Abstract
Background
Literature on prognosis of transient ischemic attack (TIA) in Chinese is scarce. The short-term prognosis of TIA and the predictive value of the ABCD2 score in Hong Kong Chinese patients attending the emergency department (ED) were studied to provide reference for TIA patient management in our ED.
Methods
A cohort of TIA patients admitted through the ED to 13 acute public hospitals in 2006 was recruited through the centralized electronic database by the Hong Kong Hospital Authority (HA). All inpatients were e-coded by the HA according to the International Classification of Diseases, Ninth Revision (ICD9). Electronic records and hard copies were studied up to 90 days after a TIA. The stroke risk of a separate TIA cohort diagnosed by the ED was compared.
Results
In the 1,000 recruited patients, the stroke risk after a TIA at days 2, 7, 30, and 90 was 0.2, 1.4, 2.9, and 4.4%, respectively. Antiplatelet agents were prescribed in 89%, warfarin in 6.9%, statin in 28.6%, antihypertensives in 39.3%, and antidiabetics in 11.9% of patients after hospitalization. Before the index TIA, the prescribed medications were 27.6, 3.7, 11.3, 27.1, and 9.7%, respectively. The accuracy of the ABCD2 score in predicting stroke risk was 0.607 at 7 days, 0.607 at 30 days, and 0.574 at 90 days. At 30 days, the p for trend across ABCD2 score levels was 0.038 (OR for every score point = 1.36, p = 0.040). Diabetes mellitus, previous stroke and carotid bruit were associated with stroke within 90 days (p = 0.038, 0.045, 0.030, respectively). A total of 45.4% of CTs of the brain showed lacunar infarcts or small vessel disease. There was an increased stroke risk at 90 days in patients with old or new infarcts on CT or MRI. Patients with carotid stenosis ≥70% had an increased stroke risk within 30 (OR = 6.335, p = 0.013) and 90 days (OR = 3.623, p = 0.050). Stroke risks at days 2, 7, 30, and 90 in the 289 TIA patients diagnosed by the ED were 0.35, 2.4, 5.2, and 6.2%, respectively.
Conclusion
The short-term stroke risk in Hong Kong Chinese TIA patients is low. The administered nonurgent treatment cannot solely explain the favorable outcome, the lower risk can be due to the different pathophysiological mechanisms of stroke between Caucasians and Chinese. The predictive value of the ABCD2 score is low in our population.
Key Words: Chinese ethnicity, Transient ischemic attack, Prognosis, Stroke, Carotid stenosis, Lacunar infarct
Introduction
In a systematic review of 18 recruited cohorts, the stroke risk was reported to be 3.1 and 5.2% on days 2 and 7, respectively, after a transient ischemic attack (TIA) [1]. Studies on stroke risk after TIA were predominantly conducted in Western populations, data in Chinese or Asians are limited. Existing data suggest that there are differences in nonmodifiable risk factors and pathophysiological mechanisms of cerebrovascular diseases between Chinese/Asians and Caucasians [2,3,4,5,6,7,8,9,10]. High levels of the apolipoprotein B/AI ratio are associated with intracranial atherosclerotic stenosis [9], and cerebral microbleeds may increase the risk for subsequent ischemic stroke after TIA within 3 months [11]. A systemic review and meta-analysis of five Western versus 4 Chinese and 1 Japanese cohort found a significant association of cerebral microbleeds with recurrent ischemic stroke in Western but not in the Chinese and Japanese cohorts [10]. Primarily, extracranial stenosis is more common in Caucasians, while intracranial stenosis predominates in Asians [2,3,12,13]. A total of 95% of the population in Hong Kong is Chinese. The objectives of this study were to explore the short-term stroke risk after a TIA and the applicability of the ABCD2 score [14] in a local Chinese population.
Methods
Setting
This study was conducted in 13 acute public hospitals managed by the Hong Kong Hospital Authority (HA). The HA is a statutory organization subsidized by the government managing all public hospitals in Hong Kong [15]. It comprises 95% of the market share of hospitalized patients, and provides universal access to primary, secondary and tertiary health care for the 6.9 million population [16]. In 2006, there were 1.9 million emergency department (ED) visits. The compulsory International Classification of Diseases, Ninth Revision (ICD9) e-coding was applied to all inpatients. The study was approved by the Ethics Committees of the 13 hospitals.
Study Design and Data Collection
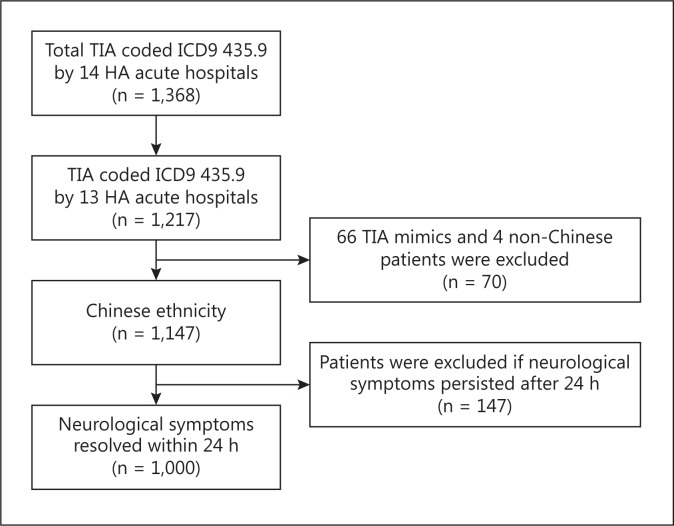
A cohort of cerebral TIA patients, with the code ICD9 435.9, admitted to 13 acute hospitals through the ED in 2006, before the publication of the ABCD2 score, was recruited through the centralized electronic database of the HA Clinical Data Analysis and Reporting System (fig. 1). The ED, inpatient and follow-up records were traced up to 90 days from the occurrence of the index TIA (i.e. the patient had the first TIA in 2006). The day of stroke was counted from the day of ED registration to the day when the stroke occurred. TIA and stroke were defined according to the WHO criteria [17].
