Abstract
Codeine is bioactivated to morphine, a strong opioid agonist, by the hepatic cytochrome P450 2D6 (CYP2D6); hence, the efficacy and safety of codeine are governed by CYP2D6 activity. Polymorphisms are a major cause of CYP2D6 variability. We summarize evidence from the literature supporting this association and provide therapeutic recommendations for codeine based on CYP2D6 genotype. This document is an update to the 2012 Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2D6 genotype and codeine therapy.
Focused Literature Review and Update
A systematic literature review focused on CYP2D6 and codeine use was conducted (Supplementary Data online). In addition to the information provided in the 2012 Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and codeine therapy,1 this document also addresses the recent US Food and Drug Administration (FDA) warning regarding codeine use in children following tonsillectomy with or without adenoidectomy, pediatric considerations, and additional considerations for use of alternative opioids metabolized by cytochrome P450 2D6 (CYP2D6). Furthermore, the accompanying Supplementary Data online has been updated.
Gene: CYP2D6
Background
More than 100 CYP2D6 alleles have been defined by the Cytochrome P450 Nomenclature Committee at http://www.cypalleles.ki.se. Clinical phenotype data are available for common alleles (Supplementary Tables S1–S5 online). However, many alleles have not been evaluated in clinical trials, and their clinical phenotypes are predicted based on the expected functional impact of their defining genetic variation or are extrapolated based on in vitro functional studies using different substrates.
Genetic test interpretation
Most clinical laboratories report CYP2D6 genotype using the star (*) allele nomenclature and may provide interpretation of the patient's predicted metabolizer phenotype. Single-nucleotide polymorphisms (SNPs) and other sequence variations, including insertions and deletions, are determined by genetic laboratory tests. The reference SNP number (rs number) for a SNP defines the specific genomic nucleotide alteration. Each star (*) allele (or haplotype) is defined by the presence of a specific combination of SNPs and/or other sequence alterations within the CYP2D6 gene locus. The key alleles are shown in Supplementary Table S1 online, and the key allele-defining SNPs and their respective impacts on CYP2D6 enzyme function are provided in Supplementary Table S2 online. Genetic results are reported as a diplotype, which includes one maternal and one paternal allele (e.g., CYP2D6*1/*4). In some cases, patients have more than two copies of the CYP2D6 gene; up to 13 gene copies have been described.2 Those alleles are denoted by an “xN” following the allele designation, e.g., CYP2D6*2x2 (duplication; see Supplementary Data online for details). Additional details about allele nomenclature and definitions can be found at http://www.cypalleles.ki.se/cyp2d6.htm, and information regarding the effects of allelic variation on CYP2D6 substrates can be found at the Pharmacogenomics Knowledgebase (http://www.pharmgkb.org/search/annotatedGene/cyp2d6/haplotype.jsp). CYP2D6 allele frequencies differ substantially among racial and ethnic groups. Supplementary Table S1 online summarizes the most important frequencies for various ethnic groups.
The combination of alleles is used to determine a patient's diplotype. CYP2D6 alleles are characterized as wild-type (normal function), reduced-function, or nonfunctional alleles based on the expected activity of the enzyme that they encode. Each allele is assigned an activity value, i.e., 0 for nonfunctional, 0.5 for reduced-function, or 1.0 for fully functional forms.3 Supplementary Tables S3 and S4 online describe the activity score values assigned to selected alleles and example diplotypes, respectively. If multiple copies of the CYP2D6 gene are detected, the activity score is multiplied by the number of copies of each allele present. The total CYP2D6 activity score is the sum of the values assigned to each allele, which typically ranges from 0 to 3.0 but may exceed 3.0 in rare cases.3,4
The CYP2D6 activity score relates to the phenotype classification system as follows (Table 1): patients with an activity score of 0 are poor metabolizers, those with a score of 0.5 are considered intermediate metabolizers, and those with a score of 1.0, 1.5, or 2.0 represent a range of extensive metabolizers. Patients with a score > 2.0 are classified as ultrarapid metabolizers. The extensive metabolizer phenotype represents normal (wild-type) enzyme activity.3,4,5 The incidence of poor and ultrarapid metabolizers varies greatly among populations (0–10% and 0–29%, respectively). Predicted phenotypes of common diplotypes are summarized in Supplementary Table S5 online.
Table 1. Assignment of likely codeine metabolism phenotypes based on cytochrome P450 2D6 (CYP2D6) diplotypes.
