IgG class, whereas, in Kanevet’s studies, the majority were IgM. In humans, most of the immunoglobulins identified as adherent to MSU crystals have been IgG. Explanations for these discrepancies might include species-specific immune variations. Alternatively, in humans at least, gout is a chronic disease, and the chronic exposure to MSU crystals might result in a persistent immune response that results in IgM-to-IgG class switching [44]. In any case, the overall model is one in which persistent exposure to MSU crystals promotes the development of anti-MSU crystal antibodies, that in turn further promote MSU crystallization.
Local Factors Dampening the Inflammatory Response to MSU
While crystallization can be affected by environmental elements, the extent of an inflammatory response to MSU crystals may also vary depending on local factors, possibly impacting the clinical phenotype of gout. Several reports indicate that molecules that may be found in synovial fluid, such as low density lipoprotein, high density lipoprotein, and apolipoprotein B, may inhibit the inflammatory reactions characteristic of the acute gout attack through a variety of mechanisms [20, 45–47]. In some circumstances, urate can crystallize on cartilage without inducing an inflammatory response at all—over the past several years, studies using musculoskeletal ultrasound have documented the presence of crystallized urate along the cartilage surfaces of diarthrodial joints, even in patients who have never had gouty attacks [48, 49]. The chemical structure of these deposits is poorly characterized, but they are presumed to be MSU. The ability of such crystals to form in the absence of resultant inflammation raises questions as to whether the structure of the crystals, their location within the cartilage, or the molecules they associate with differ in some way to render them non-inflammatory.
Kidney Stones: Crystallization of Uric Acid
Uric acid (UA) stone formation in the kidney provides a useful counterpoint to our discussion of articular urate crystallization. In contrast to the MSU crystals that form in the joints of gout patients, the urate in renal stones is mainly in the protonated form, i.e., UA, often admixed with crystals of calcium oxalate. In the urine, UA crystallizes from the aqueous solution as an anhydrous compound (most common), a dihydrate compound, a mixture of both the anhydrous and the dihydrate forms, a monohydrate form, or, rarely, UA monohydrate with sodium or ammonium urate [50]. While in-depth studies of the morphology, structure, and formation mechanisms of uric acid calculi are limited, multiple factors are understood to contribute to the formation of urinary UA crystals and stones.
Less than 5 % of circulating urate is protein-bound in the plasma, and urate is freely filtered at the glomerulus into the proximal tubule, where most of its handling occurs. In the proximal tubule, urate is reabsorbed and secreted resulting in around 90 % net urate reabsorption. High urinary UA concentration, such as results from low urine volume or hyperuricosuria, is one factor that promotes stone formation [51, 52]. Overly acidic urine is also a critical driver of UA stone formation and is an identifiable risk factor in the majority of UA stone formers [53, 54]. Whereas synovial fluid and/or serum pH are maintained within a narrow range, urine pH can vary more widely. At a urinary pH of less than 5.5, urinary urate exists largely as UA, the undissociated or protonated form. In contrast to ionized urate, UA is more hydrophobic and less soluble. Concentrations of urate that would be undersaturated as an ion become supersaturated as UA, allowing crystals to precipitate. Understanding the process of UA stone formation in a patient can guide treatment. In particular, urine alkalinization is an important approach for stone reduction. Increased fluid intake and reduction of urinary urate excretion through urate lowering medications are less important.
Urinary UA may also impact the formation of non-urate urinary crystals. In vitro work suggests that the hyperuricosuric state may promote calcium oxalate precipitation in a process called salting out [55]. This urate effect depends directly on the concurrent urinary concentrations of UA, calcium, and oxalate. In one trial, pharmacologic urate lowering (using allopurinol) decreased CaOx stone formation [56]. However, observational studies have not confirmed that higher urinary urate excretion leads to higher incidence of calcium oxalate nephrolithiasis stones [57]. Along the same lines, a role for the MSU form of urate was once thought to contribute to calcium oxalate precipitation [58], but recent studies do not support this hypothesis.