Fig. 1.
Flow chart showing the patients' recruitment. In 2006, all 14 acute public hospitals were managed by the HA according to the ICD9.
Data on patient demographics, symptomatology, investigations, medications, and clinical outcome were collected and the ABCD2 score was calculated. Two independent reviews by at least two physician coinvestigators were conducted together to ensure data accuracy. Our neurologist coauthor (W.C.F.) ascertained the diagnosis of TIA, he would be consulted when the opinions of the coinvestigators differed. A carotid bruit was coded absent unless it was specifically noted in the record. Atrial fibrillation was considered new if present in the index TIA, but absent in the past records.
We considered that some TIA patients could have a stroke during hospitalization and that these patients might not be recorded if coded stroke instead of TIA upon discharge. Therefore, we compared the stroke risk of a separate TIA cohort, with compulsory ICD9 e-coding, diagnosed by and recruited in six ED with this cohort. The TIA cohort diagnosed by the ED would give an estimate on possible missed recording of early stroke occurrence being coded stroke instead of TIA in the hospital.
Data Analysis
The association of demographic characteristics, stroke risk factors, and ABCD2 score with the risk of subsequent stroke was evaluated using the χ2 test or Fisher Exact test wherever appropriate. Cox regression analysis was used to identify factors that increased the risk of subsequent stroke after a TIA. Variables set at p < 0.1 in the initial univariate analyses and associated with stroke risk were included in the Cox regression model. The ABCD2 score was forced into the model because it was our study objective. In the final multivariate analyses, statistical significance was achieved if p < 0.05. The log rank test was used to assess the difference in stroke-free survival between groups stratified by the ABCD2 score. The test for trend was used to determine the linear relationship between stroke risk and value of the ABCD2 score. Sensitivity and specificity of stroke risk were determined at each cutoff of the ABCD2 score. In addition, the area under the receiver operating characteristic (ROC) curves was calculated. All analyses were conducted using the Statistical Package for Social Sciences, version 15.0 (SPSS Inc., Chicago, Ill., USA).
Results
A total of 1,000 patients were analyzed and 4 non-Chinese and 213 patients not meeting the TIA definition by the WHO [17] were excluded (fig. 1). Forty-five percent of these patients was treated in the acute stroke units [18], the rest was treated in medical wards and received inpatient neurology consultation. Symptom resolution upon or before arrival at the ED occurred in 483 patients (48.3%), 517 had symptom resolution in the hospital within 24 h from onset. Five patients arrived at the ED >72 h after symptom onset. In 91.1% of the 483 patients who arrived within 24 h of symptom onset, symptoms resolved before attending the ED.
Table 1 summarizes the baseline characteristics. The mean age was 67.8 years and there was a slight male predominance. The mean length of hospital stay was 3.1 days (median 2.5, standard deviation 4.3) and the most common comorbidity was hypertension. The top three presenting symptoms were focal weakness (64.9%), speech impairment (35.0%), and sensory disturbance (28.3%). In univariate analysis, history of diabetes, prior stroke and presence of carotid bruit or stenosis were significantly associated with developing stroke by 90 days (table 1). The presence of old or new infarcts on CT or MRI was associated with increased stroke risk at 90 days, but not at 7 and 30 days (table 2). In 446 patients, carotid Doppler was performed until 4 months after discharge (table 3): 58.7% of them had normal findings and 6.1% had stenosis ≥70%, and were found to have an increased 30- and 90-day stroke risk (table 4). Diabetes, previous stroke, and abnormal Doppler were selected for the final multivariate model. However, including the ABCD2 score, none of them were associated with stroke risk.
Table 1.
