Abstract
The presence of lymph node metastasis is an important prognostic factor for patients with esophageal cancer. Accurate assessment of lymph nodes in thoracic esophageal carcinoma is essential for selecting appropriate treatment and forecasting disease progression. Positron emission tomography combined with computed tomography (PET/CT) is becoming an important tool in the workup of esophageal carcinoma. Here, we evaluated the effectiveness of the maximum standardized uptake value (SUVmax) in assessing lymph node metastasis in esophageal squamous cell carcinoma (ESCC) prior to surgery. Fifty-nine surgical patients with pathologically confirmed thoracic ESCC were retrospectively studied. These patients underwent radical esophagectomy with pathologic evaluation of lymph nodes. They all had 18F-FDG PET/CT scans in their preoperative staging procedures. None had a prior history of cancer. The pathologic status and PET/CT SUVmax of lymph nodes were collected to calculate the receiver operating characteristic (ROC) curve and to determine the best cutoff value of the PET/CT SUVmax to distinguish benign from malignant lymph nodes. Lymph node data from 27 others were used for the validation. A total of 323 lymph nodes including 39 metastatic lymph nodes were evaluated in the training cohort, and 117 lymph nodes including 32 metastatic lymph nodes were evaluated in the validation cohort. The cutoff point of the SUVmax for lymph nodes was 4.1, as calculated by ROC curve (sensitivity, 80%; specificity, 92%; accuracy, 90%). When this cutoff value was applied to the validation cohort, a sensitivity, a specificity, and an accuracy of 81%, 88%, and 86%, respectively, were obtained. These results suggest that the SUVmax of lymph nodes predicts malignancy. Indeed, when an SUVmax of 4.1 was used instead of 2.5, FDG-PET/CT was more accurate in assessing nodal metastasis.
Keywords: SUVmax, esophageal squamous cell carcinoma, cutoff value, esophagectomy, lymph nodes
Fluorodeoxyglucose positron emission tomography (FDG PET) combined with computed tomography (CT) is now becoming a standard for staging esophageal carcinoma by detecting distant metastases. However, diagnosing lymph node metastasis is often difficult from its size and maximum standardized uptake value (SUVmax)[1]. The predictive value of PET/CT in primary staging in patients with esophageal carcinoma is under discussion. For patients with esophageal carcinoma, the presence of lymph node metastasis is an important prognostic factor: the prognosis of patients with lymph node involvement is dramatically worse than patients without[2].
Accurate assessment of locoregional lymph nodes in thoracic esophageal carcinoma is more complex but essential for selecting appropriate treatments and forecasting disease progression. Locoregional lymph nodes encompass any paraesophageal lymph nodes, from the cervical nodes down to the celiac nodes. Since 2005, PET/CT has become a mainstay for evaluating patients with potentially resectable esophageal carcinoma[3]. The degree of metabolic activity within the tumor is measured according to the SUVmax. Most malignant tumors have an SUVmax greater than 2.5[4],[5]. Several studies, including a recent meta-analysis[6], have suggested that tumors with an elevated SUVmax tend to be more aggressive and to be associated with a worse survival[7],[8]. However, the best cutoff value for SUVmax with which to accurately assess the status of regional lymph nodes is still under debate.
SUVmax on FDG PET/CT imaging can be used to predict mediastinal lymph node status preoperatively for patients with thoracic esophageal carcinoma. Several studies have recommended different SUVmax cutoff, ranging from 2.5 to 5.3, for mediastinal lymph nodes[9]. Therefore, the aim of this study was to determine the best cutoff value for SUVmax on 18F-FDG PET/CT for predicting malignant regional lymph node status in patients with thoracic esophageal squamous cell carcinoma (ESCC) and to apply it to a validation cohort to test the accuracy of the cutoff value obtained.
Patients and Methods
Patient selection
In this mono-institutional retrospective study, we included patients with surgically resected ESCC and divided them into a training cohort of 59 patients (44 men and 15 women), with a median age of 63 years (range, 47–83 years), and a validation cohort of 27 patients (19 men and 8 women), with a median age of 64 years (range, 48–79 years), to test and validate the results obtained. Table 1 summarizes the clinicopathologic characteristics of the patients with ESCC in the training and validation cohorts. All patients were referred to 18F-FDG PET/CT for initial staging. Other staging procedures included physical examination, laboratory tests, bronchoscopy, esophagogastroduodenoscopy, and optional CT from the neck to the upper abdomen. The exclusion criteria included the following: patients with history of other cancers; patients who did not undergo 18F-FDG PET/CT examination; and patients with cancer of the esophagogastric junction or with histologic type other than ESCC. All patients underwent radical esophagectomy at the Department of Thoracic Surgery, Sun Yat-sen University Cancer Center, between June 2005 and December 2011 for the training cohort and in 2012 for the validation cohort. Written informed consent was obtained from each patient before undergoing the examination. The study protocol was approved by the Institutional Review Board.
