Abstract
Background
Acquisition of nicotine self-administration in rodents is relatively difficult to establish and measures of acquisition rate are sometimes confounded by manipulations used to facilitate the process. This study examined acquisition of nicotine self-administration without such manipulations and used mathematical modeling to define the criterion for acquisition.
Methods
Rats were given 20 daily 2-h sessions occurring 6 days/week in chambers equipped with active and inactive levers. Each active lever press resulted in nicotine reinforcement (0–0.6 mg/kg, IV) and retraction of both levers for a 20-s time out, whereas inactive lever presses had no consequences. Acquisition was defined for individual rats by the higher likelihood of reinforcers obtained across sessions fitting a logistic over a constant function according to the corrected Akaike information criterion (AICc).
Results
For rats that acquired self-administration, an AICc-based multi-model comparison demonstrated that the asymptote (highest number of reinforcers/session) and mid-point of the acquisition curve (h; the number of sessions necessary to reach half the asymptote) varied by nicotine dose, with both exhibiting a negative relationship (the higher the dose, the lower number of reinforcers and the lower h).
Conclusions
The modeling approach used in this study provides a way of defining acquisition of nicotine self-administration that takes advantage of all data from individual subjects and the procedure used is sensitive to dose differences in the absence of manipulations that influence acquisition (e.g., food restriction, prior food reinforcement, conditioned reinforcers).
Keywords: Nicotine, Self-administration, Akaike Information Criterion, Acquisition
1. Introduction
Nicotine has relatively weak intrinsic reinforcing effects compared to other drugs of abuse despite having a high addiction liability. For instance, response-contingent cues along with nicotine infusions are typically used to establish and maintain nicotine self-administration because the behavior is much less reliable without this procedure (Caggiula et al., 2002a; Palmatier et al., 2006). During nicotine self-administration, light cues facilitate acquisition as well as progression to more demanding partial reinforcement schedules (Caggiula et al., 2002b). Stimuli commonly used in rodent drug self-administration paradigms, such as the onset or termination of a light, possess mild primary reinforcing effects (Deroche-Gamonet et al., 2002; Palmatier et al., 2006; Palmatier et al., 2007; Raiff and Dallery, 2009). Interestingly, Palmatier et al. (2006) found that control of a light cue is more reinforcing in rats than nicotine itself. Furthermore, non-contingent (i.e., experimenter delivered) nicotine enhances responding for a light stimulus, demonstrating that the primary reinforcing effects of visual stimuli are enhanced when rats are under the influence of nicotine (Chaudhriet al., 2007; Palmatieret al., 2007). After a withdrawal period, nicotine-seeking is reinstated more robustly by using the cues previously paired with the nicotine delivery than by using a nicotine priming injection (LeSage et al., 2004). A light cue also facilitates acquisition of cocaine reinforcement; however, it is important to note that a visual cue does not directly modify the primary reinforcing effects of cocaine (Deroche-Gamonetet al., 2002) as it does with nicotine (Chaudhri et al., 2006b). Thus, when investigating acquisition of nicotine reinforcement per se, it is important that co-presented, response-contingent cues lack primary reinforcing effects.
Several criteria have been used to operationally define acquisition of nicotine self-administration that rely on an arbitrary endpoint performance measure. For instance, some researchers use stability criteria (e.g., less than ±15% variation over 2 consecutive days) with a requirement for minimum number of reinforcers obtained (Belluzzi et al., 2005; Chaudhri et al., 2005; Yoshimura et al., 2007). Others determine acquisition by relative rates or ratios of active to inactive lever presses (Brower et al., 2002; Chen et al., 2007; Donny et al., 2003; Harris et al., 2009; LeSage et al., 2003; Valentine et al., 1997; Wang et al., 2008), while others use statistically significant differences between groups (Chaudhri et al., 2006a; Levin et al., 2007; O’Dell et al., 2007). These criteria have been useful for identifying factors that influence acquisition of nicotine self-administration; however, they provide limited information about the acquisition process especially with respect to individual subjects. Such changes are visible in the entire acquisition curve. Furthermore, the assessment of changes in performance requires the estimation of performance parameters from individual subjects, because changes in average performance are often not representative of changes in individual performance (Estes, 1956).
Only a few studies have employed mathematical modeling approaches that support the estimation of meaningful performance parameters from individual acquisition curves. For instance, growth curve modeling has been used to examine individual differences in acquisition of nicotine self-administration, as well as factors that influence the trajectory and shape of acquisition curves (Donny et al., 2004; Lanza et al., 2004). In these studies, however, food pre-training, food restriction and drug-associated light stimuli were employed to facilitate acquisition, and these manipulations influence nicotine self-administration acquisition rates apart from nicotine reinforcement per se.