Characteristics of 1,000 patients associated with a 90-day stroke risk
| Variable | n | % | 90-day stroke risk with characteristics, % |
P value | |
|---|---|---|---|---|---|
| present | absent | ||||
| Characteristics | |||||
| Age ≥60 | 721 | 72.1 | 4.4 | 4.3 | 0.924b |
| Mean ± SD | 67.8 ± 12.9 | ||||
| Range | 22, 96 | ||||
| Female | 432 | 43.2 | 4.4 | 4.4 | |
| Mean systolic blood pressure ± SD, mm Hg | 159.5 ± 30.0 | ||||
| Systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg | 741 | 74.1 | |||
| Medical history | |||||
| Diabetes | 273 | 27.3 | 6.6 | 3.6 | 0.038b |
| Hypertension | 583 | 58.3 | 4.3 | 4.6 | 0.838b |
| Hyperlipidemia | 176 | 17.6 | 6.3 | 4.0 | 0.187b |
| Ischemic heart disease | 106 | 10.6 | 4.7 | 4.4 | 0.803a |
| Carotid bruit and/or stenosis | 30 | 3.0 | 13.3 | 4.1 | 0.039a |
| Previous TIA | 89 | 8.9 | 5.6 | 4.3 | 0.584a |
| Previous stroke | 200 | 20.0 | 7.0 | 3.8 | 0.045b |
| Ever smoker | 379 | 37.9 | 4.5 | 4.3 | 0.918b |
| Symptoms analysis | |||||
| Symptoms on ED arrival | 517 | 51.7 | 4.3 | 4.6 | 0.817b |
| Focal weakness | 649 | 64.9 | 5.1 | 3.1 | 0.151b |
| Sensory disturbance | 283 | 28.3 | 3.2 | 4.9 | 0.237b |
| Speech impairment | 350 | 35.0 | 4.9 | 4.2 | 0.605b |
| Visual disturbance | 83 | 8.3 | 2.4 | 4.6 | 0.573a |
| Dizziness | 191 | 19.1 | 4.7 | 4.3 | 0.815b |
| Vertigo | 21 | 2.1 | 4.8 | 4.4 | 0.615a |
| Loss of consciousness | 41 | 4.1 | 0 | 4.6 | 0.253a |
| Mouth deviation/facial asymmetry | 84 | 8.4 | 4.8 | 4.4 | 0.782a |
| History of atrial fibrillation | 122 | 12.2 | 4.1 | 4.4 | 0.862b |
| New atrial fibrillation | 22 | 2.2 | 0 | 4.5 | 0.619a |
| Carotid bruit present on examination | 41 | 4.1 | 12.2 | 4.1 | 0.030a |
Fisher's exact test
χ2 test.
Table 2.
Infarct and stroke risk
| Stroke | No stroke | |
|---|---|---|
| CT and MRI, and occurrence of stroke (at 90 days)1 | ||
| Old or new infarct either on CT or MRI | 22 | 330 |
| No infarct detected on both CT and MRI | 21 | 617 |
| Total | 43 | 947 |
| CT and MRI, and occurrence of stroke (at 90 days)2 | ||
| Old infarct either on CT or MRI | 13 | 141 |
| No infarct detected on both CT and MRI | 21 | 617 |
| Total | 34 | 758 |
| 1 Fisher's exact test, p = 0.034, OR = 1.96 (1.06–3.62). | ||
| 2 χ2 test, p = 0.005, OR = 2.71 (1.33–5.54). | ||
Table 3.
Investigation results
| Variable | n (%) |
|---|---|
| Echocardiogram | 113 |
| No thrombus | 112 |
| Vegetation | 1 |
| CT of the brain/within 24 h after arrival | 994/974 |
| Normal | 546 |
| New/old lacunar infarct | 181/144 |
| Hypodensity and decreased attenuation | 114 |
| Small vessel disease | 58 |
| Periventricular white matter disease | 47 |
| Cerebral atrophy, encephalomalacia, aging changes | 10 |
| Chronic subdural hematoma/effusion | 1/1 |
| Other findings | 14 |
| MRI of the brain | 124 |
| New/old lacunar infarct | 22/11 |
| Small vessel disease | 14 |
| MRA | 77 |
| Transcranial Doppler | 44 |
| Cerebral angiography | 3 |
| Carotid Doppler | 446 |
| Normal | 262 (58.7%) |
| Stenosis | |
| <50% | 128 (28.7%) |
| 50–69% | 29 (6.5%) |
| ≥70% | 27 (6.1%) |
In patients with MRA, transcranial Doppler and cerebral angiography were performed. Fifty patients were found to have mild-to-severe, single or multiple sites of intracranial stenosis.
Table 4.
Stroke risk of carotid stenosis ≥70% compared with normal Doppler
| Stroke | No stroke | OR (95% CI) | p valuea | |
|---|---|---|---|---|
| Within 7 days | ||||
| Doppler stenosis ≥70% | 2 | 25 | 6.91 (1.10–43.30) | 0.071 |
| Normal Doppler | 3 | 259 | ||
| Within 30 days | ||||
| Doppler stenosis ≥70% | 4 | 23 | 6.34 (1.73–23.26) | 0.013 |
| Normal Doppler | 7 | 255 | ||
| Within 90 days | ||||
| Doppler stenosis ≥70% | 4 | 23 | 3.62 (1.08–12.14) | 0.050 |
| Normal Doppler | 12 | 250 |
Fisher's exact test.
Antiplatelet agents were prescribed in 89%, warfarin in 6.9%, statin in 28.6%, antihypertensives in 39.3%, and antidiabetics in 11.9% of patients after hospitalization. Before the index TIA, 27.6% of the cohort was already taking antiplatelets, 3.7% was on oral anticoagulants, 11.3% on statin, 27.1% on antihypertensives, and 9.7% on antidiabetics. Dual therapy with aspirin and clopidogrel was not yet practiced. One patient had carotid endarterectomy on day 38, 1 patient had right carotid stenosis, and >70% of patients had angioplasty and stenting on day 30.
