Abstract
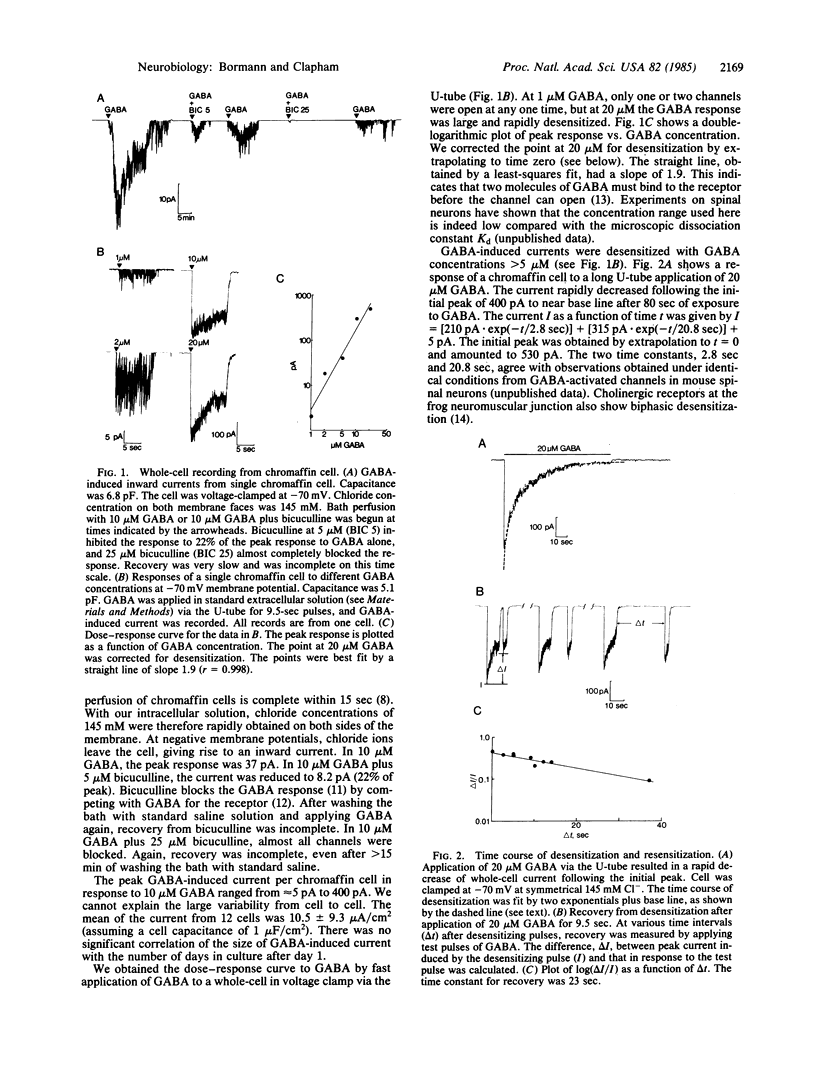
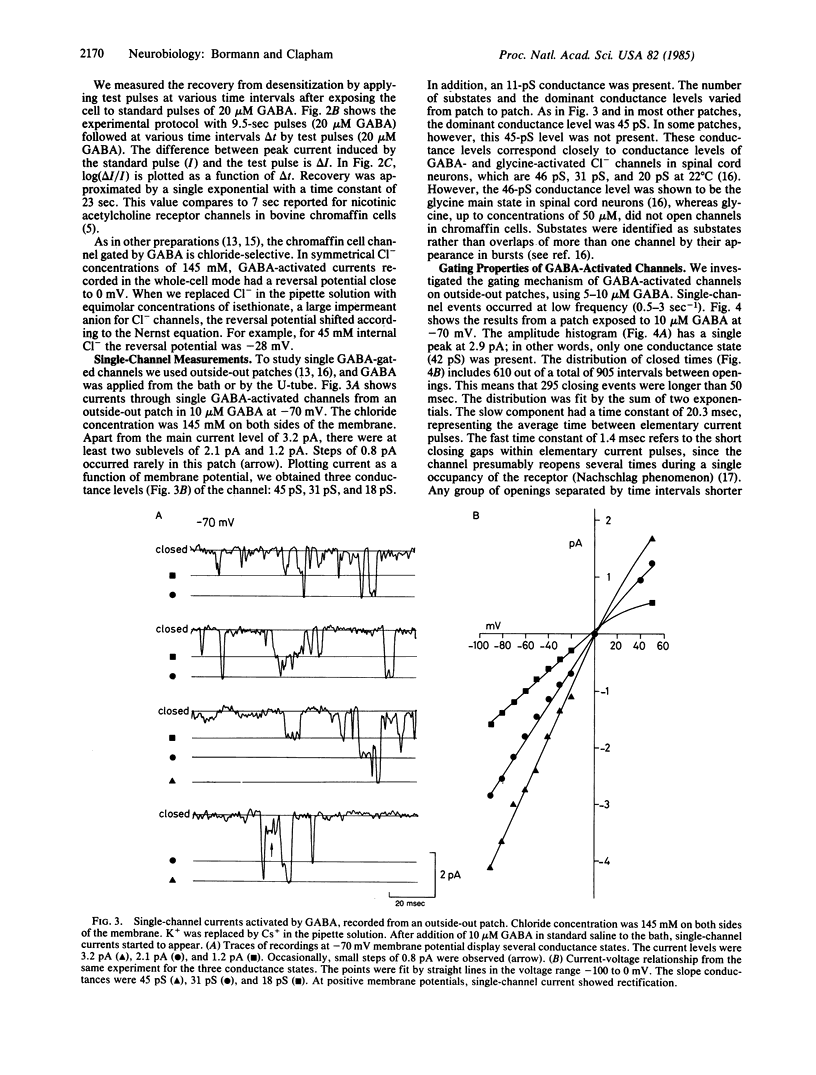
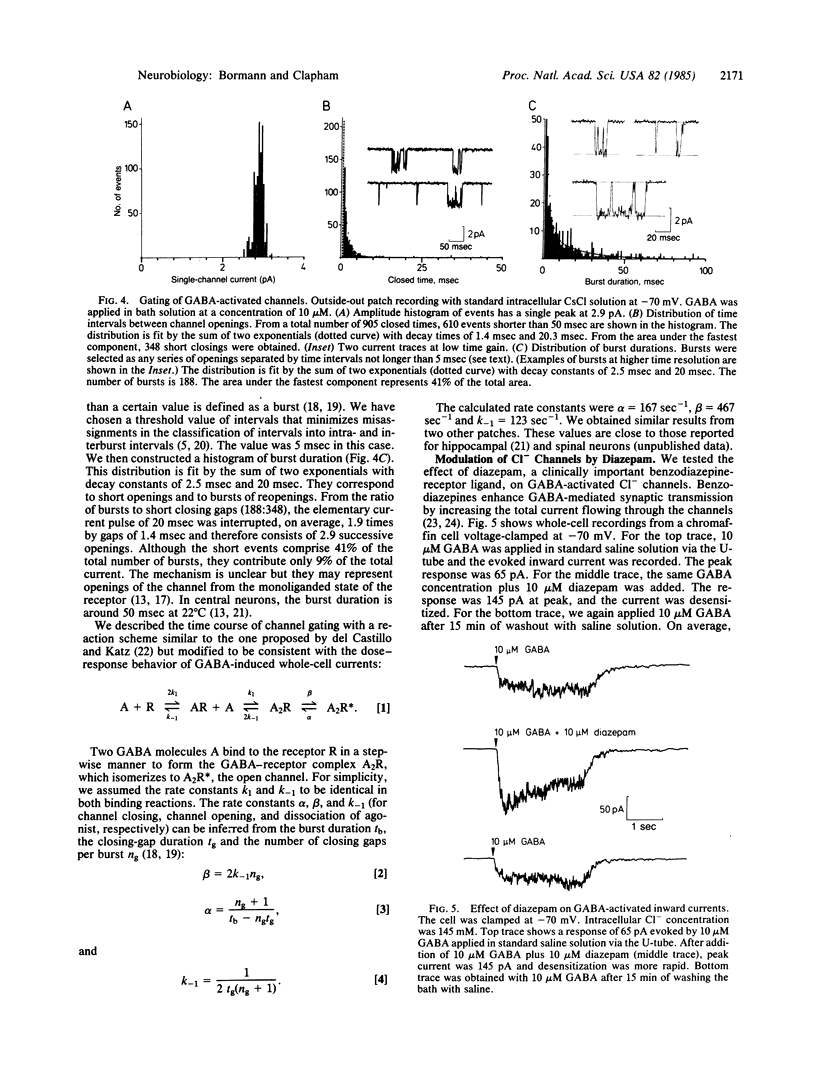
We have studied membrane channels activated by gamma-aminobutyric acid (GABA) in adrenal medullary chromaffin cells by using patch-clamp techniques. These channels share many properties with GABA-receptor channels in the central nervous system. They are chloride-selective, blocked by the GABA antagonist bicuculline, and reversibly desensitized at high GABA concentrations. The dose-response curve has a slope of 2 in the Hill plot, indicating a bimolecular binding reaction of GABA to the receptor. Single-channel currents display multiple conductance states as do glycine-activated chloride channels in mouse spinal neurons. Gating properties of GABA-activated channels, as described by a sequential model for agonist-activated channels, are similar to gating properties in central neurons. GABA-induced currents are potentiated by diazepam, indicating that anxiolytic drugs like the benzodiazepines might be involved in the regulation of anxiety states in the peripheral nervous system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J. L., Ransom B. R. Amino acid pharmacology of mammalian central neurones grown in tissue culture. J Physiol. 