This study examined an alternative mathematical modeling approach for defining an acquisition criterion for nicotine self-administration. Male rats at the transition between adolescence and young adulthood were trained for 20 sessions during which they had response-contingent access to one of three doses of intravenous nicotine. We then determined whether individual reinforcement rate data met the acquisition criterion of reasonable goodness of fit to a generalized logistic (i.e., S-shaped) function. Acquisition curves are typically S-shaped as there is an initial baseline before learning has occurred, followed by a change in performance that reaches an asymptote over time (Hartz et al., 2001). Corrected Akaike Information Criterion (AICc) was used to select between two possible models of acquisition for each rat: a logistic function demonstrating acquisition, and a constant function (i.e., a flat line) demonstrating that no acquisition had occurred. For those rats that demonstrated acquisition, AICc was applied to determine which acquisition parameters varied significantly across doses of nicotine. No response-contingent light cues were presented with reinforcer delivery nor was prior operant conditioning with a natural reinforcer or food motivation (i.e., food restriction or lever baiting) used in this experiment as these manipulations affect acquisition of self-administration apart from nicotine reinforcement (Caggiula et al., 2002a; Clemens et al., 2010; Donny et al., 1998).
2. Materials and Methods
2.1 Animals
Male Sprague-Dawley rats were pair-housed pre-surgery, then single housed post-surgery in a climate-controlled colony with a 12-h reverse light/dark cycle (lights off at 7:00 AM). Rats arrived on post-natal day (PND) 38 and were acclimated to handling for a few min each on each of 11 consecutive days. Care of the animals was in accordance with the Guide for the Care and Use of Laboratory Animals (Clark et al., 1997) and all procedures were approved by the IACUC at Arizona State University.
2.2 Drugs
(−)Nicotine hydrogen tartrate (Sigma, St. Louis, MO) was dissolved in saline, adjusted to a pH of 7.4 ± 0.1, and then filtered through a 0.2 μm filter. Doses were delivered IV at a volume of 0.1 ml and were based on the concentration of nicotine base.
2.3 Surgery
On PND 50–52, catheters were implanted intravenously as described by Pentkowski et al., (2009) under isoflurane gas (2–4%) anesthesia. The catheters were tunneled subcutaneously along the neck, exited through an incision across the skull, and were secured to the top of the skull using dental acrylic and anchor screws. To maintain catheter patency, a 0.1 ml solution of saline containing heparin sodium (70 USP U/ml; Baxter Healthcare Corporation, Deerfield, IL) and ticarcillin disodium (66.67 mg/ml; GlaxoSmithKline, Research Triangle Park, NC) was administered daily. For the first three to five days after surgery, the solution also contained the thrombolytic agent urokinase (20 mg/ml; ImaRX Therapeutics, Inc., Tucson, AZ). Rats were given buprenorphine, a partial opioid agonist, immediately prior to surgery (0.05 mg/kg SC). Catheter patency was confirmed periodically and on the last of self-administration by infusing 0.05 ml methohexital sodium (16.67 mg/ml, IV; Sigma), which produces brief anesthetic effects only when administered IV.
2.4 Apparatus
Self-administration took place in operant conditioning chambers (20 × 28 × 20 cm) equipped with two levers mounted on the front wall (Med Associates, St. Albans, VT) and housed inside sound-attenuating chambers. The self-administration chambers also had a cue light above one lever and a house light mounted on the top center of the back wall, but these features were not used in this experiment. Infusion pumps (Razel, St. Albans, VT) were located outside the chambers and contained 30 ml syringes attached via Tygon tubing to liquid swivels (Instech, Plymouth Meeting, PA). The outlet of the swivels was fastened to the catheters via Tygon tubing that ran through a metal spring leash (Plastics One, Roanoke, VA).
2.5 Nicotine self-administration dose-response without food restriction, lever baiting, or drug-paired light cues
All rats were given ad libitum access to rat chow and water in the home cage for the duration of this experiment. After 5–6 days of recovery from surgery, rats were randomly divided into groups that were either assigned to press a lever reinforced by nicotine infusions (0.015, 0.03, or 0.06 mg/kg/0.1 ml, IV; n=17, 15, 15 respectively) or saline infusions (Saline group; n = 11) beginning on PND 58. Self-administration sessions occurred daily for 2 h at the same time of day for a total of 20 sessions conducted 6 days/week. During the sessions, active lever presses on a fixed ratio 1 (FR1) schedule of reinforcement resulted in retraction of both levers, followed 0.5 s later by a 0.1-ml infusion of the assigned dose for 1.2 s. Upon completion of the 1.2-s infusion, the levers remained retracted for a 20–s time out. The house light was off for the entire session. Responses on the inactive lever were recorded but had no scheduled consequences. Designation of the right versus left lever as the active lever was counterbalanced.
Catheter patency was verified after the last session. The final number of subjects in each dose group whose catheters remained patent was n = 13 for 0.015 mg/kg dose, n = 11 for 0.03 mg/kg dose, and n = 12 for 0.06 mg/kg dose.
