Abstract
Aldehyde dehydrogenase (ALDH) can be used as a marker to isolate, propagate, and track normal and cancerous human colon stem cells. To determine their tumorigenic potential, tissues obtained from proximal (normal counterpart) and distal (cancerous) colon of colon cancer patients are implanted into NODSCID mice. In parallel, ALDHhigh and ALDHlow cells are isolated via Florescence Associated Cell Sorting (FACS) after the dissociation of distal and proximal colon tissues into a single-cell suspension. Flow cytometry for ALDHhigh and ALDHlow cells is possible with the ALDEFLUOR assay. Following cell sorting, ALDH-enriched cells are tested for their tumorigenic potential in vivo as xenografts. Owing to cancer stem cell properties, ALDHhigh cells could be propagated in vivo by serial passaging of the human tissue as xenografts and in vitro as suspension cultures called sphere cultures. In this unit, all the above-mentioned methods to isolate and propagate colon cancer stem cells using ALDH as a stem cell marker are described in detail.
Keywords: Colon cancer, Aldehyde dehydrogenase, Fluorescent activated cell sorting, Xenograft, Sphere cultures
1. Introduction
In the last decade ALDH1 has emerged as a potential universal marker for stem and progenitor cells in epithelial cancers. Our laboratory has shown that ALDH1 is a marker for both normal and cancerous colon stem cells (1). Immunostaining data by Huang et al. (1) show that rare epithelial cells at the base of the normal crypt express ALDH and that the ALDH expression expands in the epithelia of the cancerous colon. In cancer ALDH-expressing cells are no longer limited to the base of the crypt as the crypt like structure is usually disrupted, but can be found throughout the epithelium. Tumors were generated by injecting as few as twenty-five primary colon cancer cells expressing high levels of ALDH into NOD SCID mice. FACS analysis of subsequent serial passages showed that the tumors were enriched with ALDH positive cells, which was associated with increased tumorigenicity and ability to propagate as spheres in culture. Thus ALDH may be used as a marker to identify and isolate both normal and tumorigenic colon stem cells. Further, we have demonstrated that ALDH1 serves as the marker to identify and isolate the tumor initiating cells in colitis (2). Tumor-initiating cells isolated from cancer and colitis can be propagated and expanded in vitro in suspension culture, called sphere culture, an essential in vitro tool needed for studying the mechanisms involved during the tumor initiation and metastasis of stem and progenitor cells. In this chapter, we share our protocols used (1) to isolate and implant, (2) to explant and dissociate xenografts and primary tissues, (3) to isolate ALDH+ cells by FACS, and (4) to generate and propagate sphere cultures.
2. Materials
2.1. Subcutaneous Xenograft Implantation
2.2. Xenograft Explantation and Dissociation
Collagenase type IV (Worthington Biochemical Corporation, Lakewood, NJ) (see Note 3).
M199 Medium.
2.3. Dissociation of Primary Tissues
Antibiotic–Antimycotic.
Disposable 10 mL serological pipettes (VWR).
Collagenase, from Clostridium (Sigma-Aldrich) (see Note 4).
Hank’s balanced salt solution (HBSS).
Fetal bovine serum (FBS).
40 μm and 70 μm sterile cell strainers.
2.4. Sorting for Aldehyde Dehydrogenase Expressing Cells (See Note 5)
5 mL polystyrene 12 × 75 mm culture tubes with caps.
2% fetal bovine serum in HBSS.
ALDEFLUOR kit (Stem Cell Technologies, Durham, NC).
PE Mouse IgG (BD Biosciences).
1 mg/mL DAPI (4,6-diamidino-2-phenylindole) (Sigma Aldrich) reconstituted according to the manufacturer’s instructions.
2.5. Plating and Culturing Sorted Cells as Colonospheres
24-well low-attachment culture plates (Corning).
Defined medium (see Note 6), composition: DMEM F-12 50/50 Mix with L-glutamine and 15 mM HEPES, Glucose (6 mg/mL), Sodium Bicabonate (1 mg/mL), Glutamine (Glutamax) (2 mM), Albumin, from bovine serum (BSA) (4 mg/mL), Insulin Transferrin Selenium (ITS) (25 mg/mL), Progesterone (20 nM), Putrescine (9.6 μg/mL), Antimicrobial (see Note 7).
2.6. Immunohistochemistry of ALDH on Colon Spheres
4% Paraformaldehyde at pH 7.4.
