Abstract
Purpose
To report a case of the expansion of submacular retinal pigment epithelium (RPE) atrophy after using the inverted internal limiting membrane (ILM) flap technique for a persisting, large, stage IV macular hole (MH).
Case Report
A 79-year-old woman presented with a chronic large MH that remained open despite pars plana vitrectomy (PPV). The surgery was performed twice for the MH closure 14 years earlier. ILM peeling was not performed during the previous surgeries. The best-corrected visual acuity (BCVA) with the Landolt ring chart was 0.08 at her visit. The minimum MH diameter was 1,240 μm. Inverted ILM flap technique with 20% SF6 gas tamponade was performed for the MH closure. For the inverted ILM flap technique, 25-gauge PPV and ILM staining with indocyanine green were used. The ILM was peeled off for 2 disc diameters around the MH, but the ILM was not removed completely. The ILM was then inverted and covered the MH.
Results
One month after surgery, the MH was closed, accompanied by glial cell proliferation spreading from the inverted ILM flap (as reported before). On the other hand, the area of the submacular RPE atrophy, which was already observed 1 week after surgery, gradually increased in size. BCVA improved to 0.3 six months after the surgery.
Conclusions
The inverted ILM flap technique may be promising even for persisting large MH which were not closed in previous surgeries, but long-term observation is needed because the detailed behavior of the inverted ILM and the Müller cells after surgery is not yet known.
Key words: Macular hole, Inverted internal limiting membrane flap technique, Chronic macular hole, Indocyanine green, Retinal pigment epithelium atrophy
Introduction
Inverted internal limiting membrane (ILM) flap technique is a new method first reported by Michalewska and colleagues [1, 2]. After their report, this technique was successfully applied for macular holes (MH) with high myopia [3, 4]. Here, we report a case of the expansion of the submacular retinal pigment epithelium (RPE) atrophy and gliosis on the surface of the retina after the inverted ILM flap technique was used for chronic large MH.
Case Report
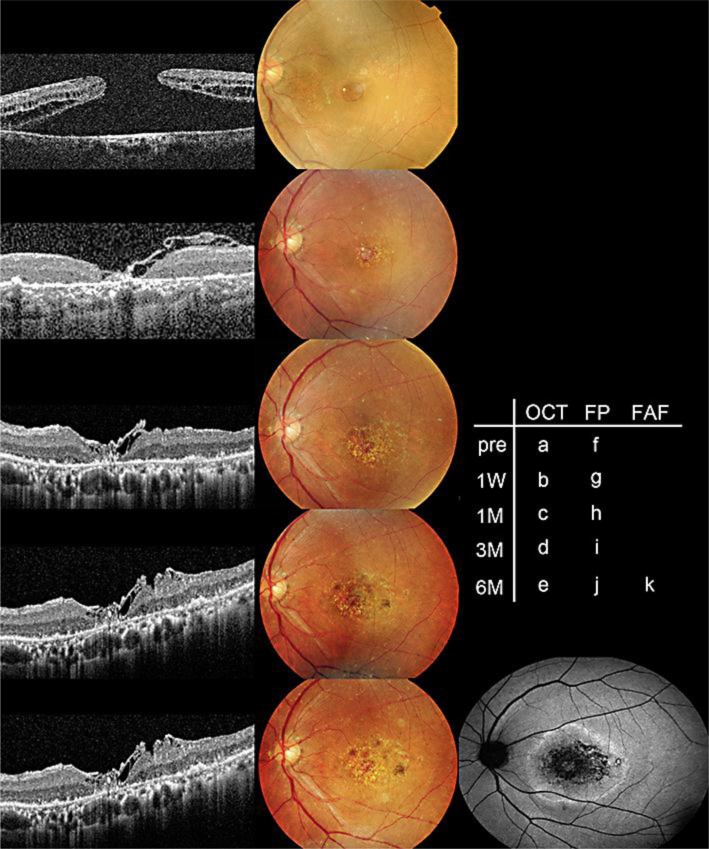
A 79-year-old woman presented with a chronic large MH which remained open despite pars plana vitrectomy (PPV). The surgery for the MH closure was performed twice 14 years earlier. ILM peeling was not done during previous surgeries. The best-corrected visual acuity (BCVA) with the Landolt ring chart was 0.08 at her visit. The minimum MH diameter was 1,240 μm. Inverted ILM flap technique with 20% SF6 gas tamponade was performed for the MH closure. For the inverted ILM flap technique, 25-gauge PPV and ILM staining with indocyanine green (ICG) (0.25%) were used. The ILM was peeled off for 2 disc diameters around the MH, but it was not completely removed. The ILM was then inverted and covered the MH. One week after surgery, the MH was closed, accompanied by glial cell proliferation, spreading from the inverted ILM flap (as reported before) (fig. 1) [1]. On the other hand, the area of the submacular RPE atrophy and gliosis on the surface of the retina, which were already observed 1 week after surgery, gradually increased in size (fig. 1). BCVA improved to 0.3 six months after surgery.