The stroke risk on days 2, 7, 30, and 90 was 0.2, 1.4, 2.9, and 4.4%, respectively. There were 43 ischemic strokes and one hemorrhagic stroke in 90 days. Forty-six patients had a recurrent TIA. Eight patients died within 90 days after the index TIA (4 deaths were stroke-related, 1 was due to bleeding from a pontine hemangioma, 1 to pancreatic cancer, 1 to chest infection, 1 to chronic renal failure, and 1 to an endarterectomy complication).
A total of 47.7% of our patients were classified as ABCD2 moderate risk score 4-5, most patients (26.8%) as score 5, and 32.7% within the high-risk scores 6-7. The log rank tests for stroke-free survival on days 7, 30, and 90 were insignificant. The test for linear trend across the ABCD2 score was only significant for stroke risk on day 30. The area under the curve (AUC) of the ROC curve for stroke on days 7, 30, and 90 was 0.607, 0.607, and 0.574, respectively (table 5). The significance test and ROC curve were not performed for day 2 stroke risk with a small number of stroke cases.
Table 5.
Stroke risk at 2, 7, 30, and 90 days stratified according to the ABCD2 score
| ABCD2 score | Patients, n | 2 days |
7 days |
30 days |
90 days |
|||
|---|---|---|---|---|---|---|---|---|
| stroke | stroke | risk, % (95% CI) | stroke | risk, % (95% CI) | stroke | risk, % (95% CI) | ||
| 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 52 | 0 | 1 | 1.9 (0–5.5) | 1 | 1.9 (0–5.7) | 1 | 1.9 (0–5.7) |
| 3 | 121 | 0 | 2 | 1.6 (0–3.9) | 2 | 1.7 (0–3.9) | 5 | 4.1 (0.6–7.7) |
| 4 | 209 | 0 | 1 | 0.5 (0–1.4) | 6 | 2.9 (0.6–5.1) | 9 | 4.3 (1.6–7.1) |
| 5 | 268 | 1 | 3 | 1.1 (0–2.4) | 6 | 2.2 (0.5–4.0) | 10 | 3.7 (1.5–6.0) |
| 6 | 249 | 0 | 3 | 1.2 (0–2.6) | 8 | 3.2 (1.0–5.4) | 12 | 4.8 (2.2–7.5) |
| 7 | 78 | 1 | 4 | 5.1 (0.2–10.0) | 6 | 7.7 (1.8–13.6) | 7 | 9.0 (2.6–15.3) |
| Total | 1,000 | 2 | 14 | 1.4 (0.7–2.1) | 29 | 2.9 (1.9–3.9) | 44 | 4.4 (3.1–5.6) |
| AUC = 0.607 (95% CI: 0.433–0.780); log rank test = 10.1, d.f. = 7, p = 0.186; p for trend across the ABCD2 score levels = 0.172; OR = 1.33 (0.88–2.02), p = 0.179 | AUC = 0.607 (95% CI: 0.498–0.715); log rank test = 8.719, d.f. = 7, p = 0.273; p for trend across the ABCD2 score levels = 0.038; OR = 1.36 (1.01–1.83), p = 0.040 | AUC = 0.574 (95% CI: 0.486–0.662); log rank test = 6.394, d.f. = 7, p = 0.495; p for trend across the ABCD2 score levels = 0.062; OR = 1.24 (0.99–1.57), p = 0.066 | ||||||
In the ED comparison cohort, there were 366 ED-coded TIA diagnosed by emergency physicians. After excluding 15 miscodings, losses to follow-up, non-Chinese patients, and 62 TIA mimics, the stroke risks of the 289 ED-diagnosed TIA were 0.35, 2.4, 5.2, and 6.2%, respectively (table 6).
Table 67.
Stroke risk of the TIA comparison cohort diagnosed by the ED
| Patients, n | 2 days |
7 days |
30 days |
90 days |
|||||
|---|---|---|---|---|---|---|---|---|---|
| stroke | stroke | risk, % (95% CI) | stroke | risk, % (95% CI) | stroke | risk, % (95% CI) | |||
| TIA mimics included | 351 | 1 | 7 | 1.99 (0.5–3.5) | 15 | 4.3 (2.2–6.4) | 18 | 5.1 (2.8–7.4) | |
| TIA mimics excluded | 289 | 1 | 7 | 2.4 (0.6–4.2) | 15 | 5.2 (2.6–7.8) | 18 | 6.2 (3.4–9.0) | |
Discussion
Misdiagnosis of TIA by non-neurologists is common [19,20]. We aimed at analyzing samples with an accurate diagnosis. With 45% of subjects admitted to the acute stroke unit, 517 subjects with symptom resolution at the hospital, and 66 TIA mimics excluded, the accuracy of TIA diagnosis in our cohort was high.
Three possibilities that could account for the low stroke risk were examined: (1) nonurgent treatments were provided, (2) strokes which happened after admission could be missed, or (3) a difference between Chinese and Caucasians was made.