2.6 Acquisition Model
A logistic model was used to estimate acquisition parameters of nicotine self-administration in individual subjects,
| (1) |
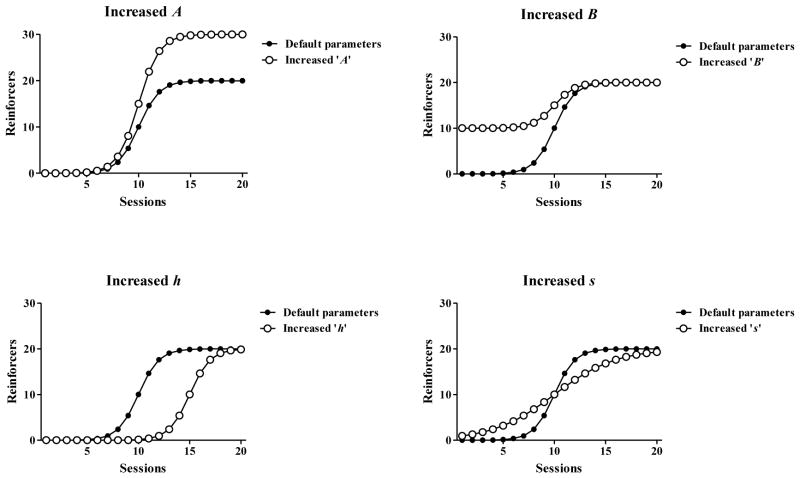
where R(t) is the number of nicotine reinforcers collected each day t, A is the asymptotic number of reinforcers/session; B is the baseline number of reinforcers/session at the beginning of training; h is the session at which half of the acquisition process is completed (i.e., R(h) = (A + B)/2); s is the slope of the acquisition process. Figure 1 illustrates how each individual parameter contributes to the shape of the logistic function. Parameters were estimated by implementing the method of least squares using the Solver add-in of Microsoft Excel 2003.
Figure 1.
Theoretical parameter fluctuations in the logistic function. The example logistic function is shown using the black symbols with A = 20 reinforcers (asymptote), B = 0 reinforcers (baseline), h = 10 sessions (half-life of the acquisition process) and s = 1 session (slope), where R is the number of nicotine reinforcers collected at each day t. The open symbols show changes in the example function as a result of selective changes in parameter values. The ‘Increased A’ graph depicts the function when the asymptote parameter is increased from 20 to 30 reinforcers, ‘Increased B’ depicts a baseline parameter increase from 0 to 10 reinforcers, ‘Increased h’ depicts an increase in mid-acquisition day from 10 to 15 sessions, and the ‘Increased s’ depicts a threefold increase in slope (1/s).
The corrected Akaike Information Criterion (AICc) was used to determine whether the 4 free parameters of Equation 1 accounted for substantially more variance in each rat’s data than the single free parameter of a constant function, R(t) = c (see Supplementary Material for details of the AICc analysis1; for further details see (Burnham and Anderson, 2002). In essence, this approach allowed us to determine whether each rat’s data were best described by an S-shaped curve that demonstrates a change in performance relative to an initial baseline (i.e., acquisition) versus a straight line demonstrating no systematic change from baseline.
To infer that acquisition had taken place, Equation 1 had to be selected by AICc (i.e., dAICc = AICc (constant) – AICc (logistic) > 4) and the estimated asymptote (A) had to be greater than the estimated baseline (B) by a minimum of 10 reinforcers (i.e., A – B>10). Because some animals had not yet reached asymptotic performance by the last session, estimates of A from the logistic model without any correction yielded unreasonable values (e.g., hundreds of reinforcers). Therefore, in such cases we used an estimate of A equal to ten percent above the highest number of reinforcers obtained by any rat in the same dose group across all self-administration sessions (capped values: 0.015 mg/kg = 60.5, 0.03 mg/kg = 47.3, and 0.06 mg/kg = 36.3). All rats had constraints imposed on A, however, only 3 rats met the cap (1 in the 0.03 mg/kg and 2 in the 0.06 mg/kg dose group). It is important to note that limiting A likely had little effect on h estimates since these estimates were already very high before capping (i.e., h occurred at the end of the function) and remained high afterwards since the limitation was constrained above what any given rat obtained per session. B was also limited to no more than 10 reinforcers which was justified because no animal exceeded 10 reinforcers on the first day of training and was necessary to avoid including misleading bends in the early part of the curve for animals that had higher responding on the first day (1 in the saline group, 2 in the 0.015 mg/kg dose group, one of which did not acquire); s was set to a minimum of 0.1 sessions, which would indicate a step-shaped learning curve. As a result of this curve-fitting procedure, animals that displayed logistic acquisition curves were designated as part of the ‘Acquired’ group, whereas animals that displayed flat acquisition curves were designated as part of the ‘Not Acquired’ group.
