Abstract
Background
Our understanding of the mother-to-child transfer of serotype-specific pneumococcal antibodies is limited in non-immunized, HIV-positive women.
Methods
We compared geometric mean antibody concentrations (GMCs), geometric mean transplacental cord:maternal ratios (GMRs) and proportions of samples with protective antibody concentration (≥ 0.35 μg/ml) to serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F, 23F between 74 HIV-infected and 98 HIV-uninfected mother-infant pairs who had not received pneumococcal immunization in South Asia. Multivariable analysis was performed to assess the influence of HIV on protective antibody concentrations.
Results
HIV-infected mothers and their infants exhibited lower GMCs and GMRs than their uninfected counterparts. This was significant for all serotypes except maternal GMC to serotype 1 and GMR for serotype 6B. In multivariate analysis, HIV was significantly associated with reduced odds of having protective pneumococcal IgG levels; 56–73% reduction for 3 maternal serotypes (4, 5, 23F) and 62–90% reduction for all cord samples except serotype 6B.
Conclusions
Maternal HIV infection is associated with lower levels of maternal pneumococcal antibodies and disproportionately lower cord antibodies, relative to maternal antibodies, suggesting that HIV infection compromises transplacental transfer. Reassessment of maternal and/or infant pneumococcal immunization strategies is needed in HIV-infected women and their infants.
Keywords: pneumococcus, antibodies, serotypes, maternal, cord, transplacental transfer, HIV, India, Bangladesh
Background
Diseases caused by Streptococcus pneumoniae (Spn), also known as pneumococcus, kill 700,000 to 1 million people annually, and contribute to 11% of all deaths in children under 5. [1] India shoulders the largest number of pneumococcal cases and deaths in children.[1, 2] Pneumonia, bacteremia, and meningitis are the most common manifestations of invasive pneumococcal disease (IPD). Spn within the respiratory tract can also cause otitis media, sinusitis or bronchitis. In non-immunized populations, Spn accounts for approximately 15–50% of community-acquired pneumonia, 30–50% of acute otitis media, and a significant proportion of meningitis and bacteremia events globally. Children less than 2 years are at greatest risk for pneumococcal infection, particularly IPD. [1, 3]
Serotypes represented in current pneumococcal vaccines (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) account for more than 80% of IPD.[4, 5] Serotypes 6B and 14 are also important in otitis media and nasopharyngeal colonization, respectively. Several factors are associated with increased risk of pneumococcal infection, including HIV disease, nasopharyngeal colonization and low anti-capsular specific IgG antibody levels.[6, 7] Protection of young infants, who are at high risk of pneumococcal disease, depends to a large extent on Spn IgG antibodies acquired from maternal-fetal transfer. Transfer of these antibodies occurs late in pregnancy and is generally protective during the first 3–6 months of life in infants (median antibody half-life is 35 days). [8, 9] The umbilical cord IgG antibody concentrations are a standard measure of maternally acquired IgG antibodies.
Previous research has shown that HIV is associated with reduced levels of pneumococcal antibodies in women, but the effect of HIV on transplacental transfer of serotype-specific antibodies—including whether the amount transferred to the infant is sufficient to protect against disease—is less clear. Studies from South Africa and Brazil reported decreased mother-to-infant transfer of total anti-polysaccharide pneumococcal IgG in HIV-infected versus uninfected women, but did not stratify by serotype. [31, 34] Another study in Brazil showed decreased transplacental antibody transfer of serotypes 6B, 9V and 14, but did not examine the impact of maternal HIV infection. [29].
India does not routinely provide pneumococcal vaccination for children or HIV-infected adults. Therefore, the objectives of our study were to: (1) determine levels of naturally occurring maternal serotype-specific Spn antibodies in HIV-infected versus HIV-uninfected pregnant women; (2) determine the degree of transplacental transfer of these antibodies from mother to infant; and (3) assess if the degree of transplacental antibody transfer should confer protection to infants against Spn serotypes associated with IPD and pneumococcal nasopharyngeal colonization. To assess the impact of HIV on transplacental antibody transfer, we also performed the same measurements in HIV-negative women in neighboring Bangladesh, for whom data were readily available. Demonstrating low levels of natural protection against pneumococcus would emphasize the need to develop novel immunization strategies for HIV-infected mothers and their HIV-exposed newborns to reduce Spn-related morbidity and mortality in these high-risk populations.
