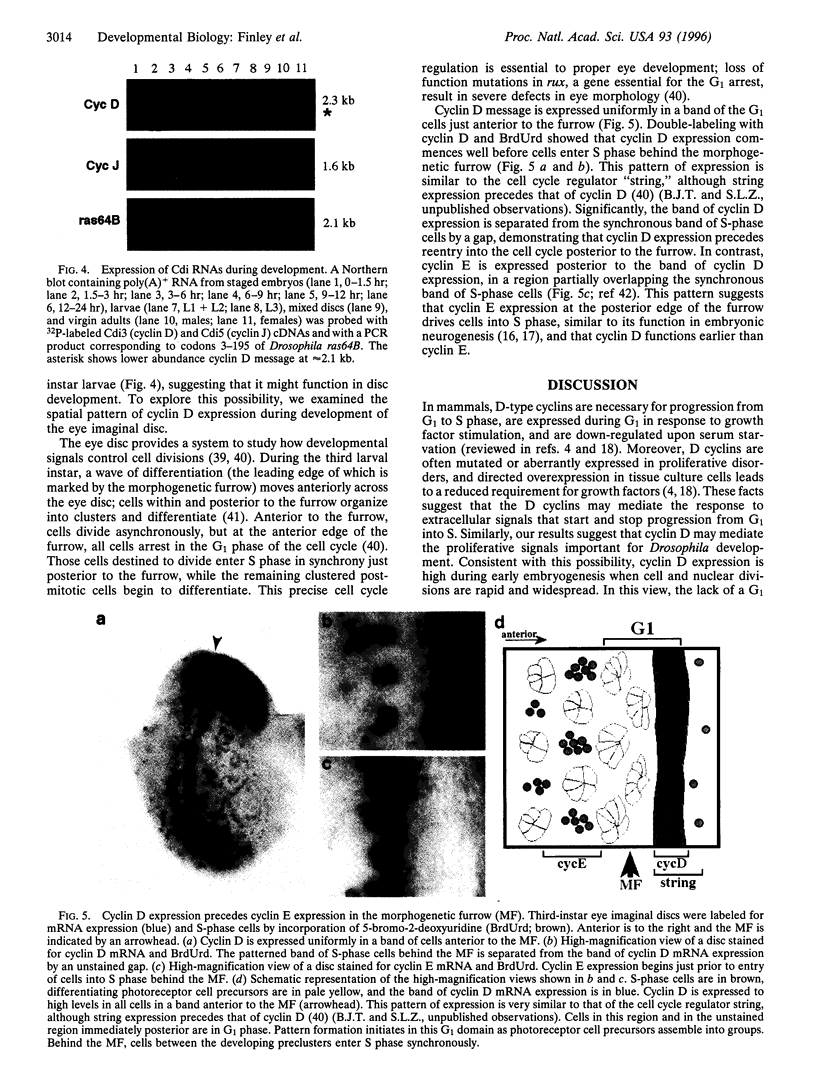
Abstract
During Drosophila development, nuclear and cell divisions are coordinated in response to developmental signals. In yeast and mammalian cells, signals that control cell division regulate the activity of cyclin-dependent kinases (Cdks) through proteins such as cyclins that interact with the Cdks. Here we describe two Drosophila cyclins identified from a set of Cdk-interacting proteins. One, cyclin J, is of a distinctive sequence type; its exclusive maternal expression pattern suggests that it may regulate oogenesis or the early nuclear divisions of embryogenesis. The other belongs to the D class of cyclins, previously identified in mammalian cells. We show that Drosophila cyclin D is expressed in early embryos and in imaginal disc cells in a pattern that anticipates cell divisions. Expression in the developing eye disc at the anterior edge of the morphogenetic furrow suggests that cyclin D acts early, prior to cyclin E, in inducing G1-arrested cells to enter S phase. Our results also suggest that, although cyclin D may be necessary, its expression alone is not sufficient to initiate the events leading to S phase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alphey L., Jimenez J., White-Cooper H., Dawson I., Nurse P., Glover D. M. twine, a cdc25 homolog that functions in the male and female germline of Drosophila. Cell. 1992 Jun 12;69(6):977–988. doi: 10.1016/0092-8674(92)90616-k. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987 Nov;6(11):3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtot C., Fankhauser C., Simanis V., Lehner C. F. The Drosophila cdc25 homolog twine is required for meiosis. Development. 1992 Oct;116(2):405–416. doi: 10.1242/dev.116.2.405. [DOI] [PubMed] [Google Scholar]
- Draetta G. Cell cycle control in eukaryotes: molecular mechanisms of cdc2 activation. Trends Biochem Sci. 1990 Oct;15(10):378–383. doi: 10.1016/0968-0004(90)90235-4. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. The three postblastoderm cell cycles of Drosophila embryogenesis are regulated in G2 by string. Cell. 1990 Aug 10;62(3):469–480. doi: 10.1016/0092-8674(90)90012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estojak J., Brent R., Golemis E. A. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995 Oct;15(10):5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley R. L., Jr, Brent R. Interaction mating reveals binary and ternary connections between Drosophila cell cycle regulators. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12980–12984. doi: 10.1073/pnas.91.26.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Glover D. M. Mitosis in the Drosophila embryo--in and out of control. Trends Genet. 1991 Apr;7(4):125–132. doi: 10.1016/0168-9525(91)90457-2. [DOI] [PubMed] [Google Scholar]
- Gyuris J., Golemis E., Chertkov H., Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993 Nov 19;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Mendenhall M. D., Reed S. I. The Saccharomyces cerevisiae CKS1 gene, a homolog of the Schizosaccharomyces pombe suc1+ gene, encodes a subunit of the Cdc28 protein kinase complex. Mol Cell Biol. 1989 May;9(5):2034–2041. doi: 10.1128/mcb.9.5.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Jimenez J., Alphey L., Nurse P., Glover D. M. Complementation of fission yeast cdc2ts and cdc25ts mutants identifies two cell cycle genes from Drosophila: a cdc2 homologue and string. EMBO J. 1990 Nov;9(11):3565–3571. doi: 10.1002/j.1460-2075.1990.tb07567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J. A., Lehner C. F. Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO J. 1993 Jan;12(1):65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J. A., Sauer K., Jones L., Richardson H., Saint R., Lehner C. F. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994 Apr 8;77(1):107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Lahue E. E., Smith A. V., Orr-Weaver T. L. A novel cyclin gene from Drosophila complements CLN function in yeast. Genes Dev. 1991 Dec;5(12A):2166–2175. doi: 10.1101/gad.5.12a.2166. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. Drosophila cdc2 homologs: a functional homolog is coexpressed with a cognate variant. EMBO J. 1990 Nov;9(11):3573–3581. doi: 10.1002/j.1460-2075.1990.tb07568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Léopold P., O'Farrell P. H. An evolutionarily conserved cyclin homolog from Drosophila rescues yeast deficient in G1 cyclins. Cell. 1991 Sep 20;66(6):1207–1216. doi: 10.1016/0092-8674(91)90043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Ordering S phase and M phase in the cell cycle. Cell. 1994 Nov 18;79(4):547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L. Developmental modification of the Drosophila cell cycle. Trends Genet. 1994 Sep;10(9):321–327. doi: 10.1016/0168-9525(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Paroush Z., Finley R. L., Jr, Kidd T., Wainwright S. M., Ingham P. W., Brent R., Ish-Horowicz D. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell. 1994 Dec 2;79(5):805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976 Oct 15;53(2):217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Richardson H. E., O'Keefe L. V., Reed S. I., Saint R. A Drosophila G1-specific cyclin E homolog exhibits different modes of expression during embryogenesis. Development. 1993 Nov;119(3):673–690. doi: 10.1242/dev.119.3.673. [DOI] [PubMed] [Google Scholar]
- Richardson H. E., Stueland C. S., Thomas J., Russell P., Reed S. I. Human cDNAs encoding homologs of the small p34Cdc28/Cdc2-associated protein of Saccharomyces cerevisiae and Schizosaccharomyces pombe. Genes Dev. 1990 Aug;4(8):1332–1344. doi: 10.1101/gad.4.8.1332. [DOI] [PubMed] [Google Scholar]
- Richardson H., O'Keefe L. V., Marty T., Saint R. Ectopic cyclin E expression induces premature entry into S phase and disrupts pattern formation in the Drosophila eye imaginal disc. Development. 1995 Oct;121(10):3371–3379. doi: 10.1242/dev.121.10.3371. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Tautz D., Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989 Aug;98(2):81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Gunning D. A., Cho J., Zipursky L. Cell cycle progression in the developing Drosophila eye: roughex encodes a novel protein required for the establishment of G1. Cell. 1994 Jul 1;77(7):1003–1014. doi: 10.1016/0092-8674(94)90440-5. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Ready D. F. Neuronal differentiation in Drosophila ommatidium. Dev Biol. 1987 Apr;120(2):366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- Whitfield W. G., González C., Sánchez-Herrero E., Glover D. M. Transcripts of one of two Drosophila cyclin genes become localized in pole cells during embryogenesis. Nature. 1989 Mar 23;338(6213):337–340. doi: 10.1038/338337a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]