Before the ABCD2 score was derived in November 2007 by Johnston, treatment and assessments were not considered urgent in our Hong Kong public hospitals, antiplatelet was not urgently prescribed and taken, a target LDL cholesterol of 2.6 was not set, and blood pressure lowering was not optimal. Dual therapy with aspirin and clopidogrel was not yet administered. Similar to the cohort patients under treatment (n = 1,707; aspirin 68%, ticlopidine 12%, and anticoagulation 14%) [14,21] in Johnston's ABCD2 score derivation study conducted in 16 ED in Northern California (1997-1998), 89% of our patients were put on antiplatelets and 6.9% on anticoagulant on discharge (table 7); however, the treatments were not immediately started both in Johnston's derivation cohort and in our cohort. First, unlike the SOS-TIA round-the-clock clinic [22] with senior vascular neurologists on duty call, providing assessments with neurological, arterial, and cardiac imaging within 4 h after admission, a target blood pressure of <130/85 mm Hg, LDL cholesterol <2.56 mmol/l, and second, unlike phase 2 of the EXPRESS [23] study which provided immediate referral, assessment and intensive treatments, with 300 mg of aspirin taken in the clinic, 84% of patients put on statin, and a median time of seeking medical attention to first prescription of 1 day [23], the assessments and treatments initiated in our ED were not as intensive, immediate and urgent in pace. In 2006, though most of our CTs of the brain were taken within 24 h after admission, most of the MRIs of the brain and vascular assessments took place many weeks after discharge from hospital. The benefit of aspirin in lowering stroke risk in our Chinese cohort was confirmed in our study, and was previously shown in the Chinese Acute Stroke Trial (CAST) [24] and the combined analysis of CAST and International Stroke Trial (IST) [25] that shows a reduction of 0.7% in recurrent ischemic stroke in the group receiving aspirin compared with the placebo group at 4 weeks.
Table 7.
Comparison of Johnston's ABCD2 derivation and validation groups with our study
| Johnston's ABCD2 derivation and validation group [14] | Hong Kong TIA study | |
|---|---|---|
| Study design | Retrospective but prospectively enrolled data from a computerized database | Retrospective but prospectively compulsory e-coded data from a computerized database and hard copies |
| Country | Oxford (UK) and California (USA) | Hong Kong |
| Year | 1998–2005 in the validation group | 2006 |
| Setting | ED cohort: multiple ED Clinic cohort | Multiple ED |
| TIA diagnosed by | ED cohort: ED physicians Clinic cohort: neurologists | Neurologists or internists |
| Age | 66–80% >60 years | 72.1% >60 years |
| Ethnicity | White 70–99% | Chinese 100% |
| Symptom onset on ED arrival or evaluation | 0–0 day and 0–3 days | 0–3 days (96%, 0–1 day) |
| Symptomatic on arrival | 50% of the California derivation group | 51.7% |
| How the ‘day of stroke’ was counted | From day of evaluation in the clinic or ED to day of stroke | From day of ED registration to day of stroke |
| Prior stroke | 0–20% | 19.9% |
| On antiplatelet on discharge | 81–99% in the validation group | 89% |
| On anticoagulation on discharge | 4–14% | 6.9% |
Surgical intervention has been shown to have no influence on the finding of low risk in our study [26]. The only patient with endarterectomy at day 38 died shortly after the operation, 1 patient with carotid angioplasty and stenting at day 30 did not suffer a stroke within 90 days.
We addressed the possibility that some TIA patients could have suffered a stroke shortly after admission, and could have been coded stroke, and therefore, failed to be recorded. After separately probing the 289 TIA cases (TIA mimics excluded) with compulsory ICD9 e-coding diagnosed in the 6 ED, we did not find the ED-diagnosed TIA cases to have a higher 2-day stroke rate shortly after admission (table 6). The effect of missed recording was insignificant.
We compared Johnston's cohorts with ours (table 7). Many similarities were observed, for example, there was a similar percentage of patients receiving antiplatelets and anticoagulant in our cohort and Johnston's ABCD2 derivation and validation groups. The major difference between the two is the patients' ethnicity. Therefore, we conclude that our finding of a lower stroke risk cannot be attributed to the nonurgent treatment alone, but to the inherent pathophysiological mechanisms including the difference in epidemiology in extra- and intracranial stenosis and the nonmodifiable factors that differ between Chinese and Caucasians [2,3,4,5,6,7,8,9,10].
In the Chinese population, intracranial stenosis and small vessel disease occur more often, and the distal branches of the intracranial cerebral vessels are commonly involved [2,3,4,5,12]. Most of the severe atherosclerotic narrowing is located in the very distal branches in the leptomeningeal surfaces [5]. Previous studies have shown that the risk of stroke was higher in patients with large artery atherosclerosis, mostly carotid stenosis [27,28]. Our CT and MRI findings are consistent with a higher prevalence of intracranial small vessel disease in Chinese (table 3). Furthermore, our low rate (4.2%) of carotid bruit and the low rate (6.1%) of carotid stenosis of ≥70% in the carotid Doppler echocardiogram and previous studies confirm that extracranial large artery disease is less common in this community [2,3,5,12,13]. A meta-analysis by Lovett et al. [28] found that recurrent stroke risk varied between subtypes of ischemic stroke, the risks being 4% at 7 days and 12.6% at 30 days in patients with large artery atherosclerosis compared with 0 and 2%, respectively, in patients with lacunar stroke. Compared with other subtypes, patients with stroke due to large artery atherosclerosis had the highest odds of recurrence at 7, 30 days, and 3 months (OR = 2.9-3.3) for stroke recurrence at 30 days for small vessel stroke (OR = 0.2) [28]. A lower short-term stroke risk in our Chinese cohort with more intracranial small vessel disease and lacunar infarcts is consistent with the findings in Lovett et al.'s [28] meta-analysis.