Table 1. Clinicopathologic characteristics of the patients with esophageal squamous cell carcinoma in training and validation cohorts.
| Variable | Training cohort (n = 59) | Validation cohort (n = 27) |
| Sex (cases) | ||
| Female | 15 | 8 |
| Male | 44 | 19 |
| Age at diagnosis (years) | ||
| Median | 63 | 64 |
| Range | 47-83 | 48-79 |
| Esophagus section (cases) | ||
| Upper third | 5 | 3 |
| Middle third | 37 | 20 |
| Lower third | 17 | 4 |
| Pathologic T category (cases) | ||
| Tis | 2 | 0 |
| T1 | 5 | 3 |
| T2 | 13 | 3 |
| T3 | 35 | 19 |
| T4a | 4 | 2 |
| Pathologic N category (cases) | ||
| N0 | 29 | 8 |
| N1 | 19 | 11 |
| N2 | 11 | 8 |
| N3 | 0 | 0 |
| Pathologic M category (cases) | ||
| M0 | 59 | 27 |
| M1 | 0 | 0 |
| Clinical staging (cases) | ||
| 0 | 2 | 0 |
| IA | 2 | 0 |
| IB | 3 | 1 |
| IIA | 12 | 5 |
| IIB | 16 | 6 |
| IIIA | 11 | 5 |
| IIIB | 10 | 8 |
| IIIC | 3 | 2 |
PET/CT imaging and SUV measurements
18F-FDG PET/CT scans were obtained with an advanced integrated PET/CT scanner (GE Discovery ST-16 PET/CT System; Wisconsin, USA). All patients fasted for at least 6 h before the PET/CT examination. PET/CT images were obtained from the head to the upper portion of the thigh 60 min after intravenous injection of the tracer. Blood glucose was measured for all diabetic patients to ensure that it was within acceptable limits (subcutaneous insulin injection was administered when necessary). SUVmax was assessed on the Xeleris® PET/CT workstation (GE Healthcare) according to the rule of the region of interest (ROI). A team of nuclear medicine physicians together with thoracic surgeons interpreted the 18F-FDG PET/CT images and recorded the number, size, SUVmax, character, and precise location of all detectable lymph nodes. Data obtained were then matched with the postsurgical pathologic report to confirm lymph node status. When there was a discrepancy between interpretations, a consensus was reached by discussion. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of FDG PET/CT for diagnosing malignant lymph nodes were calculated.
Data analysis
The software package SPSS v17.0 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis. The pathologic status and SUVmax of mediastinal lymph nodes were collected for calculating the receiver operating characteristic (ROC) curve and determining the cutoff value for SUVmax. Wilcoxon-Mann-Whitney test, chi-square test, and Student's t-test were performed for significance. Box-and-whisker plots of SUVmax were drawn for statistical summary. The P values of <0.05 were considered statistically significant.
Results
A total of 323 lymph nodes including 39 metastatic lymph nodes were evaluated in the training cohort, and 117 lymph nodes including 32 metastatic lymph nodes were evaluated in the validation cohort.
SUVmax for pathologically positive and negative lymph nodes and the cutoff value for diagnosis (training cohort)
SUVmax was measured for each lymph node and compared with the results of histopathologic examination. The median SUVmax values of pathologically negative and positive lymph nodes were 1.6 (range, 0.4–11.3) and 5.4 (range, 1.1–17.8), respectively (Figure 1). A ROC curve was drawn to determine the cutoff value for SUVmax at which sensitivity and specificity were the highest. As shown in Figure 2, the best combination between sensitivity and specificity, and thus the highest accuracy to distinguish benign from malignant lymph nodes, occurred at an SUVmax cutoff value of 4.1 (sensitivity, specificity, and accuracy of 80%, 92%, and 90%, respectively).