Methods
Study population
We retrospectively analyzed maternal-cord serum samples from 74 HIV-infected women who were enrolled into a prevention of mother-to-child HIV transmission trial (SWEN) in India. The eligibility criteria and parent study methods are described in detail elsewhere. [10] Pregnant women were at least 18 years of age, HIV-infected and enrolled at Byramjee Jeejeebhoy Government Medical College (BJGMC), a 1300-bed public hospital in Pune, India, between August 2002 and September 2007.[10] Samples were tested for Spn IgG levels as described below. None of the women had received the pneumococcal vaccine, as per standard of care in India. For the purposes of this analysis, we included the available subset of maternal serum samples that were collected within 4 weeks (median days since delivery: 0, IQR 0–1 day). The cord blood sample was collected after early clamping and cutting of the cord. Serum was separated and stored at −70°C. Socio-demographic and clinical data from antenatal visits, delivery and infant birth were collected as part of the SWEN trial. We compared the maternal and cord Spn IgG levels of our subjects to those of 98 HIV-uninfected Bangladeshi mothers and their children who participated in an influenza vaccine trial, methods of which have been described elsewhere.[11] The Bangladeshi women were enrolled between August 2004 through May 2005. Those included in this analysis received the influenza vaccine, but not pneumococcal vaccination. The maternal serum samples from the Bangladeshi women were collected and stored at the time of delivery. Cord samples were processed as described above. Based on limited available data, the serotypes and burden of pneumococcus in the Bangladeshi population is similar to that of the Indian population [12–15].
Laboratory methods
We performed multiplex bead-based immunoassay (MBIA) tests to measure maternal and cord geometric mean antibody concentrations (GMCs) and geometric mean ratio (GMR) of cord: maternal antibody concentrations of Spn IgG specific for serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F and 23F.[16] A set of standard sera with known Spn IgG concentrations were used as controls in the assay. The tested serotypes represent 9 of the 13 serotypes included in the new conjugate pneumococcal vaccines that were developed for children below 3 years of age and for which our laboratory had assays readily available. These serotypes also represent 6 of the 7 most common serotypes globally among children under 5.[17] The World Health Organization (WHO) proposes a cutoff of 0.35 μg/ml as the minimum antibody concentration conferring protection against IPD caused by any serotype.[18, 19] Other guidance suggests using a cutoff of 5.0 μg/ml for serotype 14, as this is the minimum antibody concentration conferring protection against pneumococcal nasopharyngeal colonization.[20, 21]
Statistical Analysis
Characteristics of mother-infant pairs were compared using Wilcoxon rank-sum test for skewed continuous data, and Pearson's chi-square test and Fisher's exact test for dichotomous data as appropriate. For each serotype, GMCs and GMRs were first log-transformed and then were compared using student's t-test. Proportions of maternal and cord samples with antibody levels above the proposed cutoff for protection against IPD (≥0.35 μg/ml) were compared by Chi-squared test. Six cord samples (2 HIV-infected, 4 HIV-uninfected) had undetectable levels of IgG in the assay and were assigned zero values. Multivariate logistic regression models were fit to assess the influence of maternal HIV infection on the odds of having Spn IgG level ≥0.35 μg/ml for each serotype among mothers and infants. Maternal models were adjusted for maternal age, and education greater than primary education. Infant models were adjusted for maternal age and gravidity, infant Apgar score ≥ 7 and birth weight (Kg). Among HIV-infected mother-infant pairs, clinical factors that could impact differences in serotype-specific antibody GMCs and mean GMRs were explored by using log-transformed data and student's t-test. Factors assessed included maternal HIV-1 quantitative RNA viral load level (VL) and CD4 cell count closest to delivery, infant birth weight< 2500 grams (LBW), and HIV-infection status at birth. Maternal HIV RNA VL and CD4 cell count were dichotomized close to median values at 20,000 copies/ml and 350 cells/mm3, respectively. All statistical tests were two-tailed and significance level was set 0.05.
