SUMMARY
Purpose
Refractory status epilepticus (RSE) is a life-threatening emergency, demonstrating, by definition, significant pharmacoresistance. We describe five cases of pediatric RSE treated with mild hypothermia.
Methods
Retrospective chart review was performed of records of children who received hypothermia for RSE at two tertiary-care pediatric hospitals between 2009 and 2012.
Key Findings
Five children with RSE received mild hypothermia (32–35°C). Hypothermia reduced seizure burden during and after treatment in all cases. Prior to initiation of hypothermia, four children (80%) received pentobarbital infusions to treat RSE, but relapsed after pentobarbital discontinuation. No child relapsed after treatment with hypothermia. One child died after redirection of care. Remaining four children were discharged.
Significance
This is the largest pediatric case series reporting treatment of RSE with mild hypothermia. Hypothermia decreased seizure burden during and after pediatric RSE and may prevent RSE relapse.
Keywords: Seizures, Cooling, Pharmacoresistance, Children
Status epilepticus (SE) is a life-threatening condition defined as seizure that persists longer than 5 min (Brophy et al., 2012). Annual incidence of SE is 10–41/100,000, and SE affects 120,000–200,000 people annually in the United States. SE carries a 7–39% mortality rate (DeLorenzo et al., 1996; Coeytaux et al., 2000; Knake et al., 2001; Novy et al., 2010). Refractory SE (RSE) occurs when patients fail first- and second-line anticonvulsant therapy (Novy et al., 2010; Brophy et al., 2012). RSE occurs in approximately one third of SE patients (Mayer et al., 2002; Lambrechtsen & Buchhalter, 2008). In children, RSE is associated with development of static encephalopathy and death (Barberio et al., 2012). RSE treatment typically requires anesthetic anticonvulsant levels to achieve burst suppression on electroencephalography (EEG). Hypothermia has been used occasionally as an adjunct to anticonvulsants in RSE treatment. Existing literature describes experience with mild hypothermia in adult RSE; the largest case series comprises four patients (Corry et al., 2008). Herein, we describe five children treated with mild hypothermia (32–35°C) for RSE. This is the largest case series to date describing treatment of pediatric RSE with mild hypothermia.
Methods
Subjects
Neurocritical care teams at St. Louis Children’s Hospital (SLCH) and Phoenix Children’s Hospital (PCH) treated five RSE patients with hypothermia between 2009 and 2012. We retrospectively extracted data from clinical records under institutional review board exemptions at both hospitals.
Case 1: LB
LB, a 10-year-old girl without significant medical history (PMHx), presented to an outside emergency department (ED) with 2 days of fatigue, abdominal pain, and headache without neck stiffness followed by new onset seizure. Seizure initially manifested as fixed gaze and right arm posturing and progressed to generalized tonic–clonic (GTC) activity. In the ED, LB received lorazepam and fosphenytoin but continued seizing. She was admitted to the outside pediatric intensive care unit (PICU) and placed on continuous electroencephalography (EEG) (Stellate Harmonie, Montreal, PQ, Canada). She was in SE with intermittent seizures without returning to baseline. She received fosphenytoin (600 mg/day), levetiracetam (2,400 mg/day), phenobarbital (280 mg/day), continuous midazolam infusion (0.2 mg/kg/ h, 8 mg/h), and intermittent lorazepam (4 mg). These therapies failed, and she progressed to RSE. On hospital day (HD) 7, she was put into burst suppression for 48 h with pentobarbital (3 mg/kg/h). Upon pentobarbital discontinuation, LB’s EEG demonstrated 4–15 seizures per hour. Valproic acid (4,000 mg/day) was added without effect. On HD12, pentobarbital was again added (3.5 mg/kg/h) and titrated to burst suppression. She developed hemodynamic instability and required dopamine. On HD19, pentobarbital was weaned over 24 h. Despite 7 days of burst suppression, seizures again emerged. Furthermore, seizures increased in frequency, occurring every 2–5 min. On HD27, LB was transferred to SLCH.
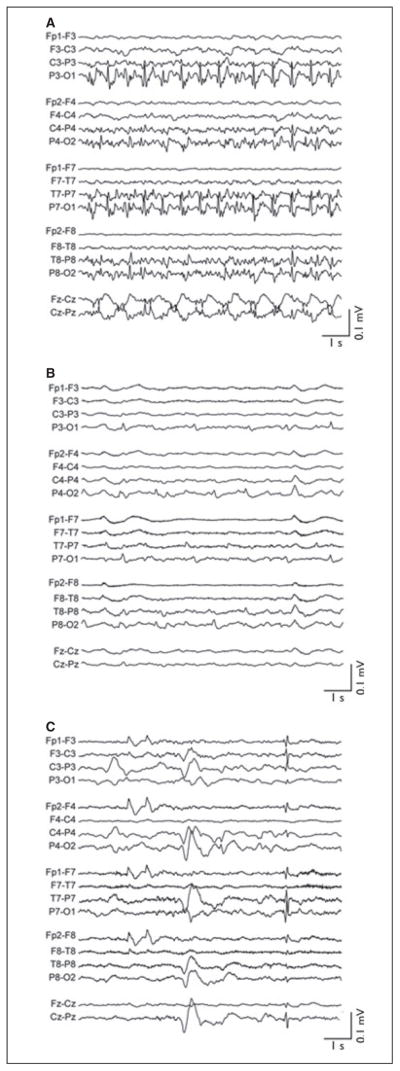
At SLCH, LB demonstrated rhythmic facial and extremity movements with epileptiform EEG activity (Fig. 1A). Despite phenobarbital (280 mg/day, peak serum level [PSL] = 36.3 μg/ml), levetiracetam (3,200 mg/day), fosphenytoin (600 mg/day, phenytoin PSL = 12.3 μg/ml), valproic acid (4,000 mg/day, PSL = 70 μg/ml), and midazolam (0.2 mg/kg/h), the patient’s RSE persisted. Given RSE severity, LB was treated with mild hypothermia using surface cooling (Arctic Sun, Bard Medical, Louisville, CO, U.S.A.) to a bladder temperature of 34°C. With induction of hypothermia, EEG progressed from parietooccipital seizures (Fig. 1A) to more blunted posterior epileptiform activity (Fig. 1B). Within 3 hours at goal temperature, EEG demonstrated low-amplitude burst suppression. All other anticonvulsants were continued during hypothermia, although midazolam was decreased to 0.05 mg/kg/h (2 mg/h). During hypothermia, phenobarbital, phenytoin, and valproate PSLs were 29.8, 18.7, and 96 μg/ml, respectively. During cooling, LB experienced only mild hypokalemia as a hypothermia-related side effect. After 24 h of hypothermia and burst suppression, LB was rewarmed by 0.5°C/day to 36.5°C. After 24 h of temperature control at 36.5°C (normothermia), the cooling device was discontinued. The patient’s EEG results remained abnormal with generalized slowing with background voltage suppression (Fig. 1C). Seizures, however, did not recur during subsequent 2-month–long hospitalization. Brain magnetic resonance images (MRI) demonstrated substantial injury, including diffusion restriction in the cortical ribbon and deep gray nuclei, diffuse edema, and transtentorial herniation. She was discharged home on levetiracetam (1,600 mg, b.i.d.) with tracheostomy and gastrostomy in a persistent vegetative state. At 2-year follow-up, she has progressed to a minimally conscious state.