1% agarose in PBS.
70% Ethanol.
Dako Target Retrieval Solution (Dako).
ALDH1 Antibody (BD Biosciences).
Secondary fluorescent antibody.
Mounting medium containing DAPI.
3. Methods
3.1. Subcutaneous Xenograft Implantation
Tissue that is to be implanted should be kept in sterile PBS or M199 medium on ice while the mouse is prepared for surgery.
Anesthesize the mouse according to your animal protocol. Place the mouse in the laminar flow hood and sterilize the flank (alcohol or chlorohexadine can be used) (see Note 8).
Implant the tissue into the flank of the mouse as follows. Use toothed forceps to gently pull skin over flank into a “teepee” shape. While holding the skin with the forceps cut a small incision (2–4 mm). Use the tip of the closed scissors to undermine a flap between the skin and the underlying muscle of the mouse. While still holding the toothed forceps with the skin away from the mouse body, use serrated micro-dissecting forceps to pick up the tissue implant and place it in the pocket. Be certain the tissue is firmly in place in the pocket! If the tissue is too near the incision it may explant slip out during suturing.
To close the incision load the sterile suture into the needle holder. A continuous running suture or several interrupted sutures are recommended. Use the toothed or serrated forceps to grip the top of the incision and pull it taut away from the mouse body. Begin sewing at the top of the incision. Pull the suture through until only about 2–3 cm of the suture end remains. Make 3–4 square knots to secure the suture in place. The skin should be occluded with no gaps. Alternatively, stapling or surgical adhesives, such as Dermabond, may be used.
3.2. Xenograft Explantation and Dissociation
3.2.1. Recovering the Xenograft and Sacrificing the Mouse
Anesthetize the mouse according to your animal protocol. Ensure the mouse is fully anesthetized per your protocol before beginning surgery. Place the mouse inside the laminar flow hood. Cleanse the tumor-containing flank(s) and abdomen with an antiseptic solution. Recover the xenograft in the following manner.
Use forceps to hold the skin of the mouse and create a 2–3 cm incision down the midline of the mouse taking care not to puncture the wall of the abdomen. Working in the direction of the xenograft, carefully separate the skin from the underlying muscle until the tumor is revealed.
Once the xenograft tumor has been reached, separate the tumor from the underlying muscle.
Then separate the tumor from the skin of the mouse. A small piece of the tumor can be saved in 4% PFA for analysis including immunohistochemistry (see IHC section below) or routine hemotoxylin and eosin (H&E) staining.
If the tumor is to be serially passaged, cut a piece of the tumor that is approximately 3 × 3 mm and place it in PBS or M199 on ice until ready for implantation. The remainder of the xenograft should be placed in a 50 mL conical tube containing 5 mL M199.
Refer to your animal protocol for proper euthanasia procedures once the tumor has been recovered (see Note 9).
3.2.2. Mincing and Treatment with Collagenase Type IV
Use mincing scissors to mince the xenograft tissue from step 3 from Subheading 3.2.1 (see Note 10). Tissue should be minced for 15–20 min.
Once the tissue is adequately minced, add M199 medium until the total volume is 15 mL.
Add 1 mL (2,000 U/mL) Collagenase type IV (Worthington) to the 50 mL conical tube containing the minced tumor.
Incubate the tube(s) horizontally in a 37°C shaking water bath for 45 min.
After incubation, washes should be carried out under sterile conditions.
Add 20–30 mL HBSS to each conical tube.
Centrifuge at 250 × g for 5 min. Pipette or decant off the supernatant (see Note 11).
Resuspend the digested tissue with 15 mL of 20% FBS in HBSS to each conical tube.
Centrifuge and pipette or decant off supernatant as above.
Resuspend in 15 mL 2% FBS in HBSS.
Centrifuge, pipette or decant, and resuspend in 10 mL 2% FBS in HBSS again.
Place tubes on ice.
Filter one sample at a time while keeping the others on ice.
3.2.3. Filtering the Tissue
Two 50 mL conicals, at least one each 70 μm and 40 μm filters, and one 10 mL syringe should be used per sample.
Place the 70 μm filter in one 50 mL conical and pipette the digested tissue from Subheading 3.2.2. step 3, into the filter.
Use the plunger from a 10 mL syringe to grind the tissue against the filter (see Note 12).
Discard the filter and the syringe in an appropriate biohazard waste container.