Figure 1. Box-and-whisker plots of maximum standardized uptake value (SUVmax) for pathologic status of lymph nodes (training cohort).
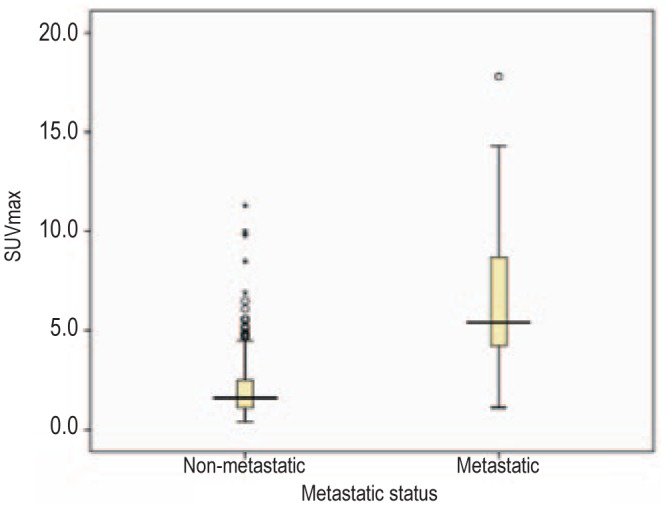
The horizontal line in each box represents the median SUVmax.
Figure 2. Receiver operating characteristic (ROC) curve for diagnosis of lymph node metastasis with fluorodeoxyglucose positron emission tomography combined with computed tomography (training cohort).
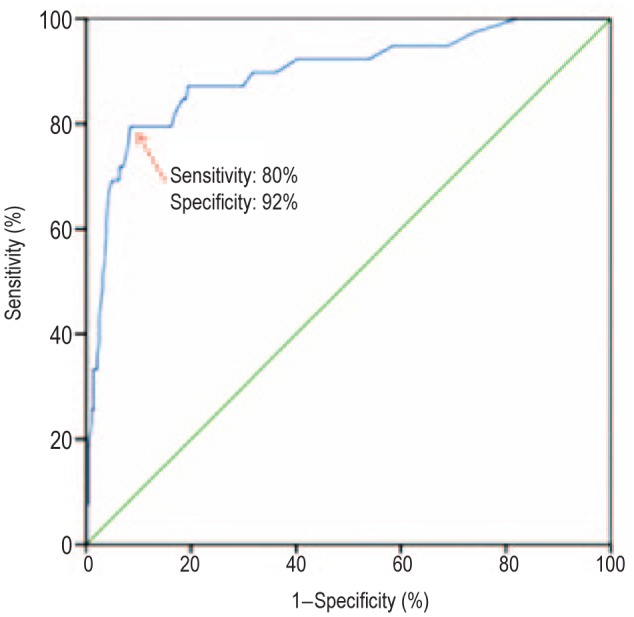
The blue line is the ROC curve from the training cohort (SUVmax), and the green line is the reference line.
To compare the sensitivities and specificities of FDG PET/CT, we used several cutoff values for FDG PET/CT-based SUVmax: 1.1, for which the sensitivity was 100%; 2.5, which is conventional[10]; 4.1, which was calculated from the ROC curve; and 6.3, the mean SUVmax of pathologically positive lymph nodes (Table 2). The cutoff points of SUVmax 2.5 and 4.1 yielded the highest sensitivity, specificity, accuracy, and positive predictive value. When all of these parameters were considered a whole, the SUVmax cutoff of 4.1 performed the best. In the validation cohort, the SUVmax of 4.1 yielded a sensitivity of 81%, a specificity of 88%, and an accuracy of 86%.
Table 2. Comparison of the diagnostic modalities of FDG PET/CT and validation using different cutoffs of maximum standardized uptake value (SUVmax).
| SUVmax cutoff value | Sensitivity (%) | Specificity (%) | Accuracy (%) | Precision (%) | NPV (%) | FNR (%) | FPR (%) | LR+ | LR− |
| Trainiyg cohort (FDG PET/CT) | |||||||||
| 1.1 | 100 | 18 | 28 | 14 | 100 | 0 | 82 | 1.22 | 0.00 |
| 2.5 | 87 | 74 | 76 | 32 | 98 | 13 | 26 | 3.39 | 0.17 |
| 4.1 | 80 | 92 | 90 | 56 | 97 | 23 | 9 | 9.10 | 0.25 |
| 6.3 | 36 | 98 | 90 | 70 | 92 | 64 | 2 | 16.99 | 0.65 |
| Validation cohort | |||||||||
| 4.1 | 81 | 88 | 86 | 72 | 93 | 19 | 12 | 6.91 | 0.21 |
FDG PET/CT, fluorodeoxyglucose positron emission tomography combined with computed tomography; NPV, negative predictive value; FNR, false negative rate; FPR, false positive rate; LR+, likelihood ratio positive; LR−, likelihood ratio negative.
