Abstract
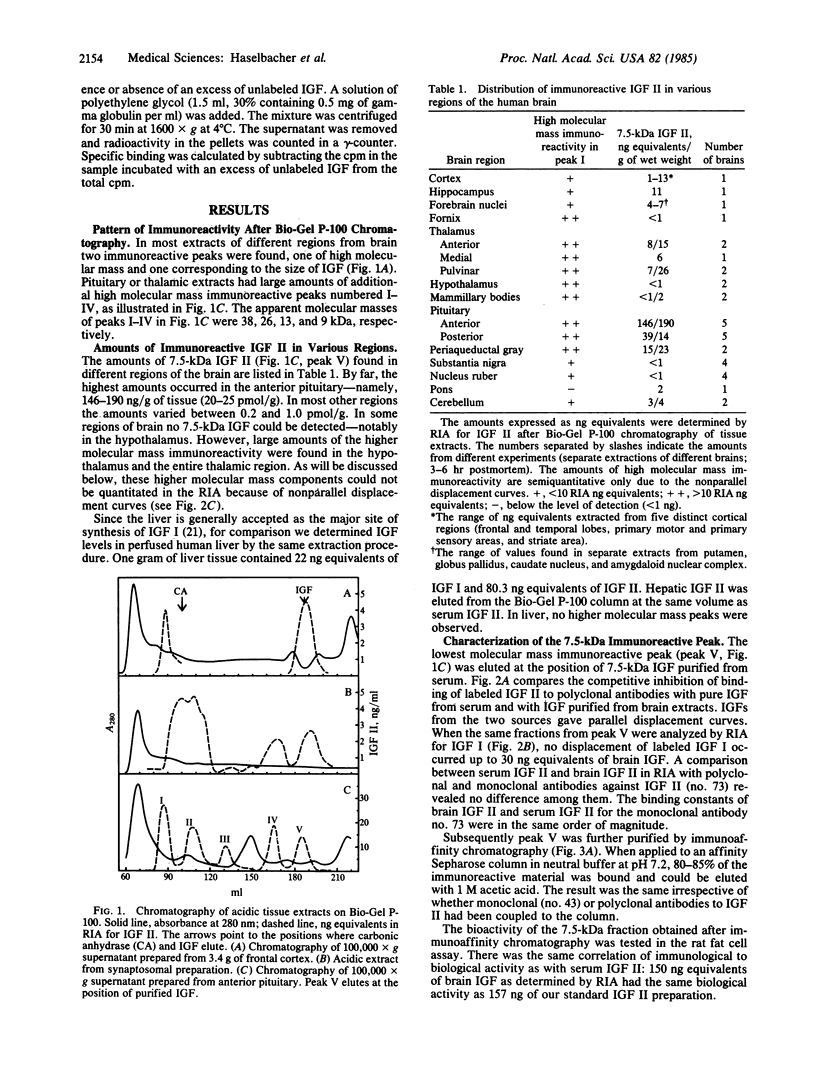
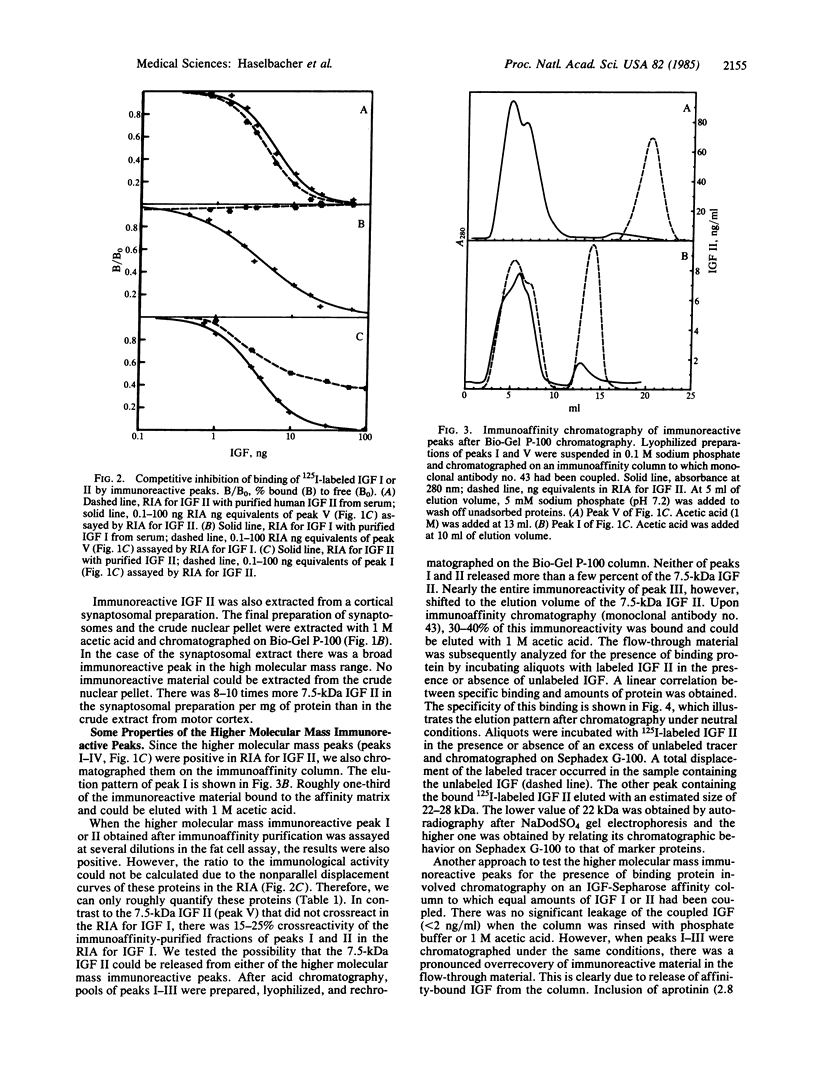
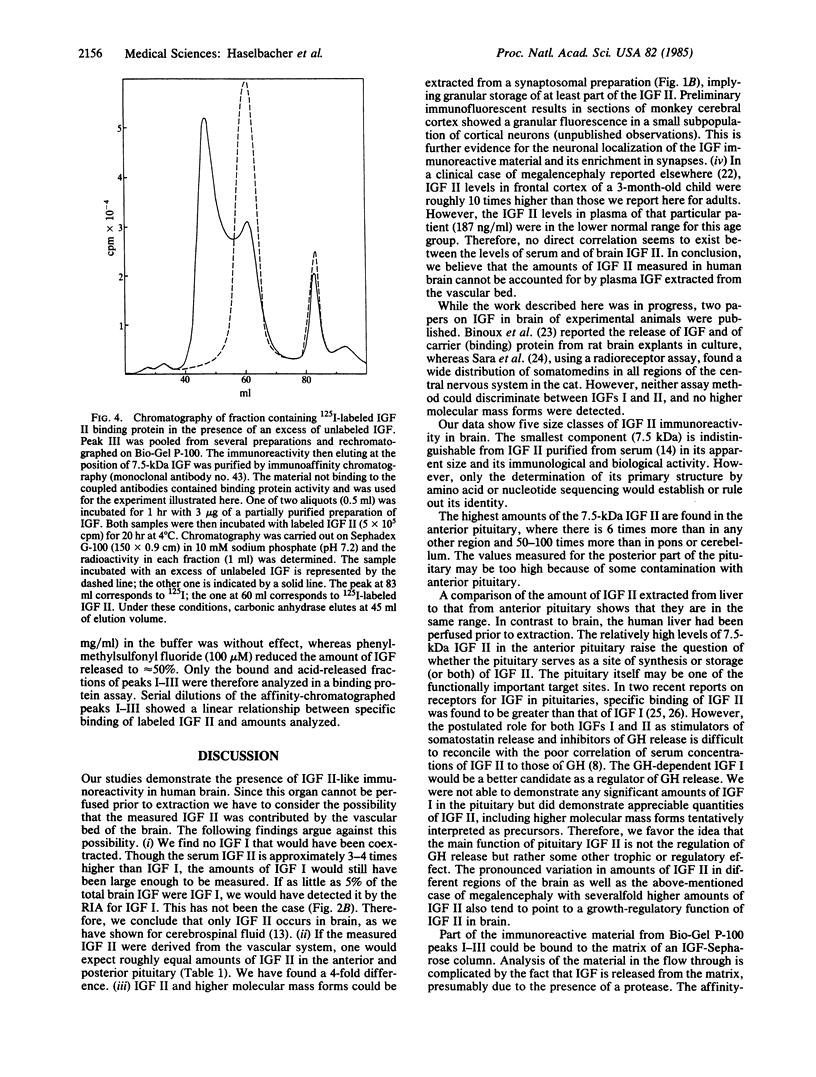
Twenty-four distinct areas of human brain were analyzed for the presence of insulin-like growth factor (IGF). As reported for cerebrospinal fluid, only IGF II-like immunoreactivity, but no significant amounts of IGF I-like immunoreactivity, could be found. Upon gel permeation chromatography, two to five distinct size classes were separated on the basis of their immunoreactivity. The smallest component had an apparent molecular mass of 7.5 kDa, identical to the one of purified IGF II from human serum. Radioimmunoassays and a bioassay also gave results indistinguishable from those of serum IGF II. The highest amounts of IGF II-like immunoreactivity occur in the anterior pituitary--namely, 20-25 pmol equivalents/g of wet weight. This is up to 100 times more than in most other brain regions analyzed. The higher molecular mass immunoreactive species were partially characterized. After immunoaffinity purification, the 38- and 26-kDa species are active in a bioassay. Specific IGF-binding protein activity could be shown after purification of the 38- and 26-kDa species on an IGF-affinity column. The 13-kDa species released significant amounts of 7.5-kDa material. The results are interpreted as evidence for the presence of IGF II synthesized locally in human brain. The structure of the larger forms of IGF II-like immunoreactive material as well as the function of IGF II in brain are not yet known.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acquaviva A. M., Bruni C. B., Nissley S. P., Rechler M. M. Cell-free synthesis of rat insulin-like growth factor II. Diabetes. 1982 Jul;31(7):656–658. doi: 10.2337/diab.31.7.656. [DOI] [PubMed] [Google Scholar]
- Adams S. O., Nissley S. P., Handwerger S., Rechler M. M. Developmental patterns of insulin-like growth factor-I and -II synthesis and regulation in rat fibroblasts. Nature. 1983 Mar 10;302(5904):150–153. doi: 10.1038/302150a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Merryweather J. P., Sanchez-Pescador R., Stempien M. M., Priestley L., Scott J., Rall L. B. Sequence of a cDNA clone encoding human preproinsulin-like growth factor II. 1984 Aug 30-Sep 5Nature. 310(5980):775–777. doi: 10.1038/310775a0. [DOI] [PubMed] [Google Scholar]