Figure 1.
EEG of patient in case 1 (LB). (A) EEG on admission to SLCH after 27 days of refractory status epilepticus. Body temperature is 36.5°C. Midazolam is at 0.2 mg/kg/h. (B) EEG 75 min after reaching goal bladder temperature of 34°C. Midazolam is at unchanged at 0.2 mg/kg/h. No additional antiseizure medications. (C) EEG prior to discharge on levetiracetam (40 mg/kg bid). EEG figures are shown in standard longitudinal bipolar montage. All data were acquired at 200 Hz, high-pass–filtered above 0.5 Hz, low-pass filtered below 50 Hz, and notch-filtered between 58 and 62 Hz. All EEG images are shown on identical time and voltage scales (abscissa and ordinate calibration bars, respectively).
Epilepsia © ILAE
Case 2: DB
DB, a 5-months-old former 27-week estimated gestation female, presented to clinic with vomiting, altered mental status, bulging fontanelle, and rhythmic movements concerning for seizures. She was transferred to an outside ED, where she received lorazepam (0.4 mg/kg) and phenobarbital (30 mg/kg) for lip smacking and right upper extremity tonic–clonic movements. Her head computerized tomography (CT) (Siemens, Malvern, PA, U.S.A.) revealed ventriculomegaly. DB was airlifted to SLCH for treatment of SE and hydrocephalus. During transfer, DB received mannitol, lorazepam (0.1 mg/kg), and phenobarbital (40 mg/kg).
At SLCH, she emergently underwent ventriculoperitoneal shunt placement. Postoperatively, DB received lorazepam (0.1 mg/kg), fosphenytoin (30 mg/kg, 120 mg), and midazolam infusion (0.1 mg/kg/h, 0.4 mg/h) for RSE. DB’s RSE continued unabated despite addition of phenobarbital (30 mg/kg), and levetiracetam (40 mg/kg/day), and increase in midazolam to 0.3 mg/kg/h (1.2 mg/h). Because of persistent RSE, pentobarbital infusion was started. At 10 mg/kg/h, pentobarbital produced complete suppression of EEG activity. She subsequently developed hypotension, requiring dopamine.
After almost 24 h of complete burst suppression (isoelectric EEG), pentobarbital was weaned by 1 mg/kg/h over 12 h. However, DB again developed clinical and electrographic RSE. Pentobarbital was restarted (5 mg/kg/ h) but was ineffective in terminating RSE. Given the ongoing hemodynamic instability and failure of multiple medications, DB was cooled from normothermia to a bladder temperature of 32°C over 1 h with Gaymar Medi-Therm (Stryker, Kalamazoo, MI, U.S.A.) cooling blanket. After several hours at goal temperature, she achieved complete burst suppression. Her pentobarbital was weaned down to 2 mg/kg/h, while hypothermia was maintained at 32–34°C.
After 24 h of hypothermia (HD5, day 4 of pentobarbital), DB developed further hemodynamic compromise and required escalation of inotropic support. She was transitioned from dopamine to epinephrine and norepinephrine infusions. Over the next 24 h, she developed abdominal hypertension and pneumatosis intestinalis. Pentobarbital was discontinued, and DB underwent decompressive laparotomy. Bowel examination demonstrated approximately 5 cm of ischemic colon requiring partial colectomy. After colectomy, her hemodynamic status normalized, and she was weaned off inotropes. Additional significant events during hypothermia and pentobarbital therapy included hypokalemia (nadir 1.8 mM) treated with intravenous supplementation, and coagulopathy (peak international normalized ratio [INR] 2.48), which did not require treatment.
After 72 h of hypothermia, DB was rewarmed by 1°C every 6 h. She did not have further clinical seizures. However, EEG remained abnormal with short, intermittent subclinical seizures and bursts of interictal epileptiform activity most prominently recorded in the left central electrodes. Phenobarbital (5 mg/kg/day) was added to DB’s maintenance regimen of fosphenytoin (6 mg/kg/day) and levetiracetam (80 mg/kg/day). Brain MRI (Siemens) on HD10 demonstrated diffuse bilateral cortical infarcts, old intraventricular and new cerebellar hemorrhages, and marked atrophy of the periventricular white matter. Given MRI findings, DB’s mother expressed significant concerns about DB’s future quality of life and requested to redirect care toward comfort measures. DB continued on phenobarbital, fosphenytoin, and levetiracetam until her death on HD35.
Case 3: EZ
EZ, an 11-month-old male infant without significant PMHx, presented to an outside hospital with sudden onset of synchronous right arm and leg jerking and intermittent staring. He received multiple doses of lorazepam, fosphenytoin, and phenobarbital (total 0.5, 40, and 60 mg/kg, respectively). He was then transferred to SLCH, where examination demonstrated nonsuppressible jerking of all extremities. Head CT was normal. Brain MRI showed mild diffusion abnormalities in left frontal gray matter and an incidental type I Chiari malformation.