Furthermore, the lower stroke risk in our study might be attributed to the good collateral compensation via leptomeningeal anastomosis in Chinese patients, in whom intracranial stenosis predominates. A study performed at the university hospital of the Chinese University of Hong Kong found that 61 of the 69 patients (88.4%) suffering from TIA or minor stroke with intracranial stenosis had a good collateral circulation score. They observed that good collateral compensations are important in patients with symptomatic intracranial stenosis and compromised antegrade flow and are associated with favorable outcome and less recurrence risk [29].
ABCD2 Score Predictability
Except for the p for trend across ABCD2 score levels at 30 days which showed a higher stroke risk with higher scores, the ABCD2 score was found to have a low predictive value (table 5). One possible reason is the overall small number of stroke cases in the sample, making this study underpowered in evaluating the usefulness of the predictive tool. Difference in patients' ethnicity, cerebrovascular pathophysiology and the prevalence of risk factors between our sample and the ABCD2 derivation cohort [14] would, however, account for the difference.
In fact, the predictive value of the ABCD2 score shows marked variations in different clinical settings, such as ED, clinics, specialist units or population base [30]. Multiple validations of the ABCD2 scoring have reported inconsistent results, from excellent predictive value [31] to little better than chance [32]. A prospective study in Canada conducted in 2011 found both low stroke risk and ABCD2 low predictive value in stroke after TIA [33]. There are also findings showing that the ABCD2 score is unable to give good predictive values in TIA confirmed by a neurologist, adjudication committee or specialist [33,34,35], but paradoxically it gives good prediction in subjects diagnosed by a nonspecialist [14,34].
The arbitrary 24-hour time- or tissue-based definition of TIA is controversial [36], and some studies include minor strokes [23,27,37,38,39], but with different definitions of minor strokes [40], this could likely be the source of the variations found [41].
Strengths
This is a multicenter cohort. The compulsory ICD9 e-coding of inpatients guaranteed complete TIA case entry. Diagnosis was made by neurologists. Our exclusion of probable TIA or TIA mimics enhanced the accuracy of stroke risk. Like in Johnston's cohorts, 51.7% of our patients had witnessed symptoms which resolved in hospital – rather than recalled symptoms – which further ensured an accurate diagnosis. A total of 95.7% of our patients arrived within 24 h from symptom onset which reinforced the 2-day stroke risk reliability.
Limitations
This is not a population-based study. This sample represents patients treated in public ED only, though all patients calling 999 for ambulance will be transported to the nearest ED. Those who suffered a stroke a few hours after a TIA might have been coded as stroke patients [42]. Those with absence of speech or motor symptoms and short duration of symptoms might have delayed seeking medical attention [43], or might have attended private practitioners, or not sought treatment at all. Only six ED with compulsory e-coding were recruited for stroke risk comparison.
Clinical Implications
It is no longer ethical to conduct a prospective study by withholding early treatments for observing strokes happening after TIA. However, noting the differences in pathophysiological mechanisms of cerebrovascular diseases between Chinese/Asian and Caucasians [2,3,4,5,6,7,8,9,10], our results serve to remind us of Giles and Rothwell's [1] statement that caution is required in extrapolating Caucasian results to nonwhite populations, as the short-term prognosis after TIA might differ.
If the short-term stroke risk after a TIA is low in the Chinese ethnicity most of our patients do not have to be immediately hospitalized when attending an ED. Joint management with the neurologist with an agreed clinical guideline on fast workups in ED, and fast-track follow-up in an outpatient TIA clinic by neurologists would be a safe and cost effective option [22,23]. Hospitalization with rapid investigations requires many resources, especially in localities with limited access to advanced imaging which cannot afford to hospitalize most TIA patients [33]. Our society is facing the challenge of an aging population, 17% of the population will be aged >65 years by 2020 [15]. To avoid undertreating the vulnerable and salvageable ones in this community, we suggest prompt antiplatelet administration after urgent CT of the brain, and early combined carotid/transcranial ultrasound to identify high-risk individuals with high-grade stenosis in ED in planning urgent and aggressive prevention therapies [32,35], for those with CT or MRI positive of infarct, or carotid Doppler showing stenosis ≥70%.
Conclusion
Hong Kong Chinese TIA patients have a lower short-term stroke risk. The nonurgent treatment started cannot solely explain the favorable outcome, the lower risk can be due to the different pathophysiological mechanisms of stroke between Caucasians and Chinese. The predictive value of the ABCD2 score in stroke risk after a TIA is low in this locality.
Appendix
Hong Kong TIA (HKTIA) Project Investigators
Participating Centers (Site Lead Coordinator and Coinvestigators). Alice Ho Miu-Ling Nethersole Hospital, North District Hospital (L.H.S. Chiu); Caritas Medical Centre (Kei-Fung Lam, MRCS Edin); Kwong Wah Hospital (Fu-Ping Sin, FHKCEM): Pamela Youde Nethersole Eastern Hospital (T.T. Au); Princess Margret Hospital (L.H.S. Chiu, C.T. Tsui); Prince of Wales Hospital (Chi-Wang Lam, FHKCEM); Queen Elizabeth Hospital (W.H. Yau); Queen Mary Hospital (L.P. Leung); Ruttonjee and Tang Shiu-Kin Hospital (K.A. Wan); Tseung Kwan O Hospital (Tak Shun Poon, FHKCEM); Tuen Mun Hospital (S.H.J. Chung); Yan Chai Hospital (P. Pang).