Place the 40 μm filter in the second 50 mL conical tube and pipette the 70 μm filtrate through the 40 μm filter.
Discard the filter and recap the tube.
After filtering, centrifuge the flow through at 250 × g for 5 min. Resuspend the pellet in a known volume of 2% FBS in HBSS and count the live cells obtained (see Note 13).
Once cells are counted they can be frozen for future FACS analysis, sorted the same day or stored overnight in an ice bath at 4°C and sorted the next day.
To freeze the cells, centrifuge the cells at 250 × g for 5 min. Add 1 mL of freezing medium (90% FBS with 10% DMSO), resuspend the pellet, and place in a cryovial for routine freezing, if sorting the same day, aliquot one to two million cells into a fresh conical tube and bring the volume of 2% FBS/HBSS up to 2–3 mL. Follow the procedure for sorting in Subheading 3.4. Any remaining cells can be frozen for later use. If sorting the next day, bring the volume of 2% FBS/HBSS up to 3–5 mL. Store cells on ice at 4°C. Follow sorting procedure in Subheading 3.4 (see Note 14).
3.3. Dissociation of Primary Tissue
Transport primary tissues in 50 mL conical tubes of M199 medium.
Before dissociation wash all primary samples in a 50 mL conical tube. Rinse the samples with sterile PBS and discard the supernatant. (During the washing steps, sections of tissue can be taken for IHC, frozen sectioning, protein isolation, RNA isolation, etc. For IHC procedure, see Subheading 3.6).
Incubate the samples in enough antimicrobial solution to cover the sample at room temperature three times for 15 min each time, discarding the supernatant after each wash.
Incubate the samples in 0.014% bleach solution for 5 min and discard the supernatant.
Rinse the samples in PBS, discarding the supernatant, and place the samples in 5 mL of M199 medium. A portion of the tissue can be dissected and set aside for xenograft implantation (see Subheading 3.1).
Mince tissue according to step 1 in Subheading 3.2.2. Use a 10 mL serological pipette to pipette the tissue up and down (see Note 14).
Bring volume of M199 medium to 15 mL.
Add 1 mL collagenase type IV (1 mg/mL) and shake tubes containing tissue at 37°C for 45 min. Every 15 min pipette the tissue up and down using a serological pipette (see Note 15).
After the incubation follow wash and filtering steps 6–13 from Subheading 3.2.2 and Subheading 3.2.3 respectively.
3.4. Sorting for Aldehyde Dehydrogenase Expressing Cells (See Note 16)
Thaw cells for sorting at 37°C. Immediately after cells thaw transfer them to a 15 mL conical tube and add 2–3 mL sterile PBS.
Centrifuge at 250 × g for 5 min and wash two more times with 2% FBS in HBSS.
Resuspend cells in 2–3 mL of 2% FBS in HBSS and count the cells. If staining cells from culture or from a fresh harvest, proceed to step 4.
Blocking: Cells should be blocked with human immunoglobulins for 20 min on ice. After incubation wash 2× with 2% FBS/HBSS and resuspend the cells in the same volume of 2% FBS/HBSS.
Controls: We recommend DAPI, autofluorescence, a mouse control, a human control, and mouse IgG as controls. We recommend using 20,000 viable cells per control. The tubes are labeled as shown in Table 1.
Staining Procedure: ALDH staining is carried out using the ALDEFLUOR Assay according to the manufacturer’s instructions. For separation of mouse and human cells during FACS, we suggest Epithelial Surface Antigen (Closely related to “Epcam”; Miltenyi Biotech) at a concentration of 2.5:100 (antibody purchased as liquid) and H2K (Southern Biotech) at a concentration of 0.5 μg/mL (for FACS settings (see Note 18)).
Flow cytometer and FACS settings: We use the FACS Aria Cell Sorter (BD Biosciences, San Jose, CA) and the FACS Diva Version 6.1.2 software. DAPI is used to discriminate live from dead cells. Fluorescence is excited at 355 nm and emission collected at 450 nm ±25 nm. A gate on the live cells is drawn on a Forward Light Scatter versus DAPI fluorescence plot, based on unstained cells (see Note 17, Fig. 1). Markers for sorting ALDEFLUOR+ cells are set based on the DEAB control sample, where approximately 99% of the sample is found to be negative. These markers are applied to the sample (tube G, Table 1). Sorting is carried out using a 100 μm nozzle and maximum flow rate is limited to 5,000 cells/s. All tubes (sample and sort collection) are maintained at 4°C during the sort.