- Binoux M., Hossenlopp P., Lassarre C., Hardouin N. Production of insulin-like growth factors and their carrier by rat pituitary gland and brain explants in culture. FEBS Lett. 1981 Feb 23;124(2):178–184. doi: 10.1016/0014-5793(81)80131-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- Dull T. J., Gray A., Hayflick J. S., Ullrich A. Insulin-like growth factor II precursor gene organization in relation to insulin gene family. 1984 Aug 30-Sep 5Nature. 310(5980):777–781. doi: 10.1038/310777a0. [DOI] [PubMed] [Google Scholar]
- Furlanetto R. W., Underwood L. E., Van Wyk J. J., D'Ercole A. J. Estimation of somatomedin-C levels in normals and patients with pituitary disease by radioimmunoassay. J Clin Invest. 1977 Sep;60(3):648–657. doi: 10.1172/JCI108816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer C. G., De Stéphano L., Lai W. H., Guyda H. J., Posner B. I. Characterization of insulin-like growth factor receptors in rat anterior pituitary, hypothalamus, and brain. Endocrinology. 1984 Apr;114(4):1187–1195. doi: 10.1210/endo-114-4-1187. [DOI] [PubMed] [Google Scholar]
- Haselbacher G., Humbel R. Evidence for two species of insulin-like growth factor II (IGF II and "big" IGF II) in human spinal fluid. Endocrinology. 1982 May;110(5):1822–1824. doi: 10.1210/endo-110-5-1822. [DOI] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., Ricker A. T., Bullock B., Woods D. E., Gabbay K. H., Nussbaum A. L., Sussenbach J. S., Van den Brande J. L. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983 Dec 8;306(5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Svoboda M. E., Van Wyk J. J. Sequence analysis of somatomedin-C: confirmation of identity with insulin-like growth factor I. Endocrinology. 1983 Jun;112(6):2215–2217. doi: 10.1210/endo-112-6-2215. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Ruppert S., Schmale H., Rehbein M., Richter D., Schütz G. Deduced amino acid sequence from the bovine oxytocin-neurophysin I precursor cDNA. Nature. 1983 Mar 24;302(5906):342–344. doi: 10.1038/302342a0. [DOI] [PubMed] [Google Scholar]