Continuous EEG-video demonstrated rhythmic left central and midline spike and wave discharges, which correlated clinically with right arm and leg jerking. Despite levetiracetam (42 mg/kg), pyridoxine (100 mg), phenobarbital (20 mg/kg, PSL, 42.9 μg/ml), and midazolam infusion (1 mg/kg/h, 12 mg/h), EZ remained in RSE. During midazolam titration, he required dopamine. After 23 h in RSE, EZ was started on pentobarbital (titrated to 3.5 mg/kg/ h, 44 mg/h), and cooled over 2 h via Arctic Sun to a bladder temperature of 34°C. He was burst suppressed on EEG by 120 min. With these interventions, EZ developed further hemodynamic instability, requiring transition from dopamine to epinephrine (max dose 0.12 μg/kg/min). After 72 h at target temperature and stable pentobarbital dose (3.5 mg/kg/h, 44 mg/h), the patient’s pentobarbital was discontinued. He continued to receive fosphenytoin (PSL, 20.5 μg/ml), levetiracetam, and phenobarbital (PSL = 50.2 μg/ml). On HD5, he was rewarmed by 0.5°C every 12 h to 36°C. He was seizure-free for the first 24 h of normothermia. On HD9, EZ had several brief right-sided seizures, which prompted initiation of phenobarbital (32 mg/day) and topiramate (150 mg/ day). In addition, his levetiracetam was increased (160 mg/ kg/day). His seizures resolved. EZ remained on levetiracetam, phenobarbital, and topiramate with one brief clinical seizure until discharge home on HD24.
An extensive diagnostic work-up for infectious, metabolic, and genetic causes of EZ’s SE revealed only a positive blood polymerase chain reaction for human herpesvirus type 6 (HHV-6). After discharge, however, EZ developed intractable focal myoclonic epilepsy and mild right hemiparesis. Thirty months after discharge, EZ was again admitted to PICU with altered mental status and elevated serum transaminases. He developed RSE, which again required pentobarbital infusion (max dose 3.5 mg/kg/h) and hypothermia to 34°C to achieve burst suppression on EEG. Intractable myoclonic epilepsy, RSE, liver dysfunction, and motor regression suggested a diagnosis of Alpers-Huttenlocher syndrome (Wolf et al., 2009; Isohanni et al., 2011). Genetic testing revealed two independent mutations in the mitochondrial DNA polymerase γ-1 (POLG-1). Given poor prognosis, EZ’s parents redirected care toward comfort measures; EZ died shortly thereafter.
Case 4: JG
JG, a 10-year-old boy with PMHx significant for epilepsy, developmental delay, and attention deficit/hyperactivity disorder (ADHD), presented to an outside hospital with GTC seizure. His home medications included levetiracetam (1,000 mg/day), lamotrigine (200 mg/day), and clonazepam (0.5 mg prn). JG received rectal diazepam (10 mg), intravenous lorazepam (6 mg), and levetiracetam (20 mg/kg) for continued left eye twitching and body stiffening. He was then transferred to an outside intensive care unit (ICU), where he received fosphenytoin (20 mg/kg), levetiracetam (20 mg/kg), and lamotrigine (200 mg/day). Despite these medications, JG’s RSE continued electro-graphically and clinically. Midazolam infusion was started (0.2 mg/kg/h), but JG remained in RSE. Pentobarbital infusion (4 mg/kg/h) achieved burst suppression on EEG, but caused hypotension and oliguria. Due to these side effects, JG’s pentobarbital dose was weaned. On HD2, he was transferred to SLCH on pentobarbital (1 mg/kg/h, PSL = 38 μg/ ml) and levetiracetam (2,000 mg/day).
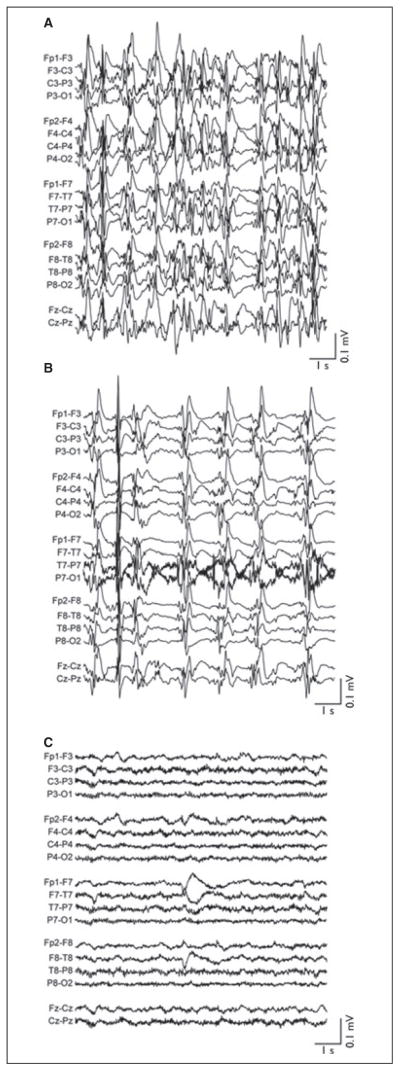
Upon arrival to SLCH, JG was in electrographic RSE (Fig. 2A). He was cooled from 37°C over 3 h with the Arctic Sun to a bladder temperature of 33°C. Hypothermia slowed electrographic burst frequency but did not achieve burst suppression (Fig. 2B). JG’s pentobarbital serum level was 54 μg/ml at this time. On HD4, lacosamide was added (200 mg/day), and pentobarbital infusion was titrated to 5 mg/kg/h, resulting in burst suppression on EEG. However, JG rapidly developed lactic acidosis (peak lactate 7 mM) despite adequate blood pressure support with dopamine and epinephrine. Lactic acidosis resolved with weaning of pentobarbital. JG remained hypothermic for 5 days. He was rewarmed by 0.5°C every 12 h beginning on HD9. He achieved normothermia on HD12. During and after rewarming, JG remained seizure-free (Fig. 2C). For the remainder of his hospitalization, he was maintained on levetiracetam (2,500 mg/day), lamotrigine (200 mg/day), and clobazam (20 mg/day).