After sorting, cells should be washed two times with sterile PBS to remove all serum from the cells. With each wash the cells should be centrifuged at 250 × g for 5 min. Cells can then be cultured or injected according to the instructions in Subheading 3.5.
Table 1.
Labelling scheme for tubes when sorting for Aldehyde Dehydrogenase expressing cells
| Label | Tubes |
|---|---|
| A | DAPI only |
| B | Autofluorescence (no treatment, except washes) |
| C | Mouse control |
| D | Human control |
| E | DEAB (negative control for ALDEFLUOR assay) |
| F | ALDEFLUOR only |
| G | Sample |
| H | IgG |
Fig. 1.
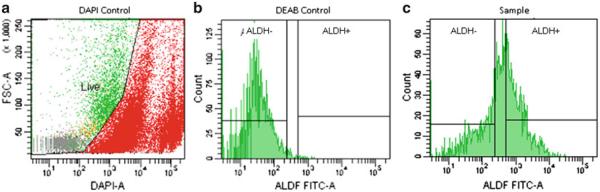
FACS ALDEFLUOR histogram depicting (a) suggested DAPI control separating live and dead populations of cells, (b) DEAB (negative) control utilized in the ALDEFLUOR assay, and (c) example analysis of a sample for ALDH+ and ALDH− fractions.
3.5. Sphere Culture and Propagation
Culturing colonospheres (see Note 19): After sorting and washing (Subheading 3.4), 25,000 to 50,000 ESA+/ALDH+ cells are plated in 24-well low attachment plate with 1 mL of defined medium (DM) (see Subheading 2.5 for the constituents) supplemented with Epidermal growth factor (EGF) (20 ng/mL) and Fibroblast growth factor-2 (FGF2) (10 ng/mL).
The cells are maintained in a 37°C incubator with 5% CO and supplemented with the EGF (20 ng/mL) and FGF2 (10 ng/mL) every other day.
In 1–2 weeks, spherical clusters of cells are formed in suspension (Fig. 2). These spheres are manually plucked under the microscope in the hood using a 200 μl pipette and are transferred to a fresh well of a 24-well plate containing 1 mL of DM supplemented with EFG and FGF-2. If the number of spheres is less than 5, then the plucking is continued until there are a sufficient number of spheres that can be trypsinized (see Note 20) and passaged. At each passage, excess cells obtained from dissociated spheres can be routinely frozen using freezing medium (90% FBS + 10% DMSO), then transferred to liquid nitrogen for long-term storage.
Injection of tumor cells into the flank of NSG mice: After sorting and washing (Subheading 3.4), cells should be resuspended in 50 μl of sterile PBS. 50 μl of matrigel is added and pipetted up and down to thoroughly mix the cells, PBS, and matrigel. The mixture is pulled into a syringe and can be injected into the flank of the mouse. These injections can be used to show tumorigenicity.
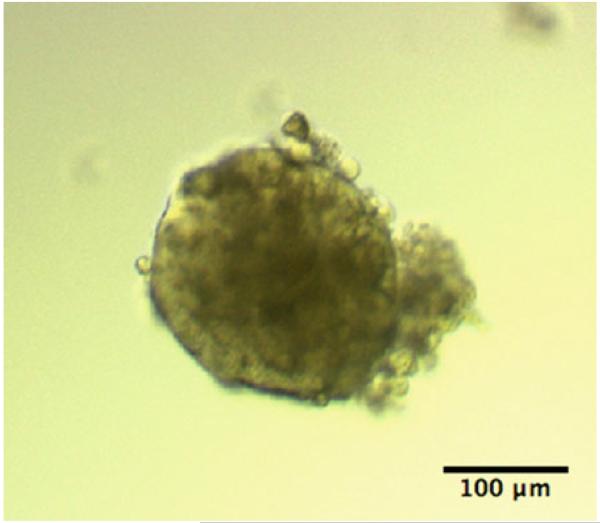
Fig. 2.
Colonosphere. The sphere obtained from ALDH-enriched colon cancer cells in defined medium.
3.6. IHC for ALDH on Spheres and Primary Tissues
3.6.1. Fixation of Colonospheres
Pipette spheres into a conical tube.
Rinse culture plate with 1× PBS and add the rinse to the conical tube containing the spheres.
Centrifuge spheres at 250 × g for 5 min. Discard the supernatant and add 2–5 mL of 4% PFA to the conical tube.