2.7 Dose-effect Models
Nicotine dose effects were further examined in the animals in the Acquired group using a multi-model comparison analysis. Fits for Equation 1 over the function of reinforcers/session were used to select between models that accounted for the effect of nicotine dose on acquisition. Model selection assessed which parameters (A, B, h) were justified to vary freely and which were likely to remain constant across dose groups. We compared 8 different models that allowed all combinations of mean parameter estimates to vary freely across dose groups. Specifically, the mean of none, only A, only B, only h, A and B, A and h, B and h, or all three parameters were allowed to vary freely across dose groups. Similar to the acquisition model, s was set to a minimum of 0.1 sessions. The standard deviation of each parameter estimate within each dose group was set to remain equal across dose groups. In addition, no caps were necessary on A or B in these models. The model that postulated no change in mean parameter estimates across doses served as the “null hypothesis.” It is important to note that parameters for individual rats were always allowed to vary freely; what distinguished one model from another were the mean parameter estimates that were constrained to be constant across groups. In this analysis, B served as our negative control parameter (i.e., we did not expect it to vary between groups). Also, the highly skewed distribution of estimates of s made it very difficult to articulate any a priori claims regarding s in relation to dose. Therefore, mean s was allowed to vary freely across dose groups in all models, and ostensible changes in mean s as a function of dose were not examined.
Models were fit to the acquisition data of each individual rat using the method of least squares. Residual sum of squares (RSS) and corrected AIC (AICc) values were computed for each model (see Supplementary Material2). The lowest AICc (AICcMIN) indicated the most likely model, corrected for the number of free parameters. AICc scores for each model (AICcj, where j indexes the model) were then rescaled as their difference relative to AICcMIN (ΔAICcj = AICcj – AICcMIN; ΔAICcMIN = 0). ΔAICcj thus indicated the likelihood of each model j, relative to the most likely model, after correcting for free parameters; lower ΔAICc were indicative of higher likelihood. Based on standard practice, the model with fewer free parameters and a ΔAICc < 4 was selected; its parameters were taken as the best estimates of the acquisition function under each dose.
Correlations were also conducted for parameter estimates of all animals that acquired. The absence of correlations between model parameters is indicative of the reliability of the individual parameter estimates.
3. Results
Twenty-one rats acquired nicotine self-administration out of 36 rats that had access to nicotine. Descriptively, the percentage of rats that acquired self-administration across dose groups exhibited an inverted U-shaped pattern with less than half acquiring at the lowest dose and the highest percentage acquiring at the intermediate dose (see Table 1). Rats that acquired nicotine self-administration had mean dAICc values of 28.07 ± 2.8 (indicative of the logistic model as the best fit); rats that did not acquire had mean dAICc values of −2.49 ± 1.5 (indicative of the constant model for the best fit). As expected, a constant function best fit the data of all saline control rats, consistent with a lack of acquisition in this group. The mean number of reinforcers per session during the last five days of self-administration is shown in Figure 2. Descriptively, these data also exhibited an inverted U-shaped pattern. Saline controls displayed the lowest number of reinforcers obtained, with the animals that received 0.015 mg/kg nicotine obtaining the highest number of reinforcers, followed by the 0.03 mg/kg group, and then the 0.06 mg/kg group.
Table 1.
Number of animals meeting the acquisition criterion by dose group.
| Group | Animals acquired*/total number in group | Proportion of group that acquired |
|---|---|---|
| Saline | 0/11 | .00 |
| 0.015 mg/kg | 6/13 | .46 |
| 0.03 mg/kg | 8/11 | .73 |
| 0.06mg/kg | 7/12 | .58 |
A – B > 10 and dAICc value ≥ 4 was used as a criterion for successful acquisition of nicotine self-administration.
Figure 2.
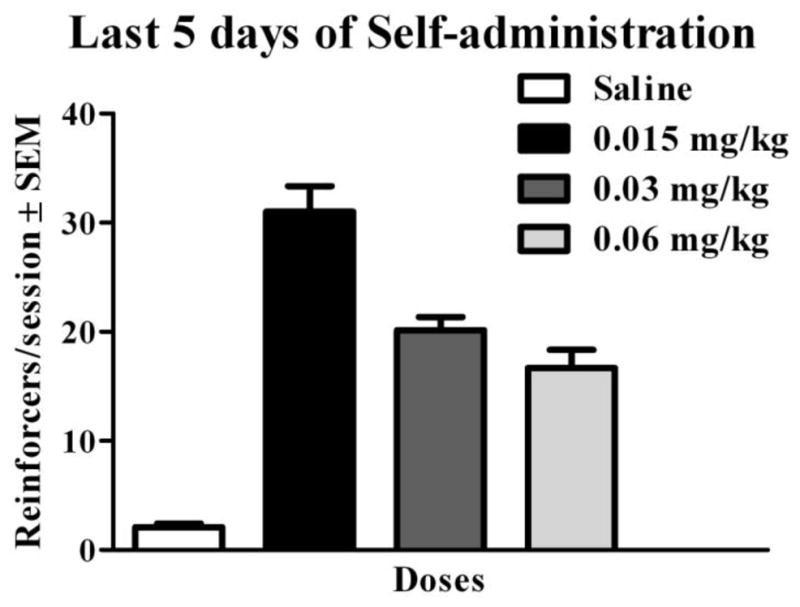
Mean number of reinforcers obtained (± SEM) during the last five days of self-administration for Acquired and Saline rats. As expected, an inverted U-shaped dose-response curve was observed.