Future Directions: Manipulation of Urate Crystallization as a Therapeutic Strategy for Gout?
At the present time, reduction of serum (and, therefore, synovial fluid) urate levels remains the only available means for preventing and/or undoing the formation of MSU crystals. Both clinical results, and advances in imaging, have documented the ability of serum urate lowering to resolve tissue deposits of urate [59, 60]. However, this process often requires prolonged therapy, and is frequently unsuccessful [61]. Given the appreciation that additional factors modulate MSU crystallization, it is reasonable to postulate that therapeutic approaches might be developed that could reduce the MSU crystal burden through mechanisms other than direct urate lowering.
The potential role of anti-MSU crystal immunoglobulins in crystal formation raises the question of whether humoral immune modulation (e.g., using the anti-B cell agent rituximab, FDA-approved for rheumatoid arthritis) might be of benefit in the treatment of patients with long-standing, recalcitrant gout, for whom conventional urate lowering therapy has failed. This remains an untested possibility. Alternatively, small molecules that bind to the MSU crystal might be developed to reduce, prevent, or reverse crystallization. Proof-of-principle for this approach may be found in atomic force microscopy studies addressing the crystallization of L-cystine, an occasional source of otherwise difficult-to-prevent kidney stones. In a series of elegant studies, Rimer et al. demonstrated that two L-cystine-related molecules, L-CDME and L-CME, have the potential to dramatically reduce the growth velocity of L-cystine crystals by binding to the crystals and frustrating the addition of L-cystine molecules [62]. In vitro, this results in reduced crystal yield and crystal size. Whether a similar approach could be applied to the problem of MSU crystallization remains to be determined.
Conclusions
MSU crystal formation is strongly influenced by an environment supersaturated with urate. However, multiple other factors can either reduce the solubility of MSU, enhance crystal nucleation, or speed up growth of existing crystals, and may thus play roles promoting urate crystallization. These relationships may prove critical to our understanding of the heterogeneity of gout, wherein a single serum urate concentration may, in different patients, result in a variety of gouty phenotypes, such as no disease, mild disease, and severe or tophaceous disease. Despite recent advances, we still have only a rudimentary understanding of how MSU crystals form and resolve within the body. Increased appreciation of both basic chemistry, and the role of proteins—particularly immunoglobulins—in the pathogenesis of MSU crystallization holds promise for the development of novel, and potentially better, treatments for patient with gout, and possibly also other crystal deposition disorders.
Acknowledgments
The authors thank David Goldfarb and Michael Pillinger for helpful input with the text, and Michael Ward for reviewing the accuracy of the figures.
This work was supported in part by grants from the Arthritis Foundation and New York Academy of Medicine (to Daria B. Crittenden), and by grant UL1 TR000038 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Footnotes
Conflict of Interest
Miguel A. Martillo, Lama Nazzal, and Daria B. Crittenden declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12(6):21. doi: 10.1186/ar3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi HK, et al. Pathogenesis of gout. Ann Intern Med. 2005;143(7):499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 4.Burns CM, WR . Disorders of purine and pyramidine metabolism. In: Longo FADL, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison’s priciples of internal medicine. McGraw-Hill; New York: 2012. [Google Scholar]
