Abstract
Background
Studies addressing the influence of early sexual maturation on the excess of body weight and height of children and adolescents are scarce. The aim of the study was to analyze the association of sexual maturation with excess body weight and height in children and adolescents.
Methods
This was a cross-sectional study performed in Florianópolis city, Brazil, in 2007, with 2339 school children, aged 8–14 years (1107 males). Selection was based on a probabilistic, cluster-stratified sampling technique. School children were classified according to the presence of excess body weight, using sex- and age-specific body mass index (BMI) cutoff points. Z-scores were calculated from height and BMI data. Sexual maturation was self-assessed according to Tanner stages of development. Subjects were ranked based on tertiles of sexual maturation (early, normal and late) for each stage of development. Poisson and linear regression models were used.
Results
Compared to the reference group (normal sexual maturation), early maturing females had higher prevalence of excess weight (adjusted prevalence ratio: 1.70; 95% CI: 1.24 to 2.33) and increased height-for-age (adjusted β: 0.37; 95% CI: 0.14 to 0.59), while late maturing females had lower prevalence of excess weight (adjusted prevalence ratio: 0.57; 95% CI: 0.37 to 0.87) and decreased height-for-age (adjusted β: −0.38; 95% CI: −0.56 to −0.20). In males, early and late sexual maturation were associated with increased (adjusted β: 0.37; 95% CI: 0.14 to 0.59) and decreased (adjusted β: −0.38; 95% CI: −0.56 to −0.20) height-for-age, respectively.
Conclusion
Early sexual maturation is associated with excess body weight in females and with greater height-for-age in both sexes.
Keywords: Obesity, Puberty, Growth, Child, Adolescent
Background
Excess body weight in the first two decades of life has been the focus of several studies, due to its consequences for health across the life course. This concern has been justified by the fact that excess weight prevalence is increasing in the United States [1], Europe [2] and Latin America [2,3]. The worldwide prevalence of childhood obesity increased from 4.2% to 6.7% between 1990 and 2010 [4]. In Brazil, data from nationwide surveys indicate that there was a sharp increase in the prevalence of overweight and obesity among children and adolescents between 1989 and 2009, despite a modest or no increase between 1974 and 1989 in all the country regions [5].
Puberty is a critical period for developing excess body weight, since hormonal changes impact directly on weight gain and linear growth. These changes comprise the chemical maturation of body tissues, including the amount and distribution of adipose tissue, and the increase in bone mass and in fat-free mass. In both sexes, there is a possibility of rapid increments in height and weight [6].
There are apparent differences between males and females in fat content and distribution, especially in adolescence [7]. In females, there is evidence that early sexual maturation is associated with a greater prevalence of overweight and obesity [8,9]. In males, few studies have been performed and findings are mixed [9,10].
Studies addressing the influence of early sexual maturation on the height of children and adolescents are scarce. In general, there is evidence that, regardless of the sex, those with early sexual maturation have greater height-for-age, compared to those with normal or late sexual maturation [11,12].
Thus, it is important to study the influence of sexual maturation on these anthropometric measures, given their relation to the health and development of children and adolescents. The aim of this study was to analyze the potential associations between sexual maturation and excess body weight or height in children and adolescents, aged 8 to 14 years, from the municipality of Florianopolis, in the State of Santa Catarina (SC), Brazil.
Methods
This was a cross-sectional study performed in the city of Florianópolis, in the State of Santa Catarina, South of Brazil, during the months from April 2007 to October 2007.
The sampling procedure is described in detail elsewhere [13-15]. Briefly, for sample size calculation, the following parameters were assumed: excess weight prevalence of 22.1% for school children aged 7–10 years [16] and 12.6% for those aged 11–14 years [17]; acceptable error of 3 percentage points; two-tailed test; confidence level of 95%; design effect of 1.5; and an addition of 10% for losses. Subjects were excluded if the parents or primary caregiver did not sign the written informed consent. The sampling procedure was probabilistic, stratified by clusters and performed in two stages (school and children). Schools were grouped in four strata, according to the geographical area and school type: center/continent public schools, center/continent private schools, beaches public and beaches private schools, considering a probability proportional to the size (number of students) of each stratum. In the first stage, schools were randomly selected from each stratum. From a total of 87 schools (33 private and 54 public), 17 schools were selected (6 private and 11 public). In the second stage, school children were randomly selected according to age. For the present study, 7 year old children (n = 421) were excluded, as there was no information on sexual maturation. The final sample consisted of 2412 school children aged 8 to 14 years (1144 males, 47.4%).