Results
Study population characteristics
Seventy-four HIV-infected mother-infant pairs and 98 HIV-uninfected mother-infant pairs were included. Among the HIV-infected pregnant women, 7 (9.4%) were taking ART. The median CD4 cell count was 365 cells/mm3, the median HIV VL at delivery was 20,285 copies/ml, and 10 (13.5%) infants were HIV-infected at birth. Compared with HIV-uninfected women, HIV-infected women were younger (median age 22 years vs 24.5 years, p<0.01), less educated (60.8% vs 95.9% received more than four years of education, p<0.01), and were less urban (91% vs 100%, p=0.002), but otherwise did not differ significantly. Compared with infants born to HIV-uninfected women, a higher proportion of infants born to HIV-infected women had low Apgar scores (< 7) at one minute postpartum (53.1% vs 9.3%, p<0.01) and had low birth weight (<2500 grams) (17.6% vs 1.0%, p<0.01) (Table 1).
Table 1.
Characteristics of the HIV-infected and HIV-uninfected mother-infant pairs
| Characteristics | HIV-infected (N=74) | HIV-uninfected (N=98) | p-value |
|---|---|---|---|
| Median maternal age (IQR) | 22 (21 – 26) | 24.5 (22 – 28) | <0.01 |
| Urban residence | 67 (91%) | 98 (100%) | 0.002 |
| Maternal education (> primary education)a | 45 (62.5%) | 94 (95.9%) | <0.01 |
| Primigravida | 31 (41.9%) | 42 (42.9%) | 0.89 |
| Taking ART | 7 (9.4%) | NA | - |
| Median maternal CD4 cells/mm3 (IQR) | 365 (253 – 496) | NA | - |
| Median maternal HIV viral load, RNA copies/ml (IQR) | 20,285 (1,727 – 50,344) | NA | - |
| Vaginal delivery | 58 (78.4%) | 58 (59.1%) | 0.008 |
| Preterm (GA<37 weeks) | 3 (4.1%) | 2 (2.0%) | 0.65 |
| Low birth weight <2500 grams | 13 (17.6%) | 1 (1.0%) | < 0.01 |
| Female infant | 40 (54.1%) | 44 (44.9%) | 0.23 |
| Apgar score at 1minute >7 | 35 (47.9%) | 88 (90.7%) | <0.01 |
| HIV infected at birthb | 10 (13.5%) | NA | - |
Abbreviations: IQR, inter-quartile range; ART: Antiretroviral therapy; GA: gestational age.
> 4 years of education
DNA PCR+ at 48 hours, confirmed by quantitative HIV RNA assay using standard methods
Spn IgG geometric mean concentrations (GMC) and transplacental transfer (GMR) by serotype and by HIV status
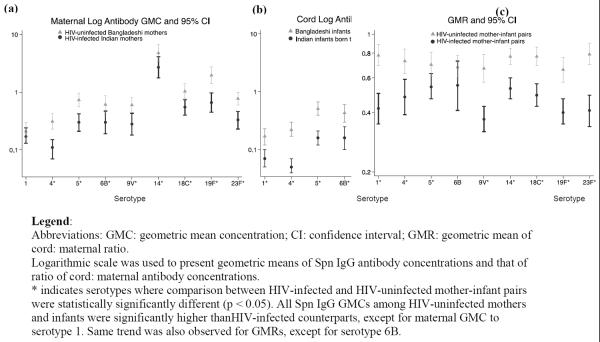
All HIV-infected maternal GMCs1 except for serotype 1 were significantly lower than those of HIV-uninfected women.(Figure 1a) Even for serotype 1, HIV-infected samples were still lower than HIV-uninfected, but not significantly so (0.17 μg/ml in HIV-infected vs. 0.21 μg/ml in HIV-uninfected, p=0.40). Similarly, among HIV-exposed infants, all cord GMCs were significantly lower than their unexposed counterparts.(Figure 1b). GMRs were significantly lower among HIV-infected pairs (range 0.37–0.55) than HIV-uninfected pairs (range 0.66–0.79). This was statistically significant for all serotypes except serotype 6B (p=0.14). The percent reduction in GMRs between HIV-infected and uninfected pairs ranged from 23% to 48% (Figure 1c).
Figure 1a–c.