- 5.Wu XW, et al. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34(1):78–84. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]
- 6.Wu XW, et al. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci U S A. 1989;86(23):9412–6. doi: 10.1073/pnas.86.23.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oda M, et al. Loss of urate oxidase activity in hominoids and its evolutionary implications. Mol Biol Evol. 2002;19(5):640–53. doi: 10.1093/oxfordjournals.molbev.a004123. [DOI] [PubMed] [Google Scholar]
- 8.Huang HY, et al. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum. 2005;52(6):1843–7. doi: 10.1002/art.21105. [DOI] [PubMed] [Google Scholar]
- 9.Sebesta I. Genetic disorders resulting in hyper- or hypouricemia. Adv Chronic Kidney Dis. 2012;19(6):398–403. doi: 10.1053/j.ackd.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Busso N, So A. Microcrystals as DAMPs and their role in joint inflammation. Rheumatology (Oxford) 2012;51(7):1154–60. doi: 10.1093/rheumatology/ker524. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 12.Pettipher ER, Higgs GA, Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986;83(22):8749–53. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger A, Schumacher HR, Agudelo CA. Urate crystals in asymptomatic metatarsophalangeal joints. Ann Intern Med. 1979;91(1):56–7. doi: 10.7326/0003-4819-91-1-56. [DOI] [PubMed] [Google Scholar]
- 14.Lin KC, Lin HY, Chou P. The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. J Rheumatol. 2000;27(6):1501–5. [PubMed] [Google Scholar]
- 15.Hall AP, et al. Epidemiology of gout and hyperuricemia. A long-term population study. Am J Med. 1967;42(1):27–37. doi: 10.1016/0002-9343(67)90004-6. [DOI] [PubMed] [Google Scholar]
- 16.Schlesinger N, Norquist JM, Watson DJ. Serum urate during acute gout. J Rheumatol. 2009;36(6):1287–9. doi: 10.3899/jrheum.080938. [DOI] [PubMed] [Google Scholar]
- 17.Mandel NS, Mandel GS. Monosodium urate monohydrate, the gout culprit. J Am Chem Soc. 1976;98(8):2319–23. doi: 10.1021/ja00424a054. [DOI] [PubMed] [Google Scholar]
- 18.Frincu MC, Fogarty CE, Swift JA. Epitaxial relationships between uric acid crystals and mineral surfaces: a factor in urinary stone formation. Langmuir. 2004;20(16):6524–9. doi: 10.1021/la049091u. [DOI] [PubMed] [Google Scholar]
- 19**.Perrin CM, et al. Monosodium urate monohydrate crystallization. CrystEngComm. 2011;13(4):1111–1117. Modern-day work in which in situ atomic force microscopy and dynamic light scattering were used to elucidate the growth of monosodium urate crystals on a molecular level. [Google Scholar]
- 20.Ortiz-Bravo E, Sieck MS, Schumacher HR., Jr Changes in the proteins coating monosodium urate crystals during active and subsiding inflammation. Immunogold studies of synovial fluid from patients with gout and of fluid obtained using the rat subcutaneous air pouch model. Arthritis Rheum. 1993;36(9):1274–85. doi: 10.1002/art.1780360912. [DOI] [PubMed] [Google Scholar]
- 21.Cherian PV, Schumacher HR., Jr Immunochemical and ultrastructural characterization of serum proteins associated with monosodium urate crystals (MSU) in synovial fluid cells from patients with gout. Ultrastruct Pathol. 1986;10(3):209–19. doi: 10.3109/01913128609032219. [DOI] [PubMed] [Google Scholar]
- 22.Kozin F, McCarty DJ. Molecular orientation of immunoglobulin G adsorbed to microcrystalline monosodium urate monohydrate. J Lab Clin Med. 1980;95(1):49–58. [PubMed] [Google Scholar]
- 23.Terkeltaub R, et al. Plasma protein binding by monosodium urate crystals. Analysis by two-dimensional gel electrophoresis. Arthritis Rheum. 1983;26(6):775–83. doi: 10.1002/art.1780260612. [DOI] [PubMed] [Google Scholar]