Table 3 displays the pathologic status of lymph nodes detected on FDG PET/CT scans according to their SUVmax. Most of the metastatic lymph nodes had an SUVmax≥4.1, whereas the vast majority of non-metastatic lymph nodes had an SUVmax cutoff value ranging from 0.0 to 2.0. The differences observed were statistically significant (P < 0.001). This table also shows the distribution of malignant lymph nodes (n = 39) according to their SUVmax and nodal diameter on FDG PET/CT images. For malignant lymph nodes ≤10 mm and > 10 mm in diameter, 81.3% (13/ 16) and 73.9% (17/ 23), respectively, had an SUVmax ≥ 4.1 (Table 3).
Table 3. Lymph node status according to SUVmax and distribution of malignant lymph nodes (LNs) according to SUVmax and their diameter.
| Parameter | SUVmax |
Total | ||
| 0.0-2.0 | 2.1-4.0 | ≥ 4.1 | ||
| Pathologic status of detected LNs | ||||
| Metastatic | 4 | 5 | 30 | 39 |
| Non-metastatic | 184 | 73 | 27 | 284 |
| Total | 188 | 78 | 57 | 323 |
| Diameter of malignant LNs (mm) | ||||
| ≤ 10 | 1 | 2 | 13 | 16 |
| > 10 | 3 | 3 | 17 | 23 |
| Total | 4 | 5 | 30 | 39 |
In 323 lymph nodes that were detected in the training cohort, the mean SUV of the malignant lymph nodes was 6.3 (n = 39; range, 1.1–17.8), and the mean SUV of the benign lymph nodes was 2.1 (n = 284; range, 0.4–11.3). The difference between the SUVs was statistically significant (P < 0.001). The mean maximum axial diameters of the malignant lymph nodes were 10.5 mm (range, 4.0–18 mm).
Sensitivity, specificity, and accuracy of SUVmax at selected lymph node sites in the training and validation cohorts
We calculated the sensitivity, specificity, and accuracy of SUVmax in characterizing lymph node status at selected lymph node sites (Table 4). High sensitivity was noted for all selected lymph node sites in the training cohort, and better specificity and accuracy were yielded for an SUVmax ≥4.1. In the validation cohort, a poor sensitivity was obtained for the right recurrent laryngeal nerve site (50%).
Table 4. Sensitivity, specificity, accuracy, and positive predictive value for different SUVmax cutoff values on FDG PET/CT scans in assessing lymph nodes at different sites.
| Site | Number of LN | Cutoff value of SUVmax | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) |
| Training cohort | ||||||
| Paraesophageal tissues | 50 | 2.5 | 92 | 54 | 64 | 41 |
| 4.1 | 85 | 89 | 88 | 73 | ||
| RRLN | 19 | 2.5 | 100 | 82 | 84 | 40 |
| 4.1 | 100 | 94 | 95 | 67 | ||
| Subcarinal tissues | 67 | 2.5 | 100 | 59 | 60 | 4 |
| 4.1 | 100 | 85 | 85 | 9 | ||
| Cardia | 18 | 2.5 | 100 | 93 | 94 | 75 |
| 4.1 | 100 | 100 | 100 | 100 | ||
| LGA | 36 | 2.5 | 80 | 92 | 89 | 80 |
| 4.1 | 70 | 96 | 89 | 88 | ||
| Validation cohort | ||||||
| Paraesophageal tissues | 16 | 4.1 | 88 | 75 | 81 | 78 |
| RRLN | 14 | 4.1 | 50 | 88 | 71 | 75 |
| Subcarinal tissues | 19 | 4.1 | 100 | 77 | 84 | 67 |
RRLN, right recurrent laryngeal nerve; LGA, left gastric artery; LN, lymph node; PPV, positive predictive value. Other footnotes as in Table 2.