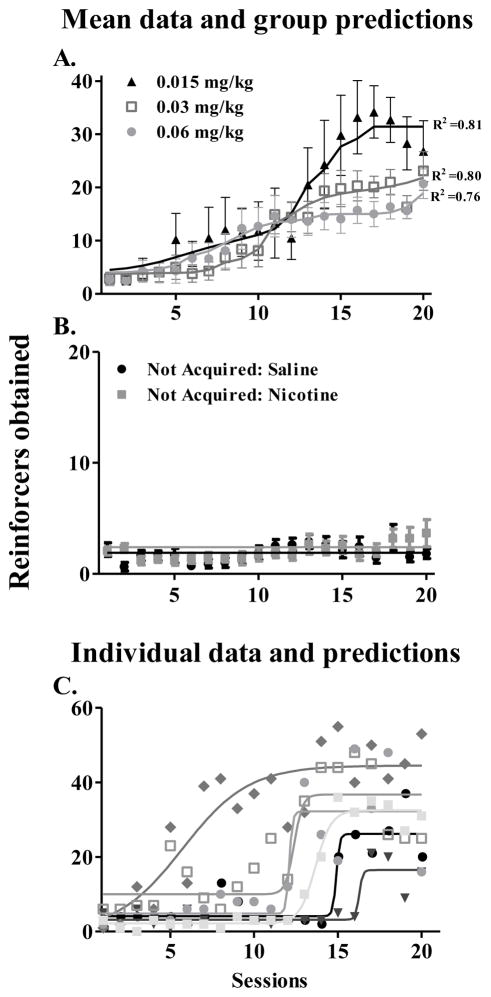
Figure 3 shows the mean acquisition data of Acquired and Not Acquired rats. As we had expected, of the 8 possible candidate models, the model in which B remains constant while A and h vary between nicotine doses was selected (Table 2). Mean traces of this model are shown as continuous lines in Figure 3. Examples of individual raw data and corresponding model predictions for rats that acquired in the 0.015 mg/kg dose group are also shown in Figure 3.
Figure 3.
Mean reinforcers obtained (± SEM) by animals that acquired nicotine self-administration in each dose group (A), animals that had not acquired pooled from nicotine dose groups and all saline animals (B), and individual data from animals that acquired in the 0.015 mg/kg dose group (C) across the 20 training sessions. Continuous lines shown in A are average fitted traces of the selected logistic model (Equation 2), which held B constant and let A and h vary across doses. As anticipated, a dose-dependent effect was obtained on the asymptotic number of reinforcers obtained, which was greater for the lowest dose, followed by the medium and high doses. Goodness-of-fit of the model to each dose-group data is shown by R2 values. Continuous lines shown in B are fitted traces of the selected constant function (Equation 1) indicating no systematic change from baseline. All saline animals failed to acquire self-administration. Data from any of the nicotine groups that had failed to acquire nicotine self-administration (Not Acquired) did not differ from saline animals. Continuous lines shown in C are fitted traces of the selected logistic model to individual self-administration data for rats that acquired in the 0.015 mg/kg dose group to illustrate the relationship of the raw individual data to model predictions.
Table 2. Model comparison and selection.
The first column specifies which mean parameter estimates were allowed to vary freely between dose groups. The row labeled None corresponds to the model in which all mean estimates were fixed. RSS is the residual sum of squares over all subjects, k is the number of free parameters in the model. As each model was fitted to individual subject data, a set of free parameters was estimated for each dose group. AICc=2k+nln (RSS/n) + [2k(k+1)/(n−k−1)], where n is the total number of observations = 21 rats × 20 sessions = 420. Data for the model with the lowest AICs are underlined and signify the selected model.
| Model | RSS | k | ΔAICc |
|---|---|---|---|
| None | 9031.94 | 79 | 48.51 |
| A | 8421.96 | 81 | 25.27 |
| B | 8879.97 | 81 | 47.51 |
| h | 8691.84 | 81 | 38.51 |
| A, B | 8352.18 | 83 | 27.97 |
| A, h | 7814.06 | 83 | 0.00 |
| B, h | 8581.68 | 83 | 39.36 |
| A, B, h | 7779.75 | 85 | 4.42 |
Mean estimates of the parameters of the selected model are shown in Table 3. A and h exhibited a negative relationship with dose. The group receiving the highest dose of nicotine most readily acquired self-administration but took fewer infusions per session at asymptote, whereas the group receiving the lowest dose acquired self-administration later but took a higher number of infusions per session at asymptote.
Table 3.