- Laubli U. K., Baier W., Binz H., Celio M. R., Humbel R. E. Monoclonal antibodies directed to human insulin-like growth factor I (IGF I). Use for radioimmunoassay and immunopurification of IGF. FEBS Lett. 1982 Nov 22;149(1):109–112. doi: 10.1016/0014-5793(82)81082-x. [DOI] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Rechler M. M., Zapf J., Nissley S. P., Froesch E. R., Moses A. C., Podskalny J. M., Schilling E. E., Humbel R. E. Interactions of insulin-like growth factors I and II and multiplication-stimulating activity with receptors and serum carrier proteins. Endocrinology. 1980 Nov;107(5):1451–1459. doi: 10.1210/endo-107-5-1451. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. Amino-terminal sequences of two polypeptides from human serum with nonsuppressible insulin-like and cell-growth-promoting activities: evidence for structural homology with insulin B chain. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4379–4381. doi: 10.1073/pnas.73.12.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. Polypeptides with nonsuppressible insulin-like and cell-growth promoting activities in human serum: isolation, chemical characterization, and some biological properties of forms I and II. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2365–2369. doi: 10.1073/pnas.73.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht E., Humbel R. E. Primary structure of human insulin-like growth factor II. FEBS Lett. 1978 May 15;89(2):283–286. doi: 10.1016/0014-5793(78)80237-3. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Ceda G., Wilson D. M., Dollar L. A., Hoffman A. R. Characterization of high affinity receptors for insulin-like growth factors I and II on rat anterior pituitary cells. Endocrinology. 1984 May;114(5):1571–1575. doi: 10.1210/endo-114-5-1571. [DOI] [PubMed] [Google Scholar]