- 24.Mullin JW. Crystallization. Butterworth-Heinemann; Oxford: 1993. Nucleation; pp. 172–201. [Google Scholar]
- 25.Fiddis RW, Vlachos N, Calvert PD. Studies of urate crystallisation in relation to gout. Ann Rheum Dis. 1983;42(Suppl 1):12–5. doi: 10.1136/ard.42.suppl_1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51(3):321–5. doi: 10.1002/art.20405. [DOI] [PubMed] [Google Scholar]
- 27.Allen DJ, Milosovich G, Mattocks AM. Inhibition of monosodium urate needle crystal growth. Arthritis Rheum. 1965;8(6):1123–33. doi: 10.1002/art.1780080611. [DOI] [PubMed] [Google Scholar]
- 28.Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15(2):189–92. doi: 10.1002/art.1780150209. [DOI] [PubMed] [Google Scholar]
- 29.Wilcox WR, et al. Solubility of uric acid and monosodium urate. Med Biol Eng. 1972;10(4):522–31. doi: 10.1007/BF02474201. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox WR, Khalaf AA. Nucleation of monosodium urate crystals. Ann Rheum Dis. 1975;34(4):332–9. doi: 10.1136/ard.34.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seegmiller JE. The acute attack of gouty arthritis. Arthritis Rheum. 1965;8(5):714–25. doi: 10.1002/art.1780080431. [DOI] [PubMed] [Google Scholar]
- 32.Abrams B. Sleep apnea as a cause of gout flares. Medscape J Med. 2009;11(1):3. [PMC free article] [PubMed] [Google Scholar]
- 33.McGill NW, Dieppe PA. Evidence for a promoter of urate crystal formation in gouty synovial fluid. Ann Rheum Dis. 1991;50(8):558–61. doi: 10.1136/ard.50.8.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tak HK, Cooper SM, Wilcox WR. Studies on the nucleation of monosodium urate at 37 degrees c. Arthritis Rheum. 1980;23(5):574–80. doi: 10.1002/art.1780230509. [DOI] [PubMed] [Google Scholar]
- 35.Burt HM, Dutt YC. Growth of monosodium urate monohydrate crystals: effect of cartilage and synovial fluid components on in vitro growth rates. Ann Rheum Dis. 1986;45(10):858–64. doi: 10.1136/ard.45.10.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurent TC. Solubility of Sodium Urate in the Presence of Chondroitin-4-Sulphate. Nature. 1964;202:1334. doi: 10.1038/2021334a0. [DOI] [PubMed] [Google Scholar]
- 37*.Pascual E, Martinez A, Ordonez S. Gout: the mechanism of urate crystal nucleation and growth. A hypothesis based in facts. Joint Bone Spine. 2013;80(1):1–4. doi: 10.1016/j.jbspin.2012.08.012. A recent review that summarizes information gleaned from current imaging of gouty joints, and a hypothesis regarding what this may teach us about monosodium urate crystallization. [DOI] [PubMed] [Google Scholar]
- 38.Katz WA. Role of proteoglycans in the development of gouty arthritis. In: Kelley WIWN, editor. Handbook of experimental pharmacology. Springer; New York: 1978. pp. 347–364. [Google Scholar]
- 39.Kippen I, et al. Factors affecting urate solubility in vitro. Ann Rheum Dis. 1974;33(4):313–7. doi: 10.1136/ard.33.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perl-Treves D, Addadi L. A structural approach to pathological crystallizations. Gout: the possible role of albumin in sodium urate crystallization. Proc R Soc Lond B Biol Sci. 1988;235(1279):145–59. doi: 10.1098/rspb.1988.0069. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko K, Maru M. Determination of urate crystal formation using flow cytometry and microarea X-ray diffractometry. Anal Biochem. 2000;281(1):9–14. doi: 10.1006/abio.2000.4543. [DOI] [PubMed] [Google Scholar]
- 42.Kam M, et al. Antibodies against crystals. Faseb J. 1992;6(8):2608–13. doi: 10.1096/fasebj.6.8.1592211. [DOI] [PubMed] [Google Scholar]
- 43.Kam M, et al. Specificity in the recognition of crystals by antibodies. J Mol Recognit. 1994;7(4):257–64. doi: 10.1002/jmr.300070404. [DOI] [PubMed] [Google Scholar]