Figure 2.
EEG of patient in case 4 (JG). (A) EEG on admission to SLCH after 2 days of refractory status epilepticus. Active antiepileptic medications are pentobarbital (1.5 mg/kg/h) and levetiracetam (2,000 mg/day). Body temperature is 36.5°C. (B) EEG upon reaching goal bladder temperature of 33°C. Active antiseizure medications are unchanged from panel A. (C) EEG prior to discharge on clobazam (20 mg/day), lamotrigine (200 mg/day), and levetiracetam (2,500 mg/day). EEG figures are shown in standard longitudinal bipolar montage. All data were acquired at 200 Hz, high-pass filtered above 0.5 Hz, low-pass–filtered below 50 Hz, and notch-filtered between 58 and 62 Hz. All EEG images are shown on identical time and voltage scales (abscissa and ordinate calibration bars, respectively).
Epilepsia © ILAE
In addition to lactic acidosis, JG experienced mild hyper-natremia (peak Na+ 155 mEq/L) and mild hypokalemia (nadir K+ 2.0 mEq/L) during hypothermia. Neither electrolyte disturbance required correction. He arrived at SLCH with methicillin-sensitive Staphylococcus aureus tracheitis, which was treated with oxacillin. No additional infections occurred. During rewarming, JG developed transient hypertension and hematuria. Hypertension was controlled with nicardipine and furosemide, which were discontinued by HD12 and HD14, respectively. Hematuria resolved spontaneously. One month after PICU discharge, JG was readmitted from the rehabilitation ward with duodenal ulcer and acute hemorrhage. The ulcer was endoscopically cauterized twice. At discharge, JG had oromotor slowing and verbal pauses, but otherwise was neurologically intact.
Case 5: JM
JM, a 15-year-old boy without significant PMHx, presented to an ED with new onset GTC seizures. He was evaluated as an outpatient and started on zonisamide. Two weeks later, he had recurrent GTC seizures and developed expressive aphasia. Zonisamide was discontinued, since it can cause aphasia (Zaccara et al., 2011). Concurrently, frequent GTC seizures prompted inpatient admission to Phoenix Children’s Hospital Diagnostic evaluation on admission included a complete metabolic panel, ammonia, lactate, complete blood count, urine drug screen, as well as head CT and brain MRI. All studies were within normal limits. Initial EEG demonstrated diffuse slowing indicative of global cerebral dysfunction. Lumbar puncture was significant for cerebrospinal fluid (CSF) pleocytosis (total cells 46, white blood cell count 42, glucose 74, and protein 34). Bacterial, Mycoplasma pneumoniae, and viral studies were negative.
Over the next 10 days, JM became more encephalopathic and developed RSE. Initial management of RSE included fosphenytoin, oxcarbazepine, and midazolam infusion (1 mg/kg/h, 85 mg/h). Due to inadequate seizure control, JM was transitioned from midazolam to pentobarbital infusion (max dose 3.5 mg/kg/h, 297.5 mg/h). Over the next 4 weeks, however, JM remained in RSE. Multiple combinations of antiepileptic medications—including propofol, ketamine, valproic acid, and topiramate—were tried and abandoned due to side effects and ineffectiveness. Eventually, JM was maintained on continuous infusions of pentobarbital (2.5 mg/kg/h) and midazolam (0.2 mg/kg/h), as well as levetiracetam (4,250 mg/day), zonisamide (425 mg/day), and phenobarbital (PSL = 42 μg/ ml)—a combination that diminished but did not fully suppress his seizures.
Following further evaluation, JM was diagnosed with anti–N-methyl-D-aspartate (NMDA) receptor encephalitis. He was treated with a 5-day course of steroids, plasmapheresis, and intravenous immunoglobulin (IVIg; 400 mg/kg/ day). He also received rituximab (850 mg/dose for four doses) and cyclophosphamide (750 mg/m2). Despite these measures, JM remained in RSE. At this point, hypothermia was induced with Gaymar Medi-Therm cooling blanket targeting esophageal temperature of 33–35°C. The patient reached goal temperature after 13 h. During hypothermia, ictal discharges on EEG became shorter in duration and less frequent. No electrolyte abnormalities, arrhythmias, or coagulopathies occurred during hypothermia. JM did develop a catheter-related urinary tract infection, which was treated with antibiotics. After 5 days of hypothermia, JM was rewarmed over 8 h to normothermia (36°C) without an increase in seizure frequency. Ketogenic diet was initiated, and pentobarbital and midazolam infusions were discontinued. JM required tracheostomy and gastrostomy. Six months after admission, JM was discharged to a long-term care facility on zonisamide (10 mg/kg/day, 850 mg/day), levetiracetam (0.12 g/kg/day, 10.20 g/day), and phenobarbital (0.6 mg/kg/day, 51 mg/day). One year after hospital discharge, JM continues on zonisamide and levetiracetam for focal seizures. He has mild cognitive and behavioral deficits but has returned to premorbid level of function.
Discussion
This is the largest pediatric case series reporting use of mild hypothermia (32–35°C) to treat RSE. Hypothermia, ranging from 20 to 35°C, has been occasionally used to treat RSE in adults (Sourek & Travnicek, 1970; Karkar et al., 2002; Corry et al., 2008). Several case reports describe its use to treat RSE in children (Orlowski et al., 1984; Elting et al., 2010; Lin et al., 2012; Shein, 2012). Table 1 summarizes clinical data available for published pediatric cases. SE and RSE are neurologic emergencies, requiring aggressive treatment. SE may proceed for hours if left untreated, resulting in neuronal injury and cell death (Fountain & Lothman, 1995; Fujikawa et al., 2000; Chen et al., 2007). Animal models indicate that efficacy of anticonvulsants in terminating SE decreases as SE duration increases (Mazarati et al., 1998; Rajasekaran et al., 2010). Simply put, the longer the seizures last, the harder they are to stop and the more damage they inflict. Hence, current guidelines recommend rapid escalation of treatment using different drug classes to terminate seizures (Shorvon et al., 2008; Shearer & Riviello, 2011; Brophy et al., 2012). Notably, hypothermia in our patients terminated seizures even after hours to weeks of RSE.