Anthropometric measures were taken following the protocol of the World Health Organization [18], as proposed by Lohman [19]. Body weight was measured to the nearest of 50g using an electronic scale (model PP 180, Marte®, Minas Gerais, Brazil), capacity of 180 kg; height was measured to the nearest of 1 mm by Alturexata® stadiometer.
The team responsible for data collection was composed of 10 people previously trained in a workshop in the period of 2006 September to 2007 March. This workshop consisted of a theoretical and practical course in anthropometric measures, as well as of a pilot study in two schools (one private and one public), in which the intra- and inter- observer errors in anthropometric measures were assessed [20]. Both schools were excluded from the sampling of the study.
Sex-specific body mass index (BMI)-for-age growth charts were used to assess excess body weight, according to the World Health Organization criteria [4]. The variable excess body weight comprised the sum of overweight and obesity derived from BMI, in which 0 represents no excess of body weight and 1 represents having excess body weight. Z-scores for height-for-age, weight-for-age and BMI-for-age were also calculated for each sex separately, using LMS values. The LMS sums up the data in smoothed curves that are specific to each stratum, which in this case are the ages and genders. Parameter M is the median value of the index observed inside each stratum; parameter S is the coefficient of variation for each stratum; and parameter L is the Box-Cox coefficient employed for the mathematical transformation of the values of the variable in question in order to obtain a normal distribution in each stratum [21]. The cutoff point for biologically implausible values was 5 z-scores [4]. To calculate the z-score, LMS values were used, by age and gender, according to the following formula:
Sexual maturation was determined based on the stages of development proposed by Tanner, comprising 5 stages for genital (males) and breast (females) development [22]. School children were asked to perform a self-evaluation; the instructions were given individually.
For each sex, the age distribution (in decimals) of each sexual maturation stage was used to define tertiles of age. Based on this, individuals were classified in three groups: early sexual maturation (below first tertile of age), late sexual maturation (second tertile or more) and normal sexual maturation or reference group (between the first and the second tertile) [23]. This criterium considers that a younger age at a sexual maturation stage indicates precocious sexual maturation.
The birth weight of school children was reported by the parent or primary caregiver, who was asked to check the child’s health record. The subjects were classified into low (< 2.500 g), normal (≥ 2.500 g - 3.999 g) and high birth weight (≥ 4.000 g).
The weight status of the mother was evaluated by BMI, using self-reported weight and height. Excess body weight classification (≥ 25 kg/m2) followed the recommendation of the World Health Organization [24].
Information on the mode of commuting to school was collected by an illustrated questionnaire and subjects were classified as active (walking and biking) or inactive (car, bus, passenger in motorcycle or bicycle).
Further information (name, date of birth, school grade and type of school) were obtained in documents provided by the school. The type of school refers to public or private.
Given the non-normal distribution of anthropometric data (Shapiro-Wilk test, p < 0.05), quantitative variables (age, BMI Z score, height Z score, weight Z score) were described as median, 25th and 75th percentiles. Qualitative variables (excess body weight, sexual maturation classification, maternal excess body weight, birth weight, active commuting to school, school type) were described by relative frequencies (%). To compare quantitative variables between groups, Mann–Whitney (female vs. male) and Kruskal-Wallis (early, normal or late sexual maturation) tests were used. The associations among qualitative variables were assessed by Rao-Scott test. The relation between the independent variables and excess body weight (0-absence; 1-presence) was tested with univariate and multivariate Poisson regression models, using robust variance and stepwise forward strategy (multivariate model). The association between the independent variables and height was evaluated using a linear regression model (homoscedasticity checked by Breusch-Pagan and Cook-Weisberg test). Stata 11.0® was used in all statistical analysis, including the command svy to allow for sampling weights and stratification [25,26].
This study was approved by the Ethics Committee on Human Research of the Santa Catarina University (UFSC) (protocol number 028/06).