Discussion
The introduction of 18F-FDG PET/CT has greatly improved preoperative staging of esophageal carcinoma, as it has become possible to assess regional metabolism noninvasively with PET and metabolic tracers. When used with 18F-FDG, PET/CT provides information on the focal increase in glucose metabolism associated with malignancies, thus facilitating the assessment of metastases in detectable lymph nodes[11]. In other words, PET/CT, in which FDG PET images are fused with CT images, has an increasing role in detecting diseased lymph nodes that appear normal with CT alone[12].
Nodal staging has a significant effect on survival rates. The 5-year overall survival rate is 42%–72% in patients with negative lymph nodes and 10%–12% in patients with positive lymph nodes[13].
In this study, we evaluated the role of FDG PET/CT in diagnosing lymph node metastasis in thoracic ESCC, as metastasis to the lymph nodes is the most important prognostic factor in esophageal carcinoma[14]. Both the number and the location of involved lymph nodes have been considered important prognostic factors[15]. Several studies previously demonstrated the advantage of PET over CT with respect to sensitivity in detecting nodal metastases[11],[16],[17]. Furthermore, we know from recent studies that combined PET/CT images are superior to side-by-side PET and CT images in assessing lymph node metastases in patients with thoracic ESCC. Therefore, this approach may improve staging. Additionally, significant improvements in N category with PET/CT were reported by Yuan et al.[18] in 2006 in a study of 45 cases. The imaging findings were corroborated by pathologic assessment.
Of the 59 patients included in our study, 30 had regional nodal metastases. A total of 323 metastatic lymph nodes were evaluated, and SUVmax ranged from 0.4 to 17.8. We used the ROC curve to determine the cutoff value of SUVmax that most effectively predicted the mediastinal lymph node metastatic status. An SUVmax cutoff value of 4.1 showed optimal overall performance, with a sensitivity of 80% and a specificity of 92%. Our results were similar to those reported in previous studies[18],[19], but the value of our best cutoff value for malignant LNs was higher than the previously reported values 2.5 and 3.3[20]. When the SUVmax cutoff value of 4.1 was applied to the validation cohort, the sensitivity, specificity, and accuracy were 81%, 88%, and 86%, respectively. This result is acceptable because the values obtained showed a high accuracy in preoperatively predicting lymph node metastasis.
We also compared the sensitivities, specificities, and accuracies, calculated for SUVmax cutoff values of 2.5 and 4.1, in assessing metastasis for lymph nodes at selected sites. The sensitivity, specificity, and accuracy varied according to the location of the lymph nodes; they were lower for paraesophageal lymph nodes than those for lymph nodes in other sites. On the other hand, sensitivity, specificity, and accuracy were relatively high for lymph nodes located around the right recurrent laryngeal nerve, cardia, and left gastric artery, as well as for subcarinal lymph nodes. The lower precision observed for paraesophageal, right recurrent laryngeal nerve, and subcarinal sites was probably due to the limited number of pathologically positive lymph nodes. This could be of great importance in patients with suspected locally advanced ESCC, and crucial for the choice of the most appropriate therapeutic management.
Several drawbacks limited our study. We found the sensitivity of FDG PET/CT in detecting lymph node metastasis was comparable to that reported by other groups, though with some inconsistencies. One possible cause may be the inclusion criteria of our study: only patients who underwent esophagectomy with lymph node dissection were included. Patients with advanced disease, who underwent palliative treatment, preoperative chemotherapy, or radiation therapy, were excluded. Therefore, our study included more cases of early-stage disease, de facto lowering the prevalence of metastatic regional lymph nodes.
Conclusions
Integrated PET/CT is a useful tool for evaluating regional lymph node status preoperatively in ESCC located within the thoracic cavity. As noted in previous studies, the SUVmax of individual lymph nodes predicts malignancy. Definitive biopsies are still the cornerstone in confirming cancer irrespective of the SUVmax. Nevertheless, when an SUVmax cutoff value of 4.1 is used instead of the traditional value of 2.5, the accuracy of FDG PET/CT in assessing nodal metastasis can be significantly improved.