Incubate on ice for 10 min.
Centrifuge spheres at 250 × g for 5 min.
Remove all but a small amount of the PFA (leave approximately 50 μl). Discard the waste PFA according to your institution’s policies.
Pipette remaining PFA and spheres into a round-bottom glass tube and place on ice.
Heat 1% agarose until completely fluid. Allow the agarose to cool so that it is not very hot, but still remains liquid.
Use a 1 mL syringe (without a needle) to pull approximately 1 cc of 1% agarose into the syringe. Quickly dispense agarose into tube containing the spheres and pull the agarose/sphere mixture back into the syringe. Immediately place the syringe on ice and do not disturb it until the agarose has completely solidified (approximately 10–15 min).
Cut the tip of the syringe and dispense the plug gently into a 15 mL conical tube containing 5 mL of 4% PFA. Leave overnight at 4°C.
Discard PFA and add 70% ethanol to the plug. Incubate overnight at 4°C.
Paraffin-embed the plug for sectioning.
Section the paraffin block in 5 μm sections for staining.
3.6.2. Retrieval
Preheat Coplin staining jar containing 50 mL of Dako target retrieval solution to 95°C in a water bath. The water level should reach to approximately 1 cm below the lid of the container. Place the container in a rack or beaker to keep it stable. Allow the temperature of the Coplin jar to reach 95°C before carrying out the retrieval.
Quickly place slides in Coplin staining jar, loosely close the lid, and incubate 20 min at 95°C and 20 min on the bench.
Remove slides from retrieval solution and dip them in deionized water.
Rinse the slides with 1× TBS-T and place them in 1× TBS-T for 5 min.
3.6.3. Blocking and Primary Antibody Treatment
Block slides in TBS-T containing 2% horse serum for 30 min.
Apply the primary ALDH1 antibody at 0.5–1 μg/mL. Incubate overnight at 4°C.
Wash the slides three times for 5 min at room temperature in 1× TBS-T. Then apply the secondary antibody.
3.6.4. Secondary Antibody Treatment
Be sure to keep all reagents and slides in the dark as much as possible.
Apply the secondary antibody using 1× TBS-T with 2% horse serum as a diluent for 1 h at room temperature (we use donkey anti-mouse Alexafluor 488 at a concentration of 4 μg/mL).
Wash slides three times for 3 min at room temperature in 1× TBS-T. Use gauze to wipe away excess buffer being careful not to disturb the cells on the slide. Allow slides to dry completely in the dark before mounting.
Mount slides using a small amount (one drop is usually more than sufficient for an agarose plug) of mounting medium with DAPI. Slides can then be visualized.
Acknowledgments
The authors would like to thank Jason E. Cline for preparation of this chapter and Neal Benson for his expertise in flourescence-activated cell sorting. This work was supported by NCI R01 142808, the University of Florida and Shands Cancer Center, and the University of Florida Seed Fund (EHH).
Footnotes
4. Notes
Unless noted otherwise “surgical instruments” refers to toothed forceps, serrated microdissecting forceps, curved microdissecting scissors, needle holder, and operating (mincing) scissors.
Quintana et al. (3) showed that NOD/SCID mice underestimate the tumorigenicity of cancer cell lines. They show that use of “NSG” mice (NOD/SCID interleukin-2 receptor gamma chain null mice) in place of NOD/SCID mice increases the sensitivity of xenograft assays. The use of NSG mice allows a marked improvement for the assay to be able to detect tumorigenic cells. See also reference (4).
Collagenase should be stored at 4°C until reconstituted. It should be reconstituted with sterile PBS at a concentration of 2,000 U/mL. Once reconstituted it should be stored in 1 mL aliquots at −20°C.
Collagenase should be stored at −20°C and only the necessary amount reconstituted each time it is used. It should be reconstituted at 1 mg/mL with PBS and then filtered through a 0.22 μm filter to sterilize it before use.
All antibodies reconstituted and stored according to the manufacturer’s instructions.
All liquid reagents and BSA are kept at 4°C, except progesterone, which should be stored at −20°C. All components should be added to the basal medium and mixed thoroughly before being passed through a 0.22 μm filter.
We use Antibiotic–Antimycotic solution from Gibco. Penicillin–Streptomycin or others may be used instead to prevent contamination of cell cultures.