Results
Seventy-three subjects were excluded from the analysis (loss of 3.03%) for the following reasons: height-for-age Z-score greater than 5 (1 female) and missing or inconsistent data on sexual maturation (36 males and 36 females). This resulted in a final sample of 2339 school children aged 8–14 years (1107 males). There were no statistically significant differences in excess weight prevalence based on BMI, BMI Z-score, height Z-score and the independent variables in the group excluded from the study.
Males comprised 47.7% of the study sample. When compared to females, they had higher values of BMI Z-score (p < 0.001), height Z-score (p = 0.002), greater prevalence of current excess weight (34.4 vs. 24.0%, p < 0.001) and of high birth weight (14.4 vs. 8.1%, p < 0.001). No significant differences were found in the remaining variables (Table 1).
Table 1.
Anthropometric characteristic and mode of commuting to school in children aged 8–14 years old from Florianópolis, South of Brazil, 2007
|
Study variables |
Males (47.7%) |
Females (52.3%) |
p
§
|
|---|---|---|---|
| Median (p25 ; p75)* | |||
| Age (years) |
11 (10; 13) |
12 (10; 13) |
0.529 |
| BMI Z score |
0.51 (−0.22; 1.42) |
0.21 (−0.41; 0.96) |
<0.001 |
| Height Z score |
0.25 (−0.41; 0.99) |
0.11 (−0.52; 0.79) |
0.002 |
| Weight Z score |
0.38 (−0.20; 1.30) |
0.23 (−0.55; 0.69) |
0.004 |
| |
% |
|
|
| Excess body weight |
34.4 |
24.0 |
<0.001 |
| Sexual maturation classification |
|
|
0.937 |
| Early |
33.1 |
32.5 |
|
| Late |
32.2 |
32.7 |
|
| Maternal excess body weight |
31.0 |
33.4 |
0.313 |
| Birth weight (grams) |
|
|
<0.001 |
| < 2,500 |
5.8 |
8.3 |
|
| ≥ 4,000 |
14.4 |
8.1 |
|
| Active commuting to school |
43.6 |
45.0 |
0.558 |
| School type |
|
|
0.975 |
| Public |
65.4 |
65.5 |
|
| Private | 34.6 | 34.5 | |
*p25 e p75: 25th and 75th percentiles, respectively;
§Mann–Whitney test (quantitative variables) and Rao-Scott test (qualitative variables).
In males, sexual maturation was associated with significant differences in BMI Z-score (p < 0.001), height Z-score (p < 0.001), weight Z-score (p < 0.001) and excess weight prevalence (p = 0.027). Similar results were found in females (p < 0.001). There was also a trend towards increased BMI Z-score, height Z-score, weight Z-score and excess weight prevalence with the precocity of sexual maturation (p for trend < 0.001) (Table 2).
Table 2.
Hypothesis tests and descriptive statistics of anthropometric variables according to sexual maturation of school children aged 8–14 years from Florianópolis, South of Brazil, 2007
| |
Sexual maturation classification |
|
|
Sexual maturation classification |
|
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Early | Normal | Late | p § | p † | Early | Normal | Late | p § | p † |
| |
Males |
|
Females |
|
||||||
| |
Median (p25; p75)* |
|
|
Median (p25; p75)* |
|
|
||||
| BMI Z score |
0.67 (−0.13; 1.56) |
0.53 (−0.17; 1.41) |
0.26 (−0.37; 1.11) |
<0.001 |
<0.001 |
0.60 (0.01; 1.33) |
0.14 (−0.40; 0.84) |
−0.10 (−0.81; 0.54) |
<0.001 |
<0.001 |
| Height Z score |
0.44 (−0.23; 1.30) |
0.19 (−0.42; 0.90) |
0.02 (−0.61; 0.77) |
<0.001 |
<0.001 |
0.43 (−0.17; 1.07) |
0.03 (−0.46; 0.57) |
−0.22 (0.89; 0.61) |
<0.001 |
<0.001 |
| Weight Z score |
0.57 (−0.09: 1.44) |
0.39 (−0.19; 1.32) |
0.14 (−0.38; 1.01) |
<0.001 |
<0.001 |
0.46 (−0.31; 1.03) |
0.24 (−0.70; 0.66) |
−0.04 (−0.56; 0.49) |
<0.001 |
<0.001 |
| |
% |
|
|
% |
|
|
||||
| Excess body weight | 37.5 | 37.8 | 26.9 | 0.027 | <0.001 | 31.7 | 19.4 | 13.4 | <0.001 | <0.001 |
*p25 e p75: 25th and 75th percentiles, respectively;
§Mann–Whitney test (quantitative variables) and Rao-Scott test (qualitative variables).