- 44.Kanevets U, et al. A role of IgM antibodies in monosodium urate crystal formation and associated adjuvanticity. J Immunol. 2009;182(4):1912–8. doi: 10.4049/jimmunol.0803777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terkeltaub R, et al. Low density lipoprotein inhibits the physical interaction of phlogistic crystals and inflammatory cells. Arthritis Rheum. 1986;29(3):363–70. doi: 10.1002/art.1780290309. [DOI] [PubMed] [Google Scholar]
- 46.Scanu A, et al. High-density lipoproteins downregulate CCL2 production in human fibroblast-like synoviocytes stimulated by urate crystals. Arthritis Res Ther. 2010;12(1):11. doi: 10.1186/ar2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGill NW, Hayes A, Dieppe PA. Morphological evidence for biological control of urate crystal formation in vivo and in vitro. Scand J Rheumatol. 1992;21(5):215–9. doi: 10.3109/03009749209099227. [DOI] [PubMed] [Google Scholar]
- 48.Thiele RG. Role of ultrasound and other advanced imaging in the diagnosis and management of gout. Curr Rheumatol Rep. 2011;13(2):146–53. doi: 10.1007/s11926-010-0156-4. [DOI] [PubMed] [Google Scholar]
- 49.Howard RG, et al. Reproducibility of musculoskeletal ultrasound for determining monosodium urate deposition: Concordance between readers. Arthritis Care & Research. 2011;63(10):1456–1462. doi: 10.1002/acr.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hesse A, et al. Uric acid dihydrate as urinary calculus component. Invest Urol. 1975;12(5):405–9. [PubMed] [Google Scholar]
- 51.Sakhaee K, et al. Assessment of the pathogenetic role of physical exercise in renal stone formation. J Clin Endocrinol Metab. 1987;65(5):974–9. doi: 10.1210/jcem-65-5-974. [DOI] [PubMed] [Google Scholar]
- 52.Asplin JR. Uric acid stones. Semin Nephrol. 1996;16(5):412–24. [PubMed] [Google Scholar]
- 53.Sakhaee K, et al. Pathophysiologic basis for normouricosuric uric acid nephrolithiasis. Kidney Int. 2002;62(3):971–9. doi: 10.1046/j.1523-1755.2002.00508.x. [DOI] [PubMed] [Google Scholar]
- 54.Pak CY, et al. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int. 2001;60(2):757–61. doi: 10.1046/j.1523-1755.2001.060002757.x. [DOI] [PubMed] [Google Scholar]
- 55.Grover PK, V, Marshall R, Ryall RL. Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: implications for calcium oxalate stone genesis. Chem Biol. 2003;10(3):271–8. doi: 10.1016/s1074-5521(03)00057-7. [DOI] [PubMed] [Google Scholar]
- 56.Ettinger B, et al. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986;315(22):1386–9. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- 57.Curhan GC, Taylor EN. 24-h uric acid excretion and the risk of kidney stones. Kidney Int. 2008;73(4):489–96. doi: 10.1038/sj.ki.5002708. [DOI] [PubMed] [Google Scholar]
- 58.Pak CY, et al. Mechanism for calcium urolithiasis among patients with hyperuricosuria: supersaturation of urine with respect to monosodium urate. J Clin Invest. 1977;59(3):426–31. doi: 10.1172/JCI108656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pascual E, Sivera F. Time required for disappearance of urate crystals from synovial fluid after successful hypouricaemic treatment relates to the duration of gout. Ann Rheum Dis. 2007;66(8):1056–8. doi: 10.1136/ard.2006.060368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thiele RG, Schlesinger N. Ultrasonography shows disappearance of monosodium urate crystal deposition on hyaline cartilage after sustained normouricemia is achieved. Rheumatol Int. 2010;30(4):495–503. doi: 10.1007/s00296-009-1002-8. [DOI] [PubMed] [Google Scholar]
- 61.Becker MA, Chohan S. We can make gout management more successful now. Curr Opin Rheumatol. 2008;20(2):167–72. doi: 10.1097/BOR.0b013e3282f54d03. [DOI] [PubMed] [Google Scholar]
- 62.Rimer JD, et al. Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science. 2010;330(6002):337–41. doi: 10.1126/science.1191968. [DOI] [PMC free article] [PubMed] [Google Scholar]