Always refer to your institution’s policies regarding mouse use and care including surgery policies such as the type of anesthesia your institution prefers. Your institution may also require the use of a heating pad be used through the duration of the surgery to prevent hypothermia of the mouse. Sterilization of the flank is essential as NSG mice are highly immune deficient and, therefore, susceptible to infection. The flank may be shaven to facilitate sterilization and implantation of the xenograft. Antibiotic treatment post-surgery is suggested to aid in the prevention of infection.
There are multiple ways to euthanize a mouse after the recovery of a xenograft. The method we use is to administer an overdose of anesthesia followed by confirmation using cervical dislocation. Any researcher attempting this or any other euthanasia procedure should be thoroughly trained in the technique(s) used.
An automated dissociator may be used for large batches of samples, but should not be used routinely. Automatic dissociation can decrease cell viability. Also, colon samples contain mucin, can clog the automated dissociator making cell sorting much more time consuming due to the increased viscosity of the cell suspension. Primary human samples should always be hand-minced as automated dissociators will become clogged with tissue and cannot adequately mince the sample.
Pipetting off the supernatant is recommended if the pellet does not stick well to the conical tube after centrifugation. Often normal samples, or tumors with a large quantity of mucin, will not pellet completely when centrifuged. In general, there is less cell loss involved with all samples when pipetting rather than decanting the supernatant.
Filtering the tissue aids in the release of epithelial cells from the underlying tissue and should be done thoroughly. After grinding the tissue on the filter it can be rinsed with the flow-through to collect as many epithelial cells as possible.
To count the cells, dilute them 1:10 with Trypan blue and use a hemocytometer to quantify them. Only count larger, round cells. Many tiny cells will be visible, but these are red blood cells and other cells that should not be included in the enumeration.
If sorting longer than 24–36 h after dissociation, freeze cells and thaw them right before sorting according to the procedures in Subheading 3.2.3, step 9 and Subheading 3.4.
It is necessary to pipette the tissue up and down in order to ensure the tissue is properly minced and to aid the release of epithelial cells during the incubation with collagenase.
The ALDEFLUOR kit detects the enzymatic activity of the family of aldehyde dehydrogenases, not exclusively ALDH1. All incubation steps should be performed on ice unless otherwise indicated to prevent cell death during the incubations.
If using ESA and H2K the following conditions may be used: ESA fluorescence (PE) and H2K fluorescence PE-CY5 are excited with a 488 nm laser. PE emission is collected at 575 ± 12.5 nm and PE-Cy5 emission is collected at 710 ± 25 nm. A gate for the ESA+ cells was applied using a ESA versus H2K dot plot.
Sorting notes: ESA fluorescence (PE) and H2K fluorescence PE-CY5 were excited with a 488 nm laser. PE emission was collected at 575 ± 12.5 nm and PE-Cy5 emission was collected at 710 ± 25 nm. A gate for the ESA+ cells was applied using a ESA versus H2K dot plot. ESA+ cells were plotted on a histogram showing FITC fluorescence, which was excited at 488 nm and emission collected at 530 ± 15 nm.
Sphere culture is initiated only after three or four passages of the human colon cancer tissue as xenografts in NOD-SCID mice as mentioned in Subheading 3.1. Passaging in vivo eliminates progenitors, as they are unable to propagate beyond a few passages. The passaging enriches the stem cell population, which can be readily propagated as spheres.
To trypsinize spheres, first centifuge them at 250 × g for 5 min at 4°C. Pipette or decant off the supernatant and resuspend the pellet in 1 mL of 0.25% trypsin with EDTA. The spheres are incubated in the trypsin with EDTA for 3–5 min and pipetted up and down every minute or so to aid dissociation of the spheres. The trypsinization is stopped using medium or PBS containing 10% FBS. The sphere cells are washed twice with plain PBS to remove any remaining serum from the cells. After the final wash, cells are resuspended in DM containing growth factors and are transferred into low attachment plate for further propagation.
References
- 1.Huang E, Hynes M, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. ALDH1 is a marker for normal and malignant human colonic stem cells and tracks stem cell overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, Huang EH. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumor formation by single melanoma cells. Nature. 2009;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishizawa K, Rasheed ZA, Karisch R, Wang Q, Kowalski J, Susky E, Pereira K, Karamboulas C, Moghal N, Rajeshkumar NV, Hidalgo M, Tsao M, Ailles L, Waddell TK, Maitra A, Neel BG, Matsui W. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 2010;7:279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]