Comparisons of Maternal and Cord Pneumococcal Antibody Geometric Mean Concentrations (GMC) and Transplacental Antibody Transfer (GMR) between HIV-infected and HIV-uninfected Mother-Infant Pairs
Samples reaching protective levels of Spn IgG
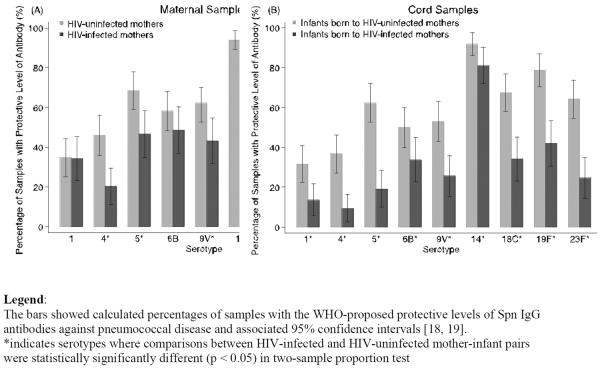
The proportions of samples that had the minimum Spn IgG for IPD protection varied by serotype and by maternal HIV status. In all mother-infant pairs, the highest proportion above 0.35 μg/ml was for serotype 14 (HIV: 85.1% maternal, 81.1% cord; HIV-uninfected: 93.9% maternal, 91.8% cord). But HIV-infected mother-infant pairs had the lowest percent protection for serotype 4 (20.3% maternal, 9.5% cord), while HIV-uninfected pairs had the lowest percent protected against serotype 1 (34.7% maternal, 31.6% cord). (Figure 2) The proportion of maternal and cord samples with Spn IgG ≥ 0.35 μg/ml was significantly lower among HIV-infected mother-infant pairs than HIV-uninfected pairs for all serotypes, except for serotypes 1, 6B and 14 among maternal samples.
Figure 2.
Percentage of Maternal and Cord Samples with Protective Level of Pneumococcal Capsular Antibody (≥ 0.35 μg/ml)
The proportion of samples with antibody concentration to serotype 14 ≥ 5.0 μg/ml, the threshold for reduced risk of pneumococcal nasopharyngeal carriage, also differed by maternal HIV status. HIV-infected maternal samples had lower protective levels than HIV-uninfected (35.1% vs 49.0%, p=0.07) and, similarly, antibody levels in HIV-infected cord samples were significantly lower than HIV-uninfected (21.6% vs 40.8%, p<0.01)
Unadjusted and adjusted analyses assessing influence of HIV on maternal and cord Spn IgG
In unadjusted logistic regression, HIV was significantly associated with reduced odds (OR 0.51–0.72) of having a maternal Spn IgG >= 0.35 μg/ml for six of nine serotypes (4, 5, 9V, 18C, 19F, 23F). HIV was also significantly associated with reduced odds (OR 0.49–0.86) of having a cord Spn IgG ≥ 0.35 μg/ml for all nine serotypes. After adjusting for maternal age and maternal education for maternal models and maternal age, gravidity, Apgar score at 1 minute and infant birth weight for cord models, HIV infection remained significantly associated with reduced odds of having protective levels (> 0.35 μg/ml) of IgG for 3 maternal serotypes (4, 5, 23F) (OR 0.56–0.73) and 8 cord serotypes (all except 6B) (OR 0.62–0.90). (Table 2)
Table 2.
Association between HIV infection and protective levels of pneumococcal capsular IgG antibodies (≥ 0.35 μg/mL) by serotype.