†p for trend.
In both crude and adjusted models (Tables 3 and 4), compared to the reference group (normal sexual maturation), early maturing females had higher prevalence of excess weight (adjusted prevalence ratio: 1.70; 95% CI: 1.24 to 2.33) and increased height-for-age (adjusted β: 0.37; 95% CI: 0.14 to 0.59), while late maturing females had lower prevalence of excess weight (adjusted prevalence ratio: 0.57; 95% CI: 0.37 to 0.87) and decreased height-for-age (adjusted β: −0.38; 95% CI: −0.56 to −0.20).
Table 3.
Estimated prevalence ratios from uni- and multivariate Poisson regression models for the prediction of excess body weight according to the sexual maturation of school children aged 8–14 years from Florianópolis, South of Brazil, 2007
| Sexual maturation classification |
Males |
Females |
||||
|---|---|---|---|---|---|---|
| Crude prevalence ratio (95% CI)* | Adjusted prevalence ratio (95% CI)* § | p § | Crude prevalence ratio (95% CI)* | Adjusted prevalence ratio (95% CI)* § | p § | |
| Early |
0.99 (0.75;1.32) |
0.98 (0.73; 1.32) |
0.891 |
1.63 (1.23; 2.16) |
1.70 (1.24; 2.33) |
0.004 |
| Normal |
1 |
1 |
- |
1 |
1 |
- |
| Late | 0.71 (0.55; 0.93) | 0.78 (0.59; 1.02) | 0.067 | 0.69 (0.51; 0.95) | 0.57 (0.37; 0.87) | 0.014 |
*95% confidence interval;
§Poisson regression model, adjusted for maternal BMI, active commuting, age, birth weight, school type and interaction between maternal BMI and school type.
Table 4.
Estimated beta coefficients from uni- and multivariate Poisson regression models for the prediction of height Z score according to the sexual maturation of school children aged 8–14 years from Florianópolis, South of Brazil, 2007
| Sexual maturation classification |
Males |
Females |
||||
|---|---|---|---|---|---|---|
| Crude beta (95% CI)* | Adjusted beta (95% CI)* § | p § | Crude beta (95% CI)* | Adjusted beta (95% CI)* § | p § | |
| Early |
0.33 (0.12; 0.54) |
0.46 (0.26; 0.66) |
<0.001 |
0.33 (0.16; 0.50) |
0.37 (0.14; 0.59) |
0.005 |
| Normal |
1 |
1 |
- |
1 |
1 |
- |
| Late |
−0.23 (−0.50; 0.04) |
−0.32 (−0.63; −0.02) |
0.039 |
−0.27 (−0.39; −0.16) |
−0.38 (−0.56; −0.20) |
0.001 |
| Variance homogeneity† | 0.303 | 0.367 | ||||
*95% confidence interval;
§Linear regression model, adjusted for maternal BMI, active commuting, age, birth weight, school type and interaction between maternal BMI and school type.
†p value from Breusch-Pagan and Cook-Weisberg test.
In males, early and late sexual maturation were associated with increased (adjusted β: 0.37; 95% CI: 0.14 to 0.59) and decreased (adjusted β: −0.38; 95% CI: −0.56 to −0.20) height-for-age, respectively (Table 4). In contrast with the findings in females, there was no association of excess weight prevalence with early (p = 0.891) or late (p = 0.067) sexual maturation (reference group: normal sexual maturation) (Table 3). The assumption of homoscedasticity was held in the regression models of height (p = 0.303 for males and 0.367 for females, Table 4).