1978 Jul;280:331–354. doi: 10.1113/jphysiol.1978.sp012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Price G. W., Hudson A. L., Hill D. R., Wilkin G. P., Turnbull M. J. GABA receptor multiplicity. Visualization of different receptor types in the mammalian CNS. Neuropharmacology. 1984 Feb;23(2B):219–231. doi: 10.1016/0028-3908(84)90063-7. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. Chlordiazepoxide selectively potentiates GABA conductance of spinal cord and sensory neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):621–631. doi: 10.1152/jn.1981.45.4.621. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neher E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J Physiol. 1984 Feb;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 24;300(1098):1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. Relaxation and fluctuations of membrane currents that flow through drug-operated channels. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):231–262. doi: 10.1098/rspb.1977.0137. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Bormann J., Sakmann B. Activation of multiple-conductance state chloride channels in spinal neurones by glycine and GABA. 1983 Oct 27-Nov 2Nature. 305(5937):805–808. doi: 10.1038/305805a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y., Gutman Y., Guidotti A., Panula P., Wroblewski J., Cosenza-Murphy D., Wu J. Y., Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Kozousek V., Mizobe F., Dean D. M. Substance P inhibits nicotinic activation of chromaffin cells. Nature. 1979 Mar 15;278(5701):256–257. doi: 10.1038/278256a0. [DOI] [PubMed] [Google Scholar]
- Nowak L. M., Young A. B., Macdonald R. L. GABA and bicuculline actions on mouse spinal cord and cortical neurons in cell culture. Brain Res. 1982 Jul 22;244(1):155–164. doi: 10.1016/0006-8993(82)90913-1. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Bormann J., Hamill O. P. Ion transport by single receptor channels. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):247–257. doi: 10.1101/sqb.1983.048.01.027. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Hamill O. P., Bormann J. Patch-clamp measurements of elementary chloride currents activated by the putative inhibitory transmitter GABA and glycine in mammalian spinal neurons. J Neural Transm Suppl. 1983;18:83–95. [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]