Parameter estimates when mean A and h varied freely across dose groups (Model A, h in Table 2) including only those animals that had acquired nicotine self-administration based on the curve-fitting criterion. Values are the asymptote (A), baseline (B), and half-life (h; i.e., sessions to reach 50% of asymptote) parameters that were obtained from the selected model. The selected model suggests that B did not vary with dose, while A and h varied dose-dependently.
| Dose group | A (reinforcers) | h (sessions) | B (reinforcers) | s (sessions) |
|---|---|---|---|---|
| 0.015 mg/kg | 31.25 | 12.32 | 3.62 | 0.58 |
| 0.03 mg/kg | 24.12 | 11.49 | 3.62 | 0.85 |
| 0.06 mg/kg | 21.37 | 11.18 | 3.62 | 1.95 |
| SD | 9.64† | 4.00† | 2.90† | 2.01* |
Dagger (†) represents the estimated standard deviation (SD) of parameter estimates in every dose group (SDs were constrained to the same value across groups).
Asterisk (*) represents the SD of the slope (s) pooled across dose groups (SD was not constrained).
The parameter estimate correlations were not significant with p values between 0.17–0.67 and correlation coefficients ranging from −0.17 to 0.31.
4. Discussion
The goal of the present study was to devise a method to determine whether or not rats acquire nicotine self-administration when given 20 days of access in the absence of prior training and food motivation manipulations, and in the presence of a motivationally neutral lever retraction cue. Therefore, we posed the question of whether a given rat’s individual data was most likely described by a constant function (indicative of no acquisition of self-administration) or by a logistic function (indicative of acquisition). As expected, all rats assigned to the saline group failed to acquire self-administration using this criterion. Nearly half to three-fourths of animals that were given access to nicotine acquired self-administration (Table 1 and Figure 3). The data from the animals that maintained robust responding demonstrate acquisition of nicotine self-administration in the absence of prior training, food restriction or motivation, and light conditioned cues that have primary reinforcement properties.
To compare the patterns of nicotine self-administration across doses, we selected among models of logistic parameter sensitivity to nicotine dose based on goodness of fit and parsimony of these models. The selected model suggests that the baseline number of reinforcers before acquisition (B) is not sensitive to nicotine dose, whereas the asymptotic number of reinforcers after acquisition (A) and session when half of the acquisition process was completed (h) were sensitive to nicotine dose. Importantly, we found no significant correlations between any of the parameters, indicating that each model parameter probably accounted for different sources of variance in the data. The fact that a single average estimate of B describes acquisition curves regardless of nicotine dose is intuitive; reinforcement rates are not expected to vary before the acquisition process occurs. As sessions progress, rats obtain more reinforcers and therefore learn the response-nicotine reinforcement contingency leading to a rise in reinforcement rates. Differences in A and h suggest that, at higher doses of nicotine, self-administration was acquired sooner (lower h) and maintained at lower levels (lower A). The mean percent of variance accounted for by the model across 20 days of self-administration for each dose group was 81%, 80% and 76% as indicated by R2 values for 0.015, 0.03 and 0.06 mg/kg, respectively. Whether these differences in performance are due to motivational processes, learning processes, or the development of tolerance is yet unknown.
The dose-dependent effects observed in the present study are consistent with previously published data reporting differences in responding for nicotine in the rodent self-administration paradigm (Chen et al., 2007; Corrigall and Coen, 1989; Cox et al., 1984; Donny et al., 1998; Donny et al., 2000; Latiff et al., 1980; Watkins et al., 1999). The observed differences in the estimates of A and h across groups was expected because previous research has shown that drug dose plays a role in acquisition and reinforcement rates in drug self-administration models. For instance, dose is positively related to rate of acquisition of cocaine self-administration (Carroll and Lac, 1997; Gerrits and van Ree, 1995). In contrast to the present study, Lanza et al. (2004) did not find differences in the rate of acquisition or the shape of the curves across doses as measured by the parameters of their growth model. However Lanza et al. transitioned rats to a partial reinforcement schedule, whereas we implemented an FR1 schedule, and these schedule differences may underlie the discrepancy across these studies. Donny et al. (1998) found that mean reinforcement rates during acquisition increased more quickly for animals receiving 0.03 mg/kg compared to 0.06 mg/kg of nicotine on both an FR1 and FR2, but not on an FR5 schedule of reinforcement. In contrast, rats receiving 0.06 mg/kg nicotine in the present study on an FR1 schedule exhibited a lower h value, suggesting more rapid acquisition than those receiving 0.03 mg/kg under the same schedule. It is likely that schedule effects contribute to the shape of the acquisition curve. Though we did not investigate changes in reinforcement schedule in the present study, in principle these effects could be analyzed using our methods. Similar to Donny et al., (2004), we believe that estimates of acquisition parameters support a precise depiction of how acquisition differs between individual animals, as well as information regarding differences in acquisition between dose groups. It is also possible that the differences in our h estimates are not indicative of all doses being different from one another, but rather one dose could be different from the others.