References
- 1.Tangoku A, Yamamoto Y, Furukita Y. The new era of staging as a key for an appropriate treatment for esophageal cancer. Ann Thorac Cardiovasc Surg. 2012;18:190–199. doi: 10.5761/atcs.ra.12.01926. [DOI] [PubMed] [Google Scholar]
- 2.Buenaventura P, Luketich JD. Surgical staging of esophageal cancer. Chest Surg Clin N Am. 2000;10:487–497. [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer network Inc (2012) NCCN practice guidelines in oncology. Esophageal cancer. Version 2. 2012. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp [accessed on November 8, 2012]
- 4.Erasmus JJ, McAdams HP, Patz EF, Jr, et al. Thoracic FDG PET: state of the art. Radiographics. 1998;18:5–20. doi: 10.1148/radiographics.18.1.9460106. [DOI] [PubMed] [Google Scholar]
- 5.Kostakoglu L, Agress H, Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics. 2003;23:315–340. doi: 10.1148/rg.232025705. [DOI] [PubMed] [Google Scholar]
- 6.Pan LL, Gu P, Huang G, et al. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2009;21:1008–1015. doi: 10.1097/MEG.0b013e328323d6fa. [DOI] [PubMed] [Google Scholar]
- 7.Rizk N, Downey RJ, Akhurst T, et al. Preoperative 18[F]-fluorodeoxyglucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg. 2006;81:1076–081. doi: 10.1016/j.athoracsur.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 8.Omloo JM, Sloof GW, Boellaard R, et al. Importance of fluorodeoxyglucose-positron emission tomography (FDG-PET) and endoscopic ultrasonography parameters in predicting survival following surgery for esophageal cancer. Endoscopy. 2008;40:464–471. doi: 10.1055/s-2008-1077302. [DOI] [PubMed] [Google Scholar]
- 9.Perigaud C, Bridji B, Roussel JC, et al. Prospective preoperative mediastinal lymph node staging by integrated positron emission tomography-computerised tomography in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg. 2009;36:731–736. doi: 10.1016/j.ejcts.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Kumar A, Dutta R, Kannan U, et al. Evaluation of mediastinal lymph nodes using 18F-FDG PET/CT scan and its histopathologic correlation. Ann Thorac Med. 2011;6:11–16. doi: 10.4103/1817-1737.74270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim K, Park SJ, Kim BT, et al. Evaluation of lymph node metastases in squamous cell carcinoma of the esophagus with positron emission tomography. Ann Thorac Surg. 2001;71:290–294. doi: 10.1016/s0003-4975(00)02221-9. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Fidias P, Hayman LA, et al. Patterns of lymphadenopathy in thoracic malignancies. Radiographics. 2004;24:419–434. doi: 10.1148/rg.242035075. [DOI] [PubMed] [Google Scholar]
- 13.Lerut T, Coosemans W, Decker G, et al. Cancer of the esophagus and the gastroesophageal junction: potentially curative therapies. Surg Oncol. 2001;10:113–122. doi: 10.1016/s0960-7404(01)00027-5. [DOI] [PubMed] [Google Scholar]
- 14.Lerut T, Flamen P, Ectors N, et al. Histopathologic validation of lymph node staging with FDG-PET scan in cancer of the esophagus and gastroesophageal junction: a prospective study based on primary surgery with extensive lymphadenectomy. Ann Surg. 2000;232:743–752. doi: 10.1097/00000658-200012000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JY, Lee KH, Shim YM, et al. Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med. 2000;41:808–815. [PubMed] [Google Scholar]
- 16.Sihvo EI, Rasanen JV, Knuuti MJ, et al. Adenocarcinoma of the esophagus and the esophagogastric junction: positron emission tomography improves staging and prediction of survival in distant but not in locoregional disease. J Gastrointest Surg. 2004;8:988–996. doi: 10.1016/j.gassur.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Kneist W, Schreckenberger M, Bartenstein P, et al. Prospective evaluation of positron emission tomography in the preoperative staging of esophageal carcinoma. Arch Surg. 2004;139:1043–1049. doi: 10.1001/archsurg.139.10.1043. [DOI] [PubMed] [Google Scholar]
- 18.Yuan S, Yu Y, Chao KS, et al. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med. 2006;47:1255–1259. [PubMed] [Google Scholar]
- 19.Kato H, Kuwano H, Nakajima M, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer. 2002;94:921–928. [PubMed] [Google Scholar]
- 20.Manabe O, Hattori N, Hirata K, et al. Diagnostic accuracy of lymph node metastasis depends on metabolic activity of the primary lesion in thoracic squamous esophageal cancer. J Nucl Med. 2013;54:1–7. doi: 10.2967/jnumed.112.110304. [DOI] [PubMed] [Google Scholar]