| Serotype | Maternal Samples | Cord Samples | ||
|---|---|---|---|---|
| OR (95%CI) | aOR (95% CI)a | OR (95%CI) | aOR (95% CI)b | |
| 1 | 0.98 (0.52–1.86) | 0.84 (0.40–1.74) | 0.34 (0.16–0.76)* | 0.20 (0.07–0.56)* |
| 4 | 0.30 (0.15–0.60)* | 0.28 (0.12–0.63)* | 0.18 (0.07–0.43)* | 0.10 (0.03–0.33)* |
| 5 | 0.40 (0.22–0.75)* | 0.44 (0.22–0.90)* | 0.14 (0.07–0.29)* | 0.13 (0.05–0.34)* |
| 6B | 0.68 (0.37–1.25) | 0.81 (0.41–1.63) | 0.51 (0.27–0.95)* | 0.61 (0.26–1.40) |
| 9V | 0.46 (0.25–0.85)* | 0.53 (0.26–1.05) | 0.31 (0.16–0.59)* | 0.38 (0.16–0.87)* |
| 14 | 0.37 (0.13–1.06) | 0.33 (0.11–1.02) | 0.38 (0.15–0.96)* | 0.26 (0.07–0.93)* |
| 18C | 0.49 (0.26–0.93)* | 0.59 (0.28–1.22) | 0.25 (0.13–0.48)* | 0.28 (0.12–0.64)* |
| 19F | 0.43 (0.20–0.90)* | 0.45 (0.19–1.02) | 0.20 (0.10–0.38)* | 0.12 (0.05–0.32)* |
| 23F | 0.28 (0.15–0.53)* | 0.27 (0.13–0.57)* | 0.19 (0.09–0.36)* | 0.15 (0.06–0.39)* |
OR: odds ratio; CI: confidence interval; aOR: adjusted odds ratio
adjusted for maternal age and maternal education (> primary education)
adjusted for maternal age, maternal gravidity, normal Apgar score at 1 minute postpartum (≥ 7) and infant birth weight (Kg)
indicates significantly reduced odds of having protective pneumococcal capsular IgG antibody level ≥ 0.35 μg/ml
Among HIV-infected mother-infant pairs, serotype-specific GMCs and GMRs did not differ significantly by maternal CD4 cell count or HIV-1 RNA VL, or infant factors such as LBW, or HIV infection at birth (data not shown). We observed lower GMRs across all serotypes among infants with LBW, although none reached statistical significance at the p < 0.05 level (data not shown).
Discussion
We found substantially lower levels of maternal, cord and transplacental transfer of pneumococcal capsular antibodies in the HIV-infected pregnant Indian cohort than in the uninfected Bangladeshi women. Furthermore, HIV-exposed infants were more likely to have antibody levels below the cutoff of protection against all serotypes associated with IPD and nasopharyngeal colonization. To our knowledge, these are the first data that specifically address the impact of HIV on transplacental transfer of pneumococcal antibodies by serotype. These are also the first data of its kind from India, a country with one of the highest burdens of pneumococcal disease in children under 5 years of age [1] and the third highest absolute burden of HIV globally. [21] Unimmunized HIV-infected Indian women and their infants remain at high risk for pneumococcal disease.
HIV, alone, is associated with increased risk of pneumococcal disease. Compared to HIV-uninfected adults and children, HIV-infected adults have a 6- to 324-fold increased risk of disease and HIV-infected children have a 9- to 43-fold increase, respectively.[22] HIV infection is also associated with increased risk of repeated episodes of pneumococcal disease. Our finding that HIV infection is associated with lower maternal and cord Spn IgG antibody concentrations supports this phenomenon. Using the WHO recommended 0.35μg/ml cutoff value against IPD, we found that HIV infection reduces the odds of having protective maternal and cord pneumococcal antibody levels by more than 50%. As HIV can impact the functional quality of antibody transferred [23], the percent of HIV-exposed infants with protective levels of antibody in our study may still be an overestimate.
Our data showing transplacental transfer of Spn IgG antibodies from 66% to 79% in Bangladeshi HIV-uninfected non-immunized women is in line with previous studies, which have found effective transplacental passage of maternal IgG antibodies of up to 85%.[9, 24, 25] In our HIV-infected mother-infant pairs, on the other hand, the transfer rate was only 37% to 55%. Studies in HIV-infected mothers from South Africa and Brazil reported a respective 15% and 76% reduction in total anti-polysaccharide pneumococcal IgG. [26, 27] Other studies in Brazil and Sub-Saharan Africa have reported similar findings for reduced transfer of measles and tetanus antibodies in HIV-infected women as well. [26–32]
Transplacental transfer of IgG antibodies is an active process mediated by Fc receptors. Several factors likely influence the process. Maternal hypergammaglobulinemia, for example, is commonly observed among HIV-infected individuals and can saturate the Fc receptor, impairing active transfer of IgG [33] High HIV VL has also been associated with reduced transfer.[ 28,34–35] Our data did not suggest any clear association between the degree of transplacental transfer of antibody and maternal HIV VL or other maternal factors such as CD4 cell count, though this study was likely underpowered to detect any such associations. There was a suggestion that LBW infants had lower Spn IgG GMRs, but this finding did not reach statistical significance. It is unclear if the association was with HIV or with LBW itself, because 13 of the 14 LBW infants were born to HIV-infected mothers. Delivery by C-section may decrease antibody transfer [36–37], though the role of delivery type in antibody transfer is debated [38–39]. In our study, HIV-uninfected mothers had a higher rate of C-sections and their infants had significantly higher levels of antibody than HIV-exposed infants. If C-sections do in fact lower antibody transfer, our results would only further emphasize the negative impact of maternal HIV on infant antibody levels.