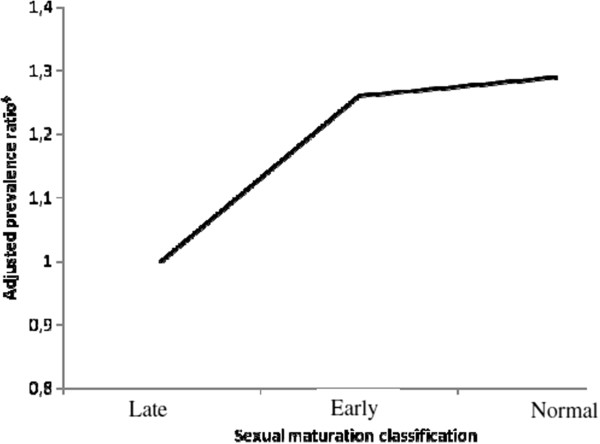
A trend of reduction in excess weight prevalence in males with late sexual maturation was also observed (Figure 1), despite non-significant values. Adjusted prevalence ratios of 1.26 (95% CI: 0.96 to 1.66) and 1.29 (95% CI: 0.99 to 1.67) were found in males with early and normal sexual maturation, respectively, when compared to those with late sexual maturation.
Figure 1.
Adjusted prevalence ratio for excess body weight by sexual maturation in male children, Brazil, 2007. §Adjusted for maternal BMI, active commuting, age, birth weight, school type and interaction between maternal BMI and school type.
Discussion
The study results indicate that early sexual maturation is associated with a higher prevalence of excess body weight in females and a higher stature in both sexes. The results observed in females are similar to those reported by previous cross-sectional studies [8,10,23,27].
Other cohort studies examined the association between anthropometric measures and sexual maturation in females, adjusted for BMI or adiposity in childhood [28-31]. Obesity in adulthood seemed to be more dependent on high BMI or adiposity in childhood and adolescence than on early sexual maturation [28-30]. However, such studies measured adiposity at the mean ages of 9 [29], 12 [30] and 14 [28], a period of life during which there is a potential influence of sexual maturation on body fat levels.
In contrast, studies that monitored BMI before sexual maturation showed different results. The study by Pierce and Leon [31] suggested that early sexual maturation is a stronger predictor of high BMI in adulthood than BMI in childhood. BMI was assessed between the ages 4 and 6 years, during which there is no influence of puberty in anthropometric variables. This finding indicated a relationship between early sexual maturation and obesity in adulthood, independent of BMI in childhood. Likewise, Demerath et al. [32] showed that higher BMI appears to be a consequence, not the cause, of puberty onset. The study followed females for 5 years (5 to 9 years old) and differences in weight were observed only after menarche.
The onset of puberty in females is associated with an increase in the amount of fat mass, as a consequence of increased blood concentration of estradiol [33]. Thus, it is plausible that the early onset of puberty in females is associated with a higher amount of adipose tissue, which may lead to a higher prevalence of overweight. It is noteworthy, however, that this relationship may be confounded by levels of adiposity at young ages. BMI in childhood is, therefore, an important confounding variable, given its capacity of predicting BMI in adulthood.
In males, evidence of the association between early sexual maturation and excess body weight is conflicting. In the present study, this association was not statistically significant, despite the fact that lower prevalences were observed in the group with late sexual maturation. In other studies, early sexual maturation was found to be risk factor [10], a protective factor [9] or not associated [23,28] with excess body weight. Cohort studies showed that males with early maturation have higher total adiposity [34], central adiposity [35] and waist and hip circumferences [12] in adulthood. Although it cannot be clearly stated, the body of evidence indicates that early maturing males can develop excess body weight.
Concerning height, the results showed that, regardless of sex, school children with early sexual maturation had greater height-for-age compared to those with normal or late sexual maturation. An increased height gain during childhood may be related to higher BMI and earlier puberty onset [36]. However, this greater stature is not observed in adulthood [11,12] and it seems to be restricted to late childhood and early adolescence, both in males [12,37] and in females [11]. The relationship between short stature in adulthood and early sexual maturation may be linked to the early onset of puberty and the decreased prepuberal period of growth, resulting in a premature gain in stature that does not translate into greater height in adulthood [12].
One possible explanation for the increased height due to early sexual maturation is the increased leptin levels during puberty. Studies have found that plasma leptin concentration in children and adolescents is closely related to the changes in height and body composition during growth [38]. Data from cell culture studies demonstrate that the administration of leptin stimulates the growth of cells from cartilaginous tissue [39], which can result in epiphyseal cartilage growth found at the ends of long bones. Other factors that have been implicated in the accelerated growth of obese children include increased adrenal androgens and insulin-like growth factor (IGF)-1. The highest prevalence of overweight observed among children with early sexual maturation can promote this condition because there is a positive association between levels of IGF-1 and adiposity [40,41]. In addition, it has been suggested that an increased adipose tissue aromatization of androgens into estrogens represents another important mechanism regulating growth in the context of obesity [6].