Table 1.
Prior reports of hypothermia in pediatric RSE
| References | No. of children | Age | Indication for cooling | Seizure duration | Other therapies attempted | Continuous infusions failed | Goal temp (°C) | Duration of HT | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Vastola et al. (1969) | 1 | 16 years | RSE due to meningoencephalitis | Not reported | Diphenylhydantoin Phenobarbital |
None attempted | 37 | 24 h | No recurrent seizures |
| Orlowski et al. (1984) | 3 | 6 years | RSE due to Reye’s syndrome | Not reported | Diazepam Phenobarbital Phenytoin |
None attempted | 30–31 | 48 h | Complete recovery, no recurrent seizures |
| 11 years | RSE due to encephalitis | Not reported | Dexamethasone Paraldehyde Phenobarbital Phenytoin |
Paraldehyde | 30–31 | 72 h | Good recovery with residual memory deficits | ||
| 18 years | RSE due to unknown neurodegenerative disease | 3 days | Carbamazepine Diazepam Paraldehyde Phenytoin Valproate |
Paraldehyde | 30–31 | 5 days | Recurrent focal seizures | ||
| Elting et al. (2010) | 1 | 5 months | Focal RSE due to hemimegalencephaly | 48 h | Ketamine Midazolam Phenytoin |
Midazolam Ketamine | 35.3a | 3 h | Discharged from ICU without RSE; hemispherectomy |
| Shein et al. (2012) | 1 | 4 months | RSE due to SCN1A mutation, Dravet syndrome | Not Reported | Diazepam Folinic acid Levetiracetam Lorazepam Midazolam Phenobarbital Phenytoin Pyridoxal-5-PO4 Topiramate |
Midazolam | 33–34 | 43 h, then again for 24 h | Seizures requiring ketogenic diet; developmental delay |
| Lin et al. (2012) | 2 | 10 years | RSE secondary to febrile-infection– related epilepsy syndrome | 1 day | Diazepam Lorazepam Midazolam Phenobarbital Phenytoin Valproic acid |
Midazolam | 33 | 5 days | Discharged home; mild cognitive and motor impairments |
| 4 years | RSE secondary to febrile-infection– related epilepsy syndrome | 10–12 h | Diazepam Lorazepam Midazolam Phenobarbital Phenytoin Valproic acid |
Midazolam | 33 | 3 days | Discharged home; mild cognitive Impairments and epilepsy |
Accidental cooling.
Multiple animal studies indicate that hypothermia is an effective primary and/or adjunct treatment for SE. In (Vastola et al. 1969). cooled cats with experimentally induced SE below 32°C, which terminated seizures in 85% of animals. In rats with SE evoked by perforant pathway stimulation, moderate hypothermia (29–33°C) reduced severity and frequency of motor seizures, albeit it was more effective when combined with diazepam (Schmitt et al., 2006). In a similar model, deep hypothermia (20°C) suppressed SE in 40% of rats (Kowski et al., 2012). Of interest, 50% of successfully treated rats were seizure free during and after rewarming (Kowski et al., 2012). In rats with seizures induced by potassium channel antagonists (Yang & Rothman, 2001) or by fluid percussive injury (D’Ambrosio et al., 2013), focal cortical cooling significantly reduced seizure frequency. In rats injected with pilocarpine, hypothermia pretreatment reduced neuronal apoptosis and increased latency to SE onset (Maeda et al., 1999; Yu et al., 2011). Hypothermia also decreased brain edema and seizure frequency in rats with kainic acid–induced SE (Wang et al., 2011). In addition, animal studies suggest that hypothermia provides neuroprotection in SE (Wang et al., 2011; Yu et al., 2011, 2012; Zhou et al., 2012). Therefore, studies across multiple mammalian epilepsy models suggest that hypothermia may effectively abrogate SE and may provide neuroprotection during seizures.
In addition to noting that hypothermia effectively abrogated RSE in our patients, we can make two additional clinical observations. First, none of our RSE patients treated with hypothermia relapsed into SE after rewarming. In contrast, when treated with pentobarbital at normothermia, 80% of our patients (4/5) experienced SE relapse on pentobarbital discontinuation (Table 2). The one patient that did not relapse (EZ) was treated with pentobarbital and hypothermia simultaneously. SE relapse rate after pentobarbital coma in our series is consistent with that reported previously (Barberio et al., 2012). Second, degree of hemodynamic instability and, consequently, use of inotropic agents appeared to decrease during hypothermia in our series. In three of five patients, inotrope requirements decreased during hypothermia. Hemodynamic improvement likely reflects decreased pentobarbital dose required to achieve burst suppression during hypothermia versus normothermia. In a meta-analysis, 77% of RSE patients treated with pentobarbital developed hypotension and required inotropic support (Claassen et al., 2002). Pentobarbital depresses cardiac function (Jiang et al., 2011). Hence, it is not surprising that inotrope requirements decreased as pentobarbital dose decreased.
Table 2.