Compared to most literature on nicotine self-administration, the acquisition functions obtained in the present study reflect a slower acquisition process and fewer animals acquiring overall (Caggiula et al., 2002a; Caggiula et al., 2002b; Chaudhri et al., 2007; Cox et al., 1984; Donny et al., 1995; Donny et al., 1998; Liu et al., 2006; Palmatier et al., 2007; Paterson and Markou, 2004; Shoaib et al., 1997). Not more than 46.2 to 72.7% of animals in a given nicotine dose group acquired self-administration. The high rates of failure to acquire are not surprising given that nicotine is a relatively weak reinforcer (Palmatieret al., 2006) and we did not use food restriction, operant pre-training with natural reinforcers, or drug-paired light cues that are known to facilitate acquisition. (Caggiula et al., 2002a; Caggiula et al., 2002b; Clemens et al., 2010; Donny et al., 1998; Liu et al., 2006). It has long been known that food restriction facilitates self-administration of abused drugs (Lang et al., 1977; Singer et al., 1978). In addition, prior training with natural rewards is generally employed to facilitate acquisition of operant responding for the drug upon subsequent training sessions (Ahmed et al., 2000; Baker et al., 2003; Di Ciano et al., 2001; Meil and See, 1996; Sutton et al., 2003; Weiss et al., 2000). This procedure results in higher response rates on the first day of self-administration (Bongiovanni and See, 2008; Donny et al., 1998) leading to a more rapid acquisition process (Donny et al., 1998) that is likely coupled with extinction of the initial response-reinforcer contingency.
In evaluating the utility of the present approach for describing the acquisition of drug self-administration, there are some limitations that need to be considered. One limitation stems from the forced constraints of B and A. In some cases where animals had begun to show learning but had not yet reached asymptote, it was necessary to create a somewhat arbitrary cap for parameter A in order for the fitted curve to yield realistic estimates. We constrained A to 10% above the maximum number of reinforcers obtained within a given dose group. We posit that the applied constraints to the estimates of A were within the biologically relevant range that any given animal in a dose group would naturally meet if allowed more time to reach asymptotic responding. The present study also makes no claims or interpretations regarding the slope of the acquisition curves, indexed by parameter s. The slope is typically steep, and is not representative of underlying learning processes (Gallistel et al., 2004). Indeed, based on the selected model, for one third of the rats that acquired self-administration, reinforcement increased from baseline to asymptote in one session, yielding a step acquisition function. Thus, the distribution of s was skewed and did not vary systematically as a function of dose. The sources of variability in acquisition slopes are still unknown and, therefore, uninterpretable.
Perhaps the most important feature of our approach is that we employed a motivationally neutral lever retraction cue to signal nicotine delivery and the time out. As mentioned previously, visual light cues are known to enhance the acquisition, maintenance, and reinstatement of nicotine-seeking as well as to promote the resistance to extinction of lever pressing (Caggiula et al., 2002a; Caggiula et al., 2001, 2002b; Donny et al., 1999; LeSage et al., 2004; Liu et al., 2008; Liu et al., 2006). When these stimuli are used in self-administration paradigms, high rates of nicotine reinforcement are observed across sessions (Chaudhriet al., 2007). However, it is important to note that these stimuli possess greater reinforcing properties when presented alone compared to access to nicotine alone, and their intrinsic reinforcing effects are enhanced while animals are under the influence of nicotine (Palmatieret al., 2006; Palmatier et al., 2007). The reinforcement-enhancing effects of nicotine also vary as a function of the incentive value of the non-pharmacological reinforcer as greater enhancement is observed with stimuli possessing greater natural incentive value (Chaudhri et al., 2006a; Palmatier et al., 2007). In contrast, lever retraction used in the present study appears to be a neutral cue because it did not support responding in the absence of nicotine (i.e., failed to produce acquisition of saline self-administration) and does not reinstate previously extinguished nicotine-seeking behavior (unpublished observation). Similarly, Sorge et al. (2009) used a motivationally neutral stimulus (i.e., white noise) as a cue to promote response contingency learning, without interfering with the primary reinforcing effects of nicotine. The animals that received the neutral white noise cue with saline infusions did not maintain robust responding; however, the animals that received a white noise cue paired with nicotine infusions demonstrated robust self-administration over and above nicotine alone, suggesting that the neutral cue may serve as a ‘temporal marker’ for the operant response and the pharmacological effects of nicotine.
Our approach of using a limited nicotine access paradigm may have maximized individual differences but also resulted in a slow rate of acquisition. One procedure that may facilitate acquisition while still preserving individual differences is to increase the time spent in the self-administration chamber (i.e., extended access). Enhanced reinforcement rates that are observed with extended access to nicotine may be due to rapid development of nicotine dependence and subsequent motivation to obtain the drug (O’Dell et al., 2007; O’Dell and Koob, 2007; Paterson and Markou, 2004). Future research using extended access may help to address the slow acquisition rates observed in the present study.
In conclusion, there are distinct features of our approach that are useful for examining acquisition of nicotine self-administration. First, we minimized manipulations that facilitate acquisition (i.e., food restriction, pre-training, light stimuli). Omitting these procedures likely allowed us to capture subtle effects of drug dose. Thus, it is likely that these procedures are advantageous for examining other variables that influence acquisition, such as such as effects of age, sex, hormone cycle, and drug pre-exposure. The second feature involves implementation of mathematical modeling to characterize how individual subject data changes as a function of time. In addition, our multi-model analysis allows for detection of group effects that may be obscured when using parametric statistics of end-point measures. In our view, the present approach promotes individual variability, while simultaneously exposing group differences, ultimately revealing more information about underlying behavior that has been largely overlooked in self-administration literature.