Disclosure Statement
The authors have no conflicts of interest to disclose.
Acknowledgements
We thank C.C. Dicken Chan (statistician) for data cleaning and statistical analysis. We thank Amelia Wong (RN) for reconfirming the accuracy of all investigation results including imaging results independently.
We thank the Hong Kong College of Emergency Medicine for the support with a research fund. The College has no role in study design, data collection, analysis and manuscript writing.
References
- 1.Giles MF, Rothwell PM. Risk of stroke early after transient ischemic attack: a systemic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann E, Daneault N, Kwan E, et al. Chinese-white differences in the distribution of occlusive cerebrovascular diseases. Neurology. 1990;40:1541–1545. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- 3.Wong KS, Huang YN, Gao S, et al. Cerebrovascular disease among Chinese populations – recent epidemiological and neuroimaging studies. Hong Kong Med J. 2001;7:50–57. [PubMed] [Google Scholar]
- 4.Huang YN, Gao S, Li SW, et al. Vascular lesions in Chinese patients with transient ischemic attack. Neurology. 1997;48:524–525. doi: 10.1212/wnl.48.2.524. [DOI] [PubMed] [Google Scholar]
- 5.Leung SY, Ng TH, Yuen ST, et al. Pattern of cerebral atherosclerosis in Hong Kong Chinese. Severity in intracranial and extracranial vessels. Stroke. 1993;24:779–786. doi: 10.1161/01.str.24.6.779. [DOI] [PubMed] [Google Scholar]
- 6.Wong KS, Huang YN, Gao S, et al. Intracranial stenosis in Chinese patient with acute stroke. Neurology. 1998;50:812–813. doi: 10.1212/wnl.50.3.812. [DOI] [PubMed] [Google Scholar]
- 7.Liu HM, Tu YK, Yip PK, Su CT. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke. 1996;27:650–653. doi: 10.1161/01.str.27.4.650. [DOI] [PubMed] [Google Scholar]
- 8.Suri MFK, Johnston SC. Epidemiology of intracranial stenosis. J Neuroimaging. 2009;19(suppl 1):11S–11S. doi: 10.1111/j.1552-6569.2009.00415.x. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Hong KS, Lee EJ, Lee J, Kim DE. High levels of apolipoprotein B/AI ratio are associated with intracranial atherosclerotic stenosis. Stroke. 2011;42:3040–3046. doi: 10.1161/STROKEAHA.111.620104. [DOI] [PubMed] [Google Scholar]
- 10.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk, systemic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. doi: 10.1161/STROKEAHA.111.000038. [DOI] [PubMed] [Google Scholar]
- 11.Fluri F, Jax F, Amort M, et al. Significance of microbleeds in patients with transient ischemic attack. Eur J Neurol. 2012;19:522–524. doi: 10.1111/j.1468-1331.2011.03522.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong KS, Li H. Long-term mortality and recurrent stroke risk among Chinese stroke patients with predominant intracranial atherosclerosis. Stroke. 2003;34:2361–2366. doi: 10.1161/01.STR.0000089017.90037.7A. [DOI] [PubMed] [Google Scholar]
- 13.Wong KS, Li H, Chan YL, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. 2000;31:2641–2647. doi: 10.1161/01.str.31.11.2641. [DOI] [PubMed] [Google Scholar]
- 14.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after TIA. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 15.The Bauhinia Foundation Research Centre Health Care Study Group Development and financing of Hong Kong's future health care. http://www.legco.gov.hk/yr06-07/english/panels/hs/papers/hs0717cb2-2460-1-e.pdf (accessed August 27, 2010).
- 16.Census and Statistics Department, the Government of the Hong Kong Special Administrative Region. http://www.censtatd.gov.hk/hong_kong_statistics/statistics_by_subject/index.jsp (accessed August 27, 2010).
- 17.WHO MONICA Project Principal Investigators The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol. 1988;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 18.Hong Kong Hospital Authority, Central Committee on Stroke Service, 2006 data.