Patient and clinical course characteristics
| Patient | LB | DB | EZ | JG | JM |
|---|---|---|---|---|---|
| Age at onset | 10 years old | 5 months old | 11 months old | 10 years old | 15 years old |
| SE etiology | Unknown | Hydrocephalus, perinatal injury | POLG-1 mutation | Epilepsy | Anti-NMDAR encephalitis |
| Preexisting comorbidities | None | Prematurity | Chiari type I RAD | Epilepsy DD, ADHD | None |
| Medications attempted and failed (alphabetical order) | Fosphenytoin Levetiracetam Lorazepam Midazolam Pentobarbital Phenobarbital Valproic acid |
Fosphenytoin Levetiracetam Lorazepam Midazolam Pentobarbital Phenobarbital |
Calcium Fosphenytoin Levetiracetam Lorazepam Midazolam Pentobarbital Phenobarbital Pyridoxine |
Diazepam Fosphenytoin Lamotrigine Levetiracetam Lorazepam Midazolam Pentobarbital |
Fosphenytoin Ketamine Midazolam Oxcarbazepine Pentobarbital Propofol Topiramate Valproic acid Zonisamide |
| Initial burst suppression achieved with | Pentobarbital 3 mg/kg/h |
Pentobarbital 10 mg/kg/h |
Pentobarbital 3.5 mg/kg/h + hypothermia |
Pentobarbital 5 mg/kg/h |
Pentobarbital 2.5 mg/kg/h |
| Did SE recur after initial burst suppression? | Yes | Yes | No | Yes | N/A |
| Target temperature (°C) | 34 | 32–34 | 34 | 33 | 33–35 |
| Time to target temp (h) | 2 | 2 | 3 | 3 | 13 |
| Time at target temp (days) | 1 | 3 | 2 | 5 | 5 |
| Rewarming rate | 0.5°C/24 h | 1°C/6 h | 0.5°C/12 h | 0.5°C/12 h | 2°C/8 h |
| Acid-base abnormalities | None | Lactic acidosis with NEC and septic shock | None | Lactic acidosis with pentobarbital | None |
| Electrolyte abnormalities | None | Hyperglycemia Hypokalemia |
Hypokalemia | Hypernatremia Hypokalemia |
None |
| BP changes during cooling or rewarming | None | None | None | Hypertension | None |
| Infection during TH | None | Clinical sepsis | None | None | UTI |
| Coagulopathy (INR > 1.5) | None | Yes | None | None | None |
| Did SE recur after rewarming? | No | No | No | No | No |
| Seizures postrewarming | None | Subclinical | Two brief clinical seizures | None | None |
| Discharge medications (alphabetical order) | Levetiracetam | N/A | Levetiracetam Phenobarbital Topiramate |
Clobazam Lamotrigine Levetiracetam |
Levetiracetam Phenobarbital Zonisamide |
| Discharge disposition | Home | Death | Home (after first admission) | Home | Long-term care facility |
In two patients (EZ and DB), however, inotrope requirement escalated with initiation of hypothermia. In EZ, hypothermia and pentobarbital were started simultaneously. Therefore, it is impossible to distinguish which of these treatments, hypothermia and/or pentobarbital, contributed most to development of hemodynamic instability. In DB, escalation of inotropes coincided with development of necrotizing enterocolitis (NEC) and septic shock 24 h after initiation of hypothermia. It is unclear whether hypothermia exacerbated these complications.
In neonates treated with hypothermia for hypoxic–ischemic encephalopathy (HIE), risk of NEC appears unaffected. Multiple clinical trials of therapeutic hypothermia in neonatal HIE demonstrated similar rates of NEC in infants treated with hypothermia compared to infants treated with usual care (normothermia) (Gluckman et al., 2005; Shankaran et al., 2005; Compagnoni et al., 2008; Shankaran et al., 2008; Azzopardi et al., 2009; Jacobs et al., 2011). None of these trials, however, was powered to detect a difference in NEC incidence between hypothermic and normothermic groups. More recently, a prospective nonrandomized trial evaluated safety of mild hypothermia in infants with advanced NEC and multiorgan dysfunction (Hall et al., 2010). In this trial, rates of death, laparotomy, and intestinal perforation were similar between 15 cooled and 10 control infants. Therefore, existing data suggest that mild hypothermia does not increase NEC risk in critically ill infants.
Body cooling poses several well-documented risks, although few are clinically significant with mild hypothermia (32–35°C). Cardiac arrhythmias are unlikely at core temperature >30°C (Welton et al., 1978; Piktel et al., 2011). Coagulopathy appears mild above 33°C (Rohrer & Natale, 1992; Eicher et al., 2005; Hall et al., 2010). We did not notice increased bleeding in our patients, including in DB who underwent laparotomy and bowel resection. Hypothermia increases infection risk (Compagnoni et al., 2008; Laupland et al., 2012; Seamon et al., 2012). One catheter-related urinary tract infection occurred in our cohort. Mild hypothermia commonly causes shivering, which necessitates neuromuscular blockade. It may also cause hypotension mainly during cooling or rewarming (Sessler, 2009). Indeed, a therapeutic hypothermia trial in children with traumatic brain injury associated hypothermia with increased incidence of hypotension during rewarming (Hutchison et al., 2008). Hypotension during rewarming (and cooling) is thought to result from changes in systemic vascular resistance and from uncontrolled diuresis (Polderman, 2009; Sessler, 2009). In our case series, we anticipated hypotension by monitoring arterial blood pressure and urine output during cooling/rewarming. We also minimized hypotension likelihood by changing temperature slowly, in some cases following a protocol established for an unrelated clinical trial of therapeutic hypothermia in pediatric cardiac arrest (Moler, 2009). We did not appreciate clinically significant hypotension during cooling or rewarming in our patients.
Generalization of our observations is limited by several factors. First, this is a retrospective case series. Second, hypothermia use and initiation timing varied significantly due to current lack of standardized protocols. Variability also existed in target temperature and in hypothermia duration. Absence of standardized hypothermia protocols likely explains implementation variability (Fink et al., 2010). Despite variability, hypothermia improved seizure burden in all five patients. Nevertheless, three patients had poor outcomes. RSE carries substantial risk of morbidity and mortality in and of itself (Barberio et al., 2012), and in all five cases, hypothermia was used as a rescue therapy after multiple agents failed. Whether earlier implementation of hypothermia would improve neurologic outcomes in RSE remains unknown. Future studies of hypothermia in SE/ RSE will require development of standardized protocols for implementation, patient selection, target temperature, and therapy duration. Although this case series demonstrates potential efficacy of hypothermia in treating pediatric RSE, prospective multicenter studies are required.