Some strengths of the study reduce the possibility of selection bias, measurement bias and confusion, such as small sample loss, the validated instruments, the trained team, and the adjustment for variables known to predict excess body weight. This, in addition to the population-based characteristic of the sample, increases the internal and external validity of the study.
The determination of early sexual maturation used in the present study, although not standardized in the scientific literature, was similar to that used by other authors [10,23]. One limitation of the study may be related to the use of self-assessed sexual maturation. However, this procedure has been validated in studies with Brazilian adolescents [42] and the results showed a good correlation (r = 0.80) between the self-assessment and experienced-personnel assessment. Data in younger populations with 8 years old or more also point to the validity of the method [43,44].
Despite the fact that BMI cannot measure body fat, it has been increasingly used to identify obese children and adolescents with high specificity, yielding few false positives [45]. Nevertheless, future studies are needed to assess the association of sexual maturity with body composition and fat distribution.
Conclusion
Early sexual maturation is associated with excess body weight (females only) and height in school children aged 8–14 years. Undoubtedly, knowledge of the association between sexual maturation and overweight in a given population enables the implementation of preventive measures in order to reduce the exposure to these risk factors. These results are of great public health importance considering the relationship between these indicators and some noncommunicable chronic diseases, as well as the evidence that obesity in adulthood is associated with high BMI in childhood and adolescence.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors participated in the acquisition of data and revision of the manuscript. All authors determined the design, performed the statistical analysis, interpreted the data and drafted the manuscript. All authors read and gave final approval for the version submitted for publication.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Jucemar Benedet, Email: jucemarb@hotmail.com.
Adair da Silva Lopes, Email: adair.lopes@ufsc.br.
Fernando Adami, Email: adamifernando@uol.com.br.
Patrícia de Fragas Hinnig, Email: phinnig@yahoo.com.br.
Francisco de Assis Guedes de Vasconcelos, Email: f.vasconcelos@ufsc.br.
Acknowledgments
FA was supported by Brazilian National Council of Scientific and Technological Development (CNPq). PFH is supported by Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES).
References
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012;82:1–8. [PubMed] [Google Scholar]
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27. doi: 10.1038/nrendo.2012.199. [DOI] [PubMed] [Google Scholar]
- Amigo H. Obesidad en el niño en América Latina: situación, criterios de diagnóstico y desafíos. Cad. Saúde Pública. 2003;19:163–170. doi: 10.1590/s0102-311x2003000700017. [DOI] [PubMed] [Google Scholar]
- De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil. análise do consumo alimentar pessoal no Brasil/IBGE. Rio de Janeiro: IBGE: Coordenação de Trabalho e Rendimento; 2011. Pesquisa de orçamentos familiares 2008-2009. [Google Scholar]
- Solorzano CMB, McCartney CR. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeting HN. Gendered dimensions of obesity in childhood and adolescence. Nutr J. 2008;7:1–14. doi: 10.1186/1475-2891-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes JH, Obarzanek E, Baranowski T, Wilson DM, Rochon J, Mcclanahan BS. Early sexual maturation, body composition, and obesity in African–American girls. Obes Res. 2004;12:64–72S. doi: 10.1038/oby.2004.270. [DOI] [PubMed] [Google Scholar]
- Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–10. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- Ribeiro J, Santos P, Duarte J, Mota J. Association between overweight and early sexual maturation in Portuguese boys and girls. Ann Hum Biol. 2006;33:55–63. doi: 10.1080/00207390500434135. [DOI] [PubMed] [Google Scholar]
- Onland-Moret NC, Peeters PHM, Van Gils CH, Clavel-Chapelon F, Key T, Tjønneland A, Trichopoulou A, Kaaks R, Manjer J, Panico S, Palli D, Tehard B, Stoikidou M, Bueno-De-Mesquita HB, Boeing H, Overvad K, Lenner P, Quirós JR, Chirlaque MD, Miller AB, Khaw KT, Riboli E. Age at menarche in relation to adult height. Am J Epidemiol. 2005;162:623–32. doi: 10.1093/aje/kwi260. [DOI] [PubMed] [Google Scholar]
- Sandhu J, Shlomo YB, Cole TJ, Holly J, Smith GD. The impact of childhood body mass index on timing of puberty, adult stature and obesity: a follow-up study based on adolescent anthropometry recorded at Christ’s Hospital (1936–1964) Int J Obes. 2006;30:14–22. doi: 10.1038/sj.ijo.0803156. [DOI] [PubMed] [Google Scholar]
- Assis MA, Calvo MC, Kupek, Vasconcelos FAG, Campos VC, Machado M, Costa FF, Andrade DF. Qualitative analysis of the diet of a probabilistic sample of schoolchildren from Florianópolis, Santa Catarina State, Brazil, using the previous day food questionnaire. Cad Saúde Publica. 2010;26:1355–65. doi: 10.1590/S0102-311X2010000700014. [DOI] [PubMed] [Google Scholar]
- Bernardo CO, Vasconcelos FAG. Association of parents’ nutritional status, and sociodemographic and dietary factors with overweight/obesity in schoolchildren 7 to 14 years old. Cad. Saúde Pública. 2012;2:291–304. doi: 10.1590/s0102-311x2012000200008. [DOI] [PubMed] [Google Scholar]
- Benedet J, Assis MA, Calvo MC, Andrade DF. Overweight in adolescents: exploring potential risk factors. Rev Paul Pediatr. 2013;31:172–81. doi: 10.1590/S0103-05822013000200007. [DOI] [PubMed] [Google Scholar]
- Assis MA, Rolland-Cachera MF, Grosseman S, de Vasconcelos FA, Luna ME, Calvo MC, Barros MV, Pires MM, Bellisle F. Obesity, overweight and thinness in schoolchildren of the city of Florianopolis, Southern Brazil. Eur J Clin Nutr. 2005;59:1015–1021. doi: 10.1038/sj.ejcn.1602206. [DOI] [PubMed] [Google Scholar]
- Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescence in the United Sates, Brazil, China, and Russia. Am J Clin Nutr. 2002;75:971–977. doi: 10.1093/ajcn/75.6.971. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Physical status: the use and interpretation of anthropometry. Geneva: WHO; 1995. [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Illinois: Human Kinetics Books; 1988. [Google Scholar]
- Frainer DE, Adami F, Vasconcelos FAG, Assis MAA, Calvo MC, Kerpel R. Standardization and reliability of anthropometric measurements for population surveys. Arch Latinoam Nutr. 2007;57:335–42. [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Br Med J. 2000;320:1–6. doi: 10.1136/bmj.320.7226.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malina RM, Bouchard C. Growth, Maturation, And Physical Activity. Champaign, Illinois: Human Kinetics Books; 1991. [Google Scholar]
- Adami F, Vasconcelos FAG. Obesidade e maturação sexual precoce em escolares de Florianópolis - SC. Rev Bras Epidemiol. 2008;11:549–60. doi: 10.1590/S1415-790X2008000400004. [DOI] [Google Scholar]
- World Health Organization. Obesity: Preventing And Managing The Global Epidemic. Report Of A Who Consultation On Obesity. Geneva: WHO; 1998. [PubMed] [Google Scholar]
- Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodo. 2003;20:3–21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbaum D, Kupper L, Nizam A, Muller KE. Applied Regression Analysis and Other Multivariable Methods. Australia: Thomson Brooks/Cole; 2008. [Google Scholar]
- Biro FM, Mcmahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, Morrison JA, Barton BA, Falkner F. Impact of timing of pubertal maturation on growth in black and white female adolescents: the national heart, lung, and blood institute growth and health study. J Pediatr. 2001;138:636–43. doi: 10.1067/mpd.2001.114476. [DOI] [PubMed] [Google Scholar]