In addition to protection against invasive pneumococcal disease, we also studied protective serum Spn IgG levels against nasopharyngeal carriage. Concentration of Spn IgG to serotype 14 ≥5μg/ml is associated with protection against nasopharyngeal carriage [20]. Though this cutoff has not been validated clinically, nasopharyngeal carriage is an immediate and necessary precursor to pneumococcal disease. [40–41] Serotype 14 accounts for 19–24% of global burden of invasive pneumococcal disease among children under 5 [17], but only 21.6% of HIV-exposed infants in our study achieved protective levels to serotype 14 for nasopharyngeal carriage. This suggests a significant risk for serious pneumococcal disease in this population.
Due to the small number of samples, we did not have adequate sample size to determine other sociodemographic and clinical factors associated with low Spn IgG antibody levels. We did not, for example, assess for gamma globulin levels and malnutrition. Another limitation is that Bangladeshi women did not undergo HIV testing. In Bangladesh, however, the prevalence of HIV is <0.1% [42], making it very unlikely that more than one woman in the HIV-uninfected cohort was misclassified. Finally we were unable address the relationship between low antibody levels and development of pneumococcal disease. Despite these limitations, however, the results demonstrate inadequate natural immunity in both HIV-infected mothers and their infants.
India is estimated to account for a significant proportion of global pneumococcal cases and HIV-infected persons are at especially high risk for IPD and other manifestations of pneumococcal disease. Pneumococcal vaccination is recommended for HIV-infected persons in the US and Europe but has not been convincingly shown to be advantageous in resource-constrained settings. [43] Pneumococcal vaccination is likely safe during pregnancy [44], but an increase in maternal antibodies may impair the infant's response to the pneumococcal vaccine in the future [45]. The NIH-funded IMPAACT P1091 study is currently studying the safety and immunogenicity of the conjugated versus polysaccharide pneumococcal vaccine among HIV-infected pregnant women and their infants in an international setting, including assessment of transplacental transfer of Spn anti-capsular IgG antibodies. An alternative strategy is neonatal vaccination. HIV-exposed infants had robust responses to pneumococcal vaccination at 6 weeks. [23, 26] Vaccine administration may also be effective at birth but this would have to be weighed against potential decreased responses to other vaccinations, including tetanus and Haemophilus influenzae b [46]. These preliminary data from South Asia, where 27% of all births take place [47] and 16% of all HIV-infected people live [48], should encourage others to evaluate the use of pneumococcal and other vaccines to protect HIV-infected mothers and their children.
Highlights
We compared maternal and cord pneumococcal antibodies by HIV status in South Asia.
HIV-infected mothers had >50% reduction in anti-pneumococcal antibodies.
HIV significantly decreased transfer of antibodies from mother to infant.
HIV-exposed infants were less likely to have protective antibody levels.
Acknowledgements
We thank the study participants and study staff for their immense contribution.
Funding: This work was supported by the Bill and Melinda Gates Foundation; the U.S. National Institutes of the Health (NIH); US National Institute of Allergy and Infectious Diseases [R01AI45462]; and the NIH-Fogarty International Center Program of International Training Grants in Epidemiology Related to AIDS [D43-TW0000]. AG, NG, VK, SP, RCB are also supported by the NIH BJMC HIV Clinical trials Unit [U01 AI069497]. JSM is supported by the NIH/ Weill Cornell Clinical and Translational Science Center [UL1 TR000457].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: The authors declared no conflict of interests
One maternal-cord pair did not have serotypes 1, 5, 18C and 23F tested.