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- Barberio M, Reiter PD, Kaufman J, Knupp K, Dobyns EL. Continuous infusion pentobarbital for refractory status epilepticus in children. J Child Neurol. 2012;27:721–726. doi: 10.1177/0883073811424941. [DOI] [PubMed] [Google Scholar]
- Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, Laroche SM, Riviello JJ, Jr, Shutter L, Sperling MR, Treiman DM, Vespa PM Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiologyofstatus epilepticus. Acta Neurol Scand Suppl. 2007;186:7–15. [PubMed] [Google Scholar]
- Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43:146–153. doi: 10.1046/j.1528-1157.2002.28501.x. [DOI] [PubMed] [Google Scholar]
- Coeytaux A, Jallon P, Galobardes B, Morabia A. Incidence of status epilepticus in French-speaking Switzerland: (EPISTAR) Neurology. 2000;55:693–697. doi: 10.1212/wnl.55.5.693. [DOI] [PubMed] [Google Scholar]
- Compagnoni G, Bottura C, Cavallaro G, Cristofori G, Lista G, Mosca F. Safety of deep hypothermia in treating neonatal asphyxia. Neonatology. 2008;93:230–235. doi: 10.1159/000111101. [DOI] [PubMed] [Google Scholar]
- Corry JJ, Dhar R, Murphy T, Diringer MN. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9:189–197. doi: 10.1007/s12028-008-9092-9. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Eastman CL, Darvas F, Fender JS, Verley DR, Farin FM, Wilkerson HW, Temkin NR, Miller JW, Ojemann J, Rothman SM, Smyth MD. Mild passive focal cooling prevents epileptic seizures after head injury in rats. Ann Neurol. 2013;73:199–209. doi: 10.1002/ana.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, Garnett L, Fortner CA, Ko D. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- Eicher DJ, Wagner CL, Katikaneni LP, Hulsey TC, Bass WT, Kaufman DA, Horgan MJ, Languani S, Bhatia JJ, Givelichian LM, Sankaran K, Yager JY. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol. 2005;32:18–24. doi: 10.1016/j.pediatrneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Elting JW, Naalt JV, Fock JM. Mild hypothermia for refractory focal status epilepticus in an infant with hemimegalencephaly. Eur J Paediatr Neurol. 2010;14:452–455. doi: 10.1016/j.ejpn.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Fink EL, Kochanek PM, Clark RS, Bell MJ. How I cool children in neurocritical care. Neurocrit Care. 2010;12:414–420. doi: 10.1007/s12028-010-9334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain NB, Lothman EW. Pathophysiology of status epilepticus. J Clin Neurophysiol. 1995;12:326–342. [PubMed] [Google Scholar]
- Fujikawa DG, Shinmei SS, Cai B. Seizure-induced neuronal necrosis: implications for programmed cell death mechanisms. Epilepsia. 2000;41(Suppl 6):S9–S13. doi: 10.1111/j.1528-1157.2000.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM, Thoresen M, Whitelaw A, Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Hall NJ, Eaton S, Peters MJ, Hiorns MP, Alexander N, Azzopardi DV, Pierro A. Mild controlled hypothermia in preterm neonates with advanced necrotizing enterocolitis. Pediatrics. 2010;125:e300–e308. doi: 10.1542/peds.2008-3211. [DOI] [PubMed] [Google Scholar]
- Isohanni P, Hakonen AH, Euro L, Paetau I, Linnankivi T, Liukkonen E, Wallden T, Luostarinen L, Valanne L, Paetau A, Uusimaa J, Lönnqvist T, Suomalainen A, Pihko H. POLG1 manifestations in childhood. Neurology. 2011;76:811–815. doi: 10.1212/WNL.0b013e31820e7b25. [DOI] [PubMed] [Google Scholar]
- Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, Wright IM, Kirpalani HM, Darlow BA, Doyle LW Infant Cooling Evaluation Collaboration. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- Jiang X, Gao L, Zhang Y, Wang G, Liu Y, Yan C, Sun H. A comparison of the effects of ketamine, chloral hydrate and pentobarbital sodium anesthesia on isolated rat hearts and cardiomyocytes. J Cardiovasc Med (Hagerstown) 2011;12:732–735. doi: 10.2459/JCM.0b013e32834a6697. [DOI] [PubMed] [Google Scholar]
- Karkar KM, Garcia PA, Bateman LM, Smyth MD, Barbaro NM, Berger M. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia. 2002;43:932–935. doi: 10.1046/j.1528-1157.2002.03902.x. [DOI] [PubMed] [Google Scholar]
- Knake S, Rosenow F, Vescovi M, Oertel WH, Mueller HH, Wirbatz A, Katsarou N, Hamer HM Status Epilepticus Study Group Hessen (SESGH) Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001;42:714–718. doi: 10.1046/j.1528-1157.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- Kowski AB, Kanaan H, Schmitt FC, Holtkamp M. Deep hypothermia terminates status epilepticus–an experimental study. Brain Res. 2012;1446:119–126. doi: 10.1016/j.brainres.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Lambrechtsen FA, Buchhalter JR. Aborted and refractory status epilepticus in children: a comparative analysis. Epilepsia. 2008;49:615–625. doi: 10.1111/j.1528-1167.2007.01465.x. [DOI] [PubMed] [Google Scholar]
- Laupland KB, Zahar JR, Adrie C, Minet C, Vésin A, Goldgran-Toledano D, Azoulay E, Garrouste-Orgeas M, Cohen Y, Schwebel C, Jamali S, Darmon M, Dumenil AS, Kallel H, Souweine B, Timsit JF. Severe hypothermia increases the risk for intensive care unit-acquired infection. Clin Infect Dis. 2012;54:1064–1070. doi: 10.1093/cid/cir1033. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Lin KL, Hsia SH, Wang HS CHEESE Study Group. Therapeutic hypothermia for febrile infection-related epilepsy syndrome in two patients. Pediatr Neurol. 2012;47:448–450. doi: 10.1016/j.pediatrneurol.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Maeda T, Hashizume K, Tanaka T. Effect of hypothermia on kainic acid-induced limbic seizures: An electroencephalographic and 14C-deoxyglucose autoradiographic study. Brain Res. 1999;818:228–235. doi: 10.1016/s0006-8993(98)01269-4. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Claassen J, Lokin J, Mendelsohn F, Dennis LJ, Fitzsimmons BF. Refractory status epilepticus: Frequency, risk factors, and impact on outcome. Arch Neurol. 2002;59:205–210. doi: 10.1001/archneur.59.2.205. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Baldwin RA, Sankar R, Wasterlain CG. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res. 1998;814:179–185. doi: 10.1016/s0006-8993(98)01080-4. [DOI] [PubMed] [Google Scholar]
- Moler FW. [Accessed January 23, 2013];Therapeutic hypothermia after pediatric cardiac arrest NCT00880087. 2009 Available at: http://www.clinicaltrials.gov.
- Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51:251–256. doi: 10.1111/j.1528-1167.2009.02323.x. [DOI] [PubMed] [Google Scholar]
- Orlowski JP, Erenberg G, Lueders H, Cruse RP. Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med. 1984;12:367–372. doi: 10.1097/00003246-198404000-00006. [DOI] [PubMed] [Google Scholar]
- Piktel JS, Jeyaraj D, Said TH, Rosenbaum DS, Wilson LD. Enhanced dispersion of repolarization explains increased arrhythmogenesis in severe versus therapeutic hypothermia. Circ Arrhythm Electrophysiol. 2011;4:79–86. doi: 10.1161/CIRCEP.110.958355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- Rajasekaran K, Zanelli SA, Goodkin HP. Lessons from the laboratory: the pathophysiology, and consequences of status epilepticus. Semin Pediatr Neurol. 2010;17:136–143. doi: 10.1016/j.spen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20:1402–1405. doi: 10.1097/00003246-199210000-00007. [DOI] [PubMed] [Google Scholar]
- Schmitt FC, Buchheim K, Meierkord H, Holtkamp M. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis. 2006;23:689–696. doi: 10.1016/j.nbd.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Seamon MJ, Wobb J, Gaughan JP, Kulp H, Kamel I, Dempsey DT. The effects of intraoperative hypothermia on surgical site infection: an analysis of 524 trauma laparotomies. Ann Surg. 2012;255:789–795. doi: 10.1097/SLA.0b013e31824b7e35. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009;37:S203–S210. doi: 10.1097/CCM.0b013e3181aa5568. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Pappas A, Laptook AR, McDonald SA, Ehrenkranz RA, Tyson JE, Walsh M, Goldberg RN, Higgins RD, Das A NICHD Neonatal Research Network. Outcomes of safety and effectiveness in a multicenter randomized, controlled trial of whole-body hypothermia for neonatal hypoxic-ischemic encephalopathy. Pediatrics. 2008;122:e791–e798. doi: 10.1542/peds.2008-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer P, Riviello J. Generalized convulsive status epilepticus in adults and children: treatment guidelines and protocols. Emerg Med Clin North Am. 2011;29:51–64. doi: 10.1016/j.emc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Shein SL, Reynolds TQ, Gedela S, Kochanek PM, Bell MJ. Therapeutic hypothermia for refractory status epilepticus in a child with malignant migrating partial seizures of infancy and SCN1A mutation: a case report. Ther Hypothermia Temp Manag. 2012;2:144–149. doi: 10.1089/ther.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorvon S, Baulac M, Cross H, Trinka E, Walker M TaskForce on Status Epilepticus of the ILAE Commission for European Affairs. The drug treatment of status epilepticus in Europe: consensus document from a workshop at the first London Colloquium on Status Epilepticus. Epilepsia. 2008;49:1277–1285. doi: 10.1111/j.1528-1167.2008.01706_3.x. [DOI] [PubMed] [Google Scholar]
- Sourek K, Travnicek V. General and local hypothermia of the brain in the treatment of intractable epilepsy. J Neurosurg. 1970;33:253–259. doi: 10.3171/jns.1970.33.3.0253. [DOI] [PubMed] [Google Scholar]
- Vastola EF, Homan R, Rosen A. Inhibition of focal seizures by moderate hypothermia. A clinical and experimental study. Arch Neurol. 1969;20:430–439. doi: 10.1001/archneur.1969.00480100106015. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu PP, Li LY, Zhang HM, Li T. Hypothermia reduces brain edema, spontaneous recurrent seizure attack, and learning memory deficits in the kainic acid treated rats. CNS Neurosci Ther. 2011;17:271–280. doi: 10.1111/j.1755-5949.2010.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welton DE, Mattox KL, Miller RR, Petmecky FF. Treatment of profound hypothermia. JAMA. 1978;240:2291–2292. [PubMed] [Google Scholar]
- Wolf NI, Rahman S, Schmitt B, Taanman JW, Duncan AJ, Harting I, Wohlrab G, Ebinger F, Rating D, Bast T. Status epilepticus in children with Alpers’ disease caused by POLG1 mutations: EEG and MRI features. Epilepsia. 2009;50:1596–1607. doi: 10.1111/j.1528-1167.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- Yang XF, Rothman SM. Focal cooling rapidly terminates experimental neocortical seizures. Ann Neurol. 2001;49:721–726. doi: 10.1002/ana.1021. [DOI] [PubMed] [Google Scholar]
- Yu L, Zhou Y, Chen W, Wang Y. Mild hypothermia pretreatment protects against pilocarpine-induced status epilepticus and neuronalapoptosis in immature rats. Neuropathology. 2011;31:144–151. doi: 10.1111/j.1440-1789.2010.01155.x. [DOI] [PubMed] [Google Scholar]
- Yu L, Zhou Y, Wang Y. Effect of mild hypothermia on glutamate receptor expression after status epilepticus. Epilepsy Res. 2012;101:56–69. doi: 10.1016/j.eplepsyres.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Zaccara G, Tramacere L, Cincotta M. Drug safety evaluation of zonisamide for the treatment of epilepsy. Expert Opin Drug Saf. 2011;10:623–631. doi: 10.1517/14740338.2011.571201. [DOI] [PubMed] [Google Scholar]
- Zhou YF, Wang Y, Shao XM, Chen L, Wang Y. Effects of hypothermia on brain injury induced by status epilepticus. Front Biosci. 2012;17:1882–1890. doi: 10.2741/4025. [DOI] [PubMed] [Google Scholar]