- Bratberg GH, Nilsen TIL, Holmen TL, Vatten LJ. Early sexual maturation, central adiposity and subsequent overweight in late adolescence. A four-year follow-up of 1605 adolescent Norwegian boys and girls: the Young HUNT study. BMC Public Health. 2007;7:54. doi: 10.1186/1471-2458-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of menarcheal age to obesity in childhood and adulthood: the Bogalusa heart study. BMC Pediatr. 2003;3:1–9. doi: 10.1186/1471-2431-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Naumova EN, Phillips SM, Blum M, Dawson-Hughes B, Rand WM. Childhood overweight and maturational timing in the development of adult overweight and fatness: the Newton girls study and its follow-up. Pediatrics. 2005;116:620–7. doi: 10.1542/peds.2004-1604. [DOI] [PubMed] [Google Scholar]
- Pierce MB, Leon DA. Age at menarche and adult BMI in the Aberdeen Children of the 1950s cohort study. Am J Clin Nutr. 2005;81:733–9. doi: 10.1093/ajcn/82.4.733. [DOI] [PubMed] [Google Scholar]
- Demerath EW, Li J, Sun SS, Chumlea WC, Remsberg KE, Czerwinski SA, Towne B, Siervogel RM. Fifty-year trends in serial body mass index during adolescence in girls: the Fels Longitudinal Study. Am J Clin Nutr. 2004;80:441–6. doi: 10.1093/ajcn/80.2.441. [DOI] [PubMed] [Google Scholar]
- Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002;32:192–200. doi: 10.1016/s1054-139x(02)00485-8. [DOI] [PubMed] [Google Scholar]
- Van Lenthe FJ, Kemper HCG, Mechelen WV. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam growth and health study. Am J Clin Nutr. 1996;64:18–24. doi: 10.1093/ajcn/64.1.18. [DOI] [PubMed] [Google Scholar]
- Kindblom JM, Lorentzon M, Norjavaara E, Lonn L, Brandberg J, Angelhed JE, Hellqvist A, Nilsson S, Ohlsson C. Pubertal timing is an independent predictor of central adiposity in young adult males: the gothenburg osteoporosis and obesity determinants study. Diabetes. 2006;55:3047–52. doi: 10.2337/db06-0192. [DOI] [PubMed] [Google Scholar]
- He Q, Karlberg J. BMI in childhood and its association with height gain, timing of puberty, and final height. Pediatr Res. 2001;49:244–51. doi: 10.1203/00006450-200102000-00019. [DOI] [PubMed] [Google Scholar]
- Karpati AM, Rubin CH, Kieszak SM, Marcus M, Troiano RP. Stature and pubertal stage assessment in American boys: the 1988–1994 third national health and nutrition examination survey. J Adolesc Health. 1988–1994;2002(30):205–212. doi: 10.1016/s1054-139x(01)00320-2. [DOI] [PubMed] [Google Scholar]
- Thankamony A, Ong KK, Ahmed ML, Ness AR, Holly JM, Dunger DB. Higher levels of IGF-I and adrenal androgens at Age 8 years Are associated with earlier Age at menarche in girls. J Clin Endocrinol Metab. 2012;97:786–790. doi: 10.1210/jc.2011-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styne DM. The regulation of pubertal growth. Horm Res. 2003;60(S1):22–6. doi: 10.1159/000071222. [DOI] [PubMed] [Google Scholar]
- Marcovecchio ML, Chiarelli F. Obesity and growth during childhood and puberty. World Rev Nutr Diet. 2013;106:135–41. doi: 10.1159/000342545. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY. Endocrine control of body composition in infancy, childhood and puberty. Endocr Rev. 2005;26:114–46. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- Matsudo SM, Matsudo VK. Validade da auto- -avaliação da maturação sexual. Rev Bras Ciênc Mov. 1991;5:18–35. [Google Scholar]
- Chan NP, Sung RY, Kong AP, Goggins WB, So HK, Nelson EA. Reliability of pubertal self-assessment in Hong Kong Chinese children. J Paediatr Child Health. 2008;44:353–8. doi: 10.1111/j.1440-1754.2008.01311.x. [DOI] [PubMed] [Google Scholar]
- Slora EJ, Bocian AB, Herman-Giddens ME, Harris DL, Pedlow SE, Dowshen SA, Wasserman RC. Assessing inter-rater reliability (IRR) of Tanner staging and orchidometer use with boys: a study from PROS. J Pediatr Endocrinol Metab. 2009;22:291–9. doi: 10.1515/jpem.2009.22.4.291. [DOI] [PubMed] [Google Scholar]
- De onis M. Update on the implementation of the WHO child growth standards. World Rev Nutr Diet. 2013;106:75–82. doi: 10.1159/000342550. [DOI] [PubMed] [Google Scholar]