References
- 1.O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 3.Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- 4.Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000;30:122–40. doi: 10.1086/313609. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–21. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 6.Lockhart NJ, Daly KA, Lindgren BR, Meland M, Le CT, Giebink GS. Low cord blood type 14 pneumococcal IgG1 but not IgG2 antibody predicts early infant otitis media. J Infect Dis. 2000;181:1979–82. doi: 10.1086/315501. [DOI] [PubMed] [Google Scholar]
- 7.van der Poll T, Opal SM. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet. 2009;374:1543–56. doi: 10.1016/S0140-6736(09)61114-4. [DOI] [PubMed] [Google Scholar]
- 8.Halsey NA, Klein D. Maternal immunization. Pediatr Infect Dis J. 1990;9:574–81. doi: 10.1097/00006454-199008000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–7. doi: 10.1016/s0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- 10.Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 11.Zaman K, Roy E, Arifeen S, et al. Effectiveness of Maternal Influenza Immunization in Mothers and Infants. New Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 12.Prospective multicentre hospital surveillance of Streptococcus pneumoniae disease in India. Invasive Bacterial Infection Surveillance (IBIS) Group, International Clinical Epidemiology Network (INCLEN) Lancet. 1999;353:1216–21. [PubMed] [Google Scholar]
- 13.Arifeen SE, Saha SK, Rahman S, et al. Invasive pneumococcal disease among children in rural Bangladesh: results from a population-based surveillance. Clin Infect Dis. 2009;48(Suppl 2):S103–13. doi: 10.1086/596543. [DOI] [PubMed] [Google Scholar]
- 14.Brooks WA, Breiman RF, Goswami D, et al. Invasive pneumococcal disease burden and implications for vaccine policy in urban Bangladesh. Am J Trop Med Hyg. 2007;77:795–801. [PubMed] [Google Scholar]
- 15.Saha SK, Naheed A, El Arifeen S, et al. Surveillance for invasive Streptococcus pneumoniae disease among hospitalized children in Bangladesh: antimicrobial susceptibility and serotype distribution. Clin Infect Dis. 2009;48(Suppl 2):S75–81. doi: 10.1086/596544. [DOI] [PubMed] [Google Scholar]
- 16.Klein DL, Martinez JE, Hickey MH, Hassouna F, Zaman K, Steinhoff M. Development and characterization of a multiplex bead-based immunoassay to quantify pneumococcal capsular polysaccharide-specific antibodies. Clinical and vaccine immunology : CVI. 2012;19:1276–82. doi: 10.1128/CVI.05535-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS medicine. 2010;7(10):e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherian T. WHO expert consultation on serotype composition of pneumococcal conjugate vaccines for use in resource-poor developing countries, 26–27 October 2006, Geneva. Vaccine. 2007;25:6557–64. doi: 10.1016/j.vaccine.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Organization WH . WHO Technical Report Series: recommendation for the production and control of pneumococcal conjugate vaccines, No.927. 2005. pp. 66–92. [Google Scholar]
- 20.Goldblatt D, Hussain M, Andrews N, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis. 2005;192:387–93. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 21.UNAIDS . UNAIDS REPORT ON THE GLOBAL AIDS EPIDEMIC. UNAIDS 2013; Geneva, Switzerland: 2013. [Google Scholar]
- 22.Bliss SJ, O'Brien KL, Janoff EN, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis. 2008;8:67–80. doi: 10.1016/S1473-3099(07)70242-6. [DOI] [PubMed] [Google Scholar]
- 23.Madhi SA, Adrian P, Cotton MF, et al. Effect of HIV infection status and anti-retroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J Infect Dis. 2010;202:355–61. doi: 10.1086/653704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida Vde C, Mussi-Pinhata MM, De Souza CB, et al. Immunogenicity of 23-valent pneumococcal polysaccharide vaccine in HIV-infected pregnant women and kinetics of passively acquired antibodies in young infants. Vaccine. 2009;27:3856–61. doi: 10.1016/j.vaccine.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–5. doi: 10.1136/fn.79.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CE, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling AC. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA : the journal of the American Medical Association. 2011;305:576–84. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 28.Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–7. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho BT, Carneiro-Sampaio MM, Sole D, Naspitz C, Leiva LE, Sorensen RU. Transplacental transmission of serotype-specific pneumococcal antibodies in a Brazilian population. Clin Diagn Lab Immunol. 1999;6:50–4. doi: 10.1128/cdli.6.1.50-54.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cumberland P, Shulman CE, Maple PA, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis. 2007;196:550–7. doi: 10.1086/519845. [DOI] [PubMed] [Google Scholar]