Fig. 1.
The transition of funduscopic and optical coherence tomography (OCT) findings. The large MH (a, f) was closed 1 week after the inverted ILM flap technique (b, g). The photoreceptor defect gradually decreased in diameter (b–e). On the other hand, the area of the submacular RPE atrophy and gliosis on the surface of the retina, which was observed 1 week after surgery (g), expanded gradually (h–j). The area of the RPE atrophy was depicted as a hypoautofluorescent area on the fundus autofluorescein image 6 months after the procedure (k). FP = Fundus photography; FAF = fundus autofluorescein.
Discussion
Michalewska et al. [1] reported that the inverted ILM flap technique stimulates proliferation of glial cells that fill MH. In our case, the MH was closed successfully after 1 week and the photoreceptor defect decreased in diameter after 6 months. Finally, visual acuity improved from 0.08 to 0.3. This result might support the hypothesis that proliferation of glial cells produces an environment for the photoreceptor to assume new positions in direct proximity to the fovea [1].
On the other hand, we observed the gradual expansion of RPE atrophy which was depicted as a hypoautofluorescent area on the fundus autofluorescein image (fig. 1). One possibility for this RPE atrophy might be the effect of ICG cytotoxicity. Several reports have demonstrated cytotoxicity of ICG to the RPE and neurosensory retina [5, 6, 7, 8]. In our case, we used ICG for the ILM staining. It is possible that some quantity of ICG, which was left at the bottom of the MH and enclosed by the overlying ICG-stained ILM flap, provoked the RPE damage. Another possibility might be that several inflammatory cytokines secreted from the vitreous, like tissue necrosis growth factor-α [9, 10], induced unexpected intensive Müller cell activation, followed by both RPE atrophy and gliosis.
Conclusion
In summary, the inverted ILM flap technique may be promising even for persisting large MH which were not closed in previous multiple surgeries. Long-term observation is needed because detailed behavior of the inverted ILM and Müller cells after surgery is not yet known.
References
- 1.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117:2018–2025. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Mahalingam P, Sambhav K. Surgical outcomes of inverted internal limiting membrane flap technique for large macular hole. Indian J Ophthalmol. 2013;61:601–603. doi: 10.4103/0301-4738.121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuriyama S, Hayashi H, Jingami Y, Kuramoto N, Akita J, Matsumoto M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am J Ophthalmol. 2013;156:125–131. doi: 10.1016/j.ajo.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J: Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina, 2013, Epub ahead of print. [DOI] [PubMed]
- 5.Maia M, Haller JA, Pieramici DJ, Margalit E, de Juan E, Jr, Farah ME, Lakhanpal RR, Au Eong KG, Guven D, Humayun MS. Retinal pigment epithelial abnormalities after internal limiting membrane peeling guided by indocyanine green staining. Retina. 2004;24:157–160. doi: 10.1097/00006982-200402000-00024. [DOI] [PubMed] [Google Scholar]
- 6.Smiddy WE. The current status of macular hole surgery. Bull Soc Belge Ophtalmol. 1996;262:31–42. [PubMed] [Google Scholar]
- 7.Rodrigues EB, Penha FM, de Paula Fiod Costa E, Maia M, Dib E, Moraes M, Jr, Meyer CH, Magalhaes O, Jr, Melo GB, Stefano V, Dias AB, Farah ME. The use of vital dyes in ocular surgery. Surv Ophthalmol. 2009;54:576–617. doi: 10.1016/j.survophthal.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Burk SE, Da Mata AP, Snyder ME, Rosa RH, Jr, Foster RE. Indocyanine green-assisted peeling of the retinal internal limiting membrane. Ophthalmology. 2000;107:2010–2014. doi: 10.1016/s0161-6420(00)00375-4. [DOI] [PubMed] [Google Scholar]
- 9.Caicedo A, Espinosa-Heidmann DG, Piña Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005;81:38–47. doi: 10.1016/j.exer.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Bueno I, Pastor JC, Gayoso MJ, Alcalde I, Garcia MT. Müller and macrophage-like cell interactions in an organotypic culture of porcine neuroretina. Mol Vis. 2008;14:2148–2156. [PMC free article] [PubMed] [Google Scholar]