Supplementary Material
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIDA grants R01DA11064, R21DA023123, F31DA02746 and the Minority Graduate Education at Mountain State Alliance Program at Arizona State University. These funding sources had no further role in the study design, in the collection, analysis and interpretation of the data, in the writing of the report, or the decision to submit the paper for publication.
The authors wish to thank Lara Pockros, Nathan Pentkowski, Peter Kufahl, Lauren E. Hood, Mike R. Painter, Demi Hills, and Valeria Routt for assistance with surgery and data collection, and M. Foster Olive and Heather Bimonte-Nelson for their helpful comments as members of Natalie Peartree’s Master’s Thesis Committee.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by enterng doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Contributors
J.L. Neisewander, N.A. Peartree and K.J. Thiel designed the study and wrote the protocol. S.M. Weber collected data and assisted with their interpretation. F. Sanabria developed the modeling approach and interpretation. T.H.C. Cheung assisted with data analysis. N.A. Peartree drafted the manuscript under the direction of J.L. Neisewander and F. Sanabria. All authors contributed to preparation and approval of the final manuscript. N.A. Peartree prepared and defended a Master’s thesis based on this study.
Conflict of Interest
There are no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacol Biochem Behav. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer-Verlag; New York: 2002. [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002a;77:683–687. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002b;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i. v amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006a;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology (Berl) 2005;180:258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006b;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. Special report: the 1996 Guide for the Care and Use of Laboratory Animals. ILAR J. 1997;38:41–48. doi: 10.1093/ilar.38.1.41. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2010;211:43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Cox BM, Goldstein A, Nelson WT. Nicotine self-administration in rats. Br J Pharmacol. 1984;83:49–55. doi: 10.1111/j.1476-5381.1984.tb10118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Changes in dopamine efflux associated with extinction, CS-induced and d-amphetamine-induced reinstatement of drug-seeking behavior by rats. Behav Brain Res. 2001;120:147–158. doi: 10.1016/s0166-4328(00)00373-9. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Donny EC, Lanza ST, Balster RL, Collins LM, Caggiula A, Rowell PP. Using growth models to relate acquisition of nicotine self-administration to break point and nicotinic receptor binding. Drug Alcohol Depend. 2004;75:23–35. doi: 10.1016/j.drugalcdep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Estes WK. The problem of inference from curves based on group data. Psychol Bull. 1956;53:134–140. doi: 10.1037/h0045156. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proc Natl Acad Sci USA. 2004;101:13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrits MA, van Ree JM. Assessment of motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Pharmacol Biochem Behav. 1995;52:35–42. doi: 10.1016/0091-3057(95)00112-a. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Correlates of individual differences in compensatory nicotine self-administration in rats following a decrease in nicotine unit dose. Psychopharmacology (Berl) 2009;205:599–611. doi: 10.1007/s00213-009-1567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Ben-Shahar Y, Tyler M. Logistic growth curve analysis in associative learning data. Anim Cogn. 2001;4:185–189. [Google Scholar]
- Lang WJ, Latiff AA, McQueen A, Singer G. Self administration of nicotine with and without a food delivery schedule. Pharmacol Biochem Behav. 1977;7:65–70. doi: 10.1016/0091-3057(77)90012-0. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Donny EC, Collins LM, Balster RL. Analyzing the acquisition of drug self-administration using growth curve models. Drug Alcohol Depend. 2004;75:11–21. doi: 10.1016/j.drugalcdep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Latiff AA, Smith LA, Lang WJ. Effects of changing dosage and urinary pH in rats self-administering nicotine on a food delivery schedule. Pharmacol Biochem Behav. 1980;13:209–213. doi: 10.1016/0091-3057(80)90075-1. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196:365–375. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology (Berl) 2006;184:417–425. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Koob GF. ’Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Acosta JI, Browning JR, Hamilton EC, Neisewander JL. Stimulation of 5-HT(1B) receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict Biol. 2009;14:419–430. doi: 10.1111/j.1369-1600.2009.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behav Processes. 2009;82:95–99. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Singer G, Simpson F, Lang WJ. Schedule induced self injections of nicotine with recovered body weight. Pharmacol Biochem Behav. 1978;9:387–389. doi: 10.1016/0091-3057(78)90302-7. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Pierre VJ, Clarke PB. Facilitation of intravenous nicotine self-administration in rats by a motivationally neutral sensory stimulus. Psychopharmacology (Berl) 2009;207:191–200. doi: 10.1007/s00213-009-1647-8. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen H, Sharp BM. Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. J Neurochem. 2008;106:943–956. doi: 10.1111/j.1471-4159.2008.05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, Leslie FM, Gee KW. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. J Pharmacol Exp Ther. 2007;323:907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.