- Sara V. R., Uvnäs-Moberg K., Uvnäs B., Hall K., Wetterberg L., Posloncec B., Goiny M. The distribution of somatomedins in the nervous system of the cat and their release following neural stimulation. Acta Physiol Scand. 1982 Aug;115(4):467–470. doi: 10.1111/j.1748-1716.1982.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Hauri C., Steiner T., Froesch E. R. Comparison of in vivo effects of insulin-like growth factors I and II and of growth hormone in hypophysectomized rats. Acta Endocrinol (Copenh) 1985 Feb;108(2):167–174. doi: 10.1530/acta.0.1080167. [DOI] [PubMed] [Google Scholar]
- Schoenle E., Zapf J., Humbel R. E., Froesch E. R. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982 Mar 18;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- Schwander J. C., Hauri C., Zapf J., Froesch E. R. Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology. 1983 Jul;113(1):297–305. doi: 10.1210/endo-113-1-297. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulou-Sellin R., Phillips L. S. Extraction of somatomedin activity from rat liver. Endocrinology. 1982 Feb;110(2):582–589. doi: 10.1210/endo-110-2-582. [DOI] [PubMed] [Google Scholar]
- Zapf J., Froesch E. R., Humbel R. E. The insulin-like growth factors (IGF) of human serum: chemical and biological characterization and aspects of their possible physiological role. Curr Top Cell Regul. 1981;19:257–309. doi: 10.1016/b978-0-12-152819-5.50024-5. [DOI] [PubMed] [Google Scholar]
- Zapf J., Morell B., Walter H., Laron Z., Froesch E. R. Serum levels of insulin-like growth factor (IGF) and its carrier protein in various metabolic disorders. Acta Endocrinol (Copenh) 1980 Dec;95(4):505–517. doi: 10.1530/acta.0.0950505. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Jagars G., Sand I., Grunwald J., Froesch E. R. Inhibition of the action of nonsuppressible insulin-like activity on isolated rat fat cells by binding to its carrier protein. J Clin Invest. 1979 May;63(5):1077–1084. doi: 10.1172/JCI109377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981 Nov;68(5):1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]