- 19.Ferro JM, Falcão I, Rodrigues G, et al. Diagnosis of transient ischemic attack by the non-neurologist: a validation study. Stroke. 1996;27:2225–2229. doi: 10.1161/01.str.27.12.2225. [DOI] [PubMed] [Google Scholar]
- 20.Ghia D, Thomas PR, Cordato DJ, et al. Validation of emergency and final diagnosis coding in transient ischemic attack: South Western Sydney transient ischemic attack study. Neuroepidemiology. 2010;35:53–58. doi: 10.1159/000310338. [DOI] [PubMed] [Google Scholar]
- 21.Johnston SC, Gress DR, Browner WS, et al. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 22.Lavallee PC, Meseguer E, Abboud H, et al. A transient ischemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 23.Ramon LF, Gray AM, Rothwell PM. Effect of urgent treatment for transient ischaemic attack and minor stroke on disability and hospital costs (EXPRESS study): a prospective population-based sequential comparison. Lancet Neurol. 2009;8:235–243. doi: 10.1016/S1474-4422(09)70019-5. [DOI] [PubMed] [Google Scholar]
- 24.CAST (Chinese Acute Stroke Trial) Collaborative Group CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–1649. [PubMed] [Google Scholar]
- 25.Chen ZM, Sandercock P, Pan HC, et al. Indications for early aspirin use in acute ischaemic stroke: a combined analysis of 40,000 randomized patients from the Chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31:1240–1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 26.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJM. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 27.Rothwell PM, Buchan A, Johnston SC. Recent advances in management of transient ischaemic attacks and minor ischaemic strokes. Lancet Neurol. 2006;5:323–331. doi: 10.1016/S1474-4422(06)70408-2. [DOI] [PubMed] [Google Scholar]
- 28.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–574. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 29.Lau AYL, Wong EHC, Wong A, et al. Significance of good collateral compensation in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. 2012;33:517–524. doi: 10.1159/000337332. [DOI] [PubMed] [Google Scholar]
- 30.Giles MF, Rothwell PM. Systematic review and pooled analysis of published and unpublished validations of the ABCD and ABCD2 transient ischemic attack risk scores. Stroke. 2010;41:667–673. doi: 10.1161/STROKEAHA.109.571174. [DOI] [PubMed] [Google Scholar]
- 31.Tsivgoulis G, Spengos K, Manta P, Karandreas N, Zambelis T, Zakopoulos N, Vassilopoulos D. Validation of the ABCD score in identifying individuals at high risk of stroke after transient ischemic attack: hospital-based case series study. Stroke. 2006;37:2892–2897. doi: 10.1161/01.STR.0000249007.12256.4a. [DOI] [PubMed] [Google Scholar]
- 32.Purroy F, Molina CA, Montaner J, Alvarez-Sabin J. Absence of usefulness of ABCD score in early risk of stroke of transient ischemic attack patients. Stroke. 2007;38:855–856. doi: 10.1161/01.STR.0000257306.00512.d3. [DOI] [PubMed] [Google Scholar]
- 33.Perry JJ, Sharma M, Sivilotti MLA, Sutherland J, Symington C, Worster A, Émond M, Stotts G, Jin AY, Oczkowski WJ, Sahlas DJ, Murray HE, MacKey A, Verreault S, Wells GA, Stiell IG. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack. CMAJ. 2011;183:1137–1145. doi: 10.1503/cmaj.101668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke. 2010;41:844–850. doi: 10.1161/STROKEAHA.109.571844. [DOI] [PubMed] [Google Scholar]
- 35.Stead LG, Suravaram S, Bellolio MF, Enduri S, Rabinstein A, Gilmore RM, Bhagra A, Manivannan V, Decker WW. An assessment of the incremental value of the ABCD2 score in the emergency department evaluation of transient ischemic attack. Ann Emerg Med. 2011;57:46–51. doi: 10.1016/j.annemergmed.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albers GW. Transient ischemic attack – proposal for a new definition. N Engl J Med. 2002;347:1713–1716. doi: 10.1056/NEJMsb020987. [DOI] [PubMed] [Google Scholar]
- 37.Ray G, Wright F, Stott DJ, Langhorne P. Prospective study using the ABCD2 score in screening for minor stroke or transient ischemic attack in referrals to a fast-track clinic. Stroke. 2009;40:e467. doi: 10.1161/STROKEAHA.109.551077. [DOI] [PubMed] [Google Scholar]
- 38.Coutts SB, Eliasziw M, Hill MD, Scott JN, Subramaniam S, Buchan AM, Demchuk AM, VISION study group An improved scoring system for identifying patients at high early risk of stroke and functional impairment after TIA or minor stroke. Int J Stroke. 2008;3:3–10. doi: 10.1111/j.1747-4949.2008.00182.x. [DOI] [PubMed] [Google Scholar]
- 39.Selvarajah JR, Smith CJ, Hulme S, Georgiou RF, Vail A, Tyrrell PJ, NORTHSTAR Collaborators Prognosis in patients with TIA and minor stroke attending services in the North West of England: NORTHSTAR Study. J Neurol Neurosurg Psychiatry. 2008;79:38–43. doi: 10.1136/jnnp.2007.129163. [DOI] [PubMed] [Google Scholar]
- 40.Fischer U, Baumgartner A, Arnold M, Nedeltchev K, Gralla J, De Marchis GM, Kappeler L, Mono ML, Brekenfeld C, Schroth G, Mattle HP. What is a minor stroke? Stroke. 2010;41:661–666. doi: 10.1161/STROKEAHA.109.572883. [DOI] [PubMed] [Google Scholar]
- 41.Mullen MT, Cucchiara BL. Redefinition of transient ischemic attack improves prognosis of transient ischemic attack and ischemic stroke. An example of the Will Rogers Phenomenon. Stroke. 2011;42:3612–3613. doi: 10.1161/STROKEAHA.111.627877. [DOI] [PubMed] [Google Scholar]
- 42.Chandratheva A, Mehta Z, Geraghty OC, Marquardt L, Rothwell PM, Oxford Vascular Study Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology. 2009;72:1941–1947. doi: 10.1212/WNL.0b013e3181a826ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chandratheva A, Lasserson DS, Geraghty OC, Rothwell PM, Oxford Vascular Study Population-based study of behavior immediately after transient ischemic attack and minor stroke in 1,000 consecutive patients: lessons for public education. Stroke. 2010;41:1108–1114. doi: 10.1161/STROKEAHA.109.576611. [DOI] [PubMed] [Google Scholar]