- 31.Scott S, Cumberland P, Shulman CE, et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis. 2005;191:1854–60. doi: 10.1086/429963. [DOI] [PubMed] [Google Scholar]
- 32.de Moraes-Pinto MI, Almeida AC, Kenj G, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–84. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 33.Firan M, Bawdon R, Radu C, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 34.de Moraes-Pinto MI, Farhat CK, Fraser WD, Hart CA, Johnson PM. Human serum beta2-microglobulin levels: correlation with total serum IgG and placental IgG transfer in HIV-infected and non-HIV infected individuals. J Reprod Immunol. 1999;42:167–74. doi: 10.1016/s0165-0378(98)00083-7. [DOI] [PubMed] [Google Scholar]
- 35.Okoko BJ, Wesuperuma LH, Ota MO, et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr. 2001;19:59–65. [PubMed] [Google Scholar]
- 36.Agrawal S, Agrawal BM, Khurana K, Gupta K, Ansari KH. Comparative study of immunoglobulin G and immunoglobulin M among neonates in caesarean section and vaginal delivery. J Indian Med Assoc. 1996;94:43–4. [PubMed] [Google Scholar]
- 37.Tatra G, Placheta P. IgG levels in maternal and umbilical cord serum after vaginal delivery and after elective Caesarean section. Arch Gynecol. 1979;22:135–40. doi: 10.1007/BF02103287. [DOI] [PubMed] [Google Scholar]
- 38.Doroudchi M, Samsami Dehagani A, Emad K, Ghaderi A. Placental transfer of rubella-specific IgG in fullterm and preterm newborns. Int J Gynaecol Obstet. 2003;81:157–62. doi: 10.1016/s0020-7292(02)00442-3. [DOI] [PubMed] [Google Scholar]
- 39.Palmeira P, Quinella C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012 doi: 10.1155/2012/985646. doi: 10.1155/2012/ 985646. Epub 2011 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL. The fundamental link between pneumococcal carriage and disease. Expert review of vaccines. 2012;11:841–55. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 41.Salazar JC, Daly KA, Giebink GS, et al. Low cord blood pneumococcal immunoglobulin G (IgG) antibodies predict early onset acute otitis media in infancy. Am J Epidemiol. 1997;145:1048–56. doi: 10.1093/oxfordjournals.aje.a009061. [DOI] [PubMed] [Google Scholar]
- 42.2008 UNGASS country progress report—Bangladesh; reporting period: January 2006-December 2007. Dhaka: National AIDS/STD Programme, Ministry of Health and Family Welfare, Government of Bangladesh; Bangladesh: 2008. New 18. Ministry of Health and Family Welfare. Directorate General of Health Services. National AIDS/STD Programme; p. 42. [Google Scholar]
- 43.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double blind, randomized and placebo controlled trial. Lancet. 2000;355:2106–11. doi: 10.1016/s0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 1997;46(No.RR-8):6. [Google Scholar]
- 45.O'Brien KL, Moïsi J, Moulton LH, et al. Predictors of Pneumococcal Conjugate Vaccine Immunogenicity among Infants and Toddlers in an American Indian PnCRM7 Efficacy Trial. J Infect Dis. 2007;196:104–14. doi: 10.1086/518438. [DOI] [PubMed] [Google Scholar]
- 46.Baro-On ES, Goldberg E, Hellman S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenza B (HIB) Cochrane Database Syst Rev. 2012;4:CD005530. doi: 10.1002/14651858.CD005530.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.United Nations Children's Fund [Accessed September 28, 2013];The State of the World's Children. 2013 Available: http://data.un.org/Data.aspx?d=PopDiv&f=variableID%3A54.
- 48.World Bank . AIDS in South Asia. World Bank; Washington, United States of America: 2006. Available: http://siteresources.worldbank.org/SOUTHASIAEXT/Resources/Publications/448813-1155152122224/southasia_aids.pdf. [Google Scholar]