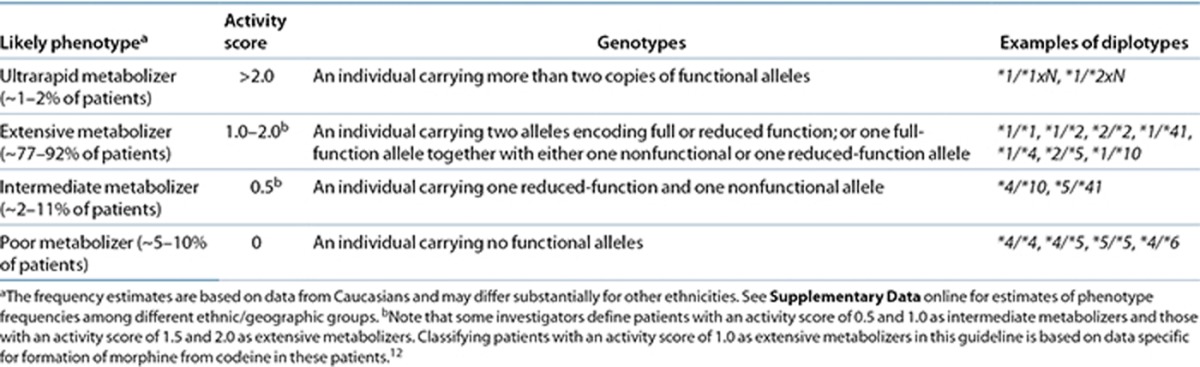
Subjects with genotypes giving rise to an activity score of 1.0, which can be due to a diplotype containing one functional and one nonfunctional allele or containing two reduced-function alleles, are classified by some investigators as intermediate metabolizers. Regardless of the term used to describe such individuals (extensive or intermediate metabolizer), their CYP2D6 activity is lower as compared with subjects having two fully functional alleles (with an activity score of 2.0) and higher as compared with subjects having one reduced and one nonfunctional allele (with an activity score of 0.5). Because there is no gold standard for phenotype classification, genotypes that result in CYP2D6 activity scores of 1.0, 1.5, and 2.0 are grouped together as extensive metabolizers in this guideline based on data specific for codeine metabolism (Supplementary Table S6 online).
Reference laboratories providing clinical CYP2D6 genotyping may use varying methods to assign phenotypes. Therefore, it is advisable to note a patient's CYP2D6 diplotype and to calculate an activity score before making therapeutic decisions about codeine therapy.
Available genetic test options
A number of academic and commercial clinical laboratories offer genetic testing for CYP2D6. Specific information on several of these laboratories, assays, and links to the product labels can be found at the Pharmacogenomics Knowledgebase (http://www.pharmgkb.org/resources/forScientificUsers/pharmacogenomic_tests.jsp) and the Genetic Testing Registry of the National Institutes of Health (http://www.ncbi.nlm.nih.gov/gtr/tests/?term=cyp2d6).
Incidental findings
Currently, there are no diseases or conditions that are known to be linked to variation in the CYP2D6 gene independently of drug metabolism and response. Although there are some isolated reports of CYP2D6 genetic effects on phenotypes outside of drug response,6,7 these findings have not been consistently reproduced in other studies.
Other considerations
Modification of the predicted phenotype by drug interactions. CYP2D6 metabolizer phenotype may be altered in a patient who is taking drugs that inhibit CYP2D6 activity. A list of CYP2D6 inhibitors can be found at http://medicine.iupui.edu/clinpharm/ddis/table.aspx. For this purpose, drugs are classified as strong, moderate, or weak inhibitors based on the FDA guidance on drug interaction studies (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ DrugInteractionsLabeling/ucm093664.htm#classInhibit). Borges et al. demonstrated that incorporating the impact of drug interactions with CYP2D6 inhibitors improves the ability of the activity score to predict the extent of tamoxifen metabolism by CYP2D6.4 For patients on strong inhibitors, the CYP2D6 activity score is adjusted to 0, and the predicted phenotype is a poor metabolizer. For patients who are on weak or moderate CYP2D6 inhibitors, the activity score is multiplied by 0.5 and then converted to the predicted phenotype.4 CYP2D6 does not appear to be induced by any medications; however, there are limited data showing that CYP2D6 enzyme activity increases during pregnancy (see Supplementary Data online for further discussion).
Drug: Codeine
Background
Codeine is an opioid analgesic indicated for the relief of mild to moderately severe pain. The analgesic properties of codeine stem from its conversion to morphine and morphine-6-glucuronide because codeine has a 200-fold weaker affinity for µ-opioid receptors than does morphine.8,9 Both codeine and morphine also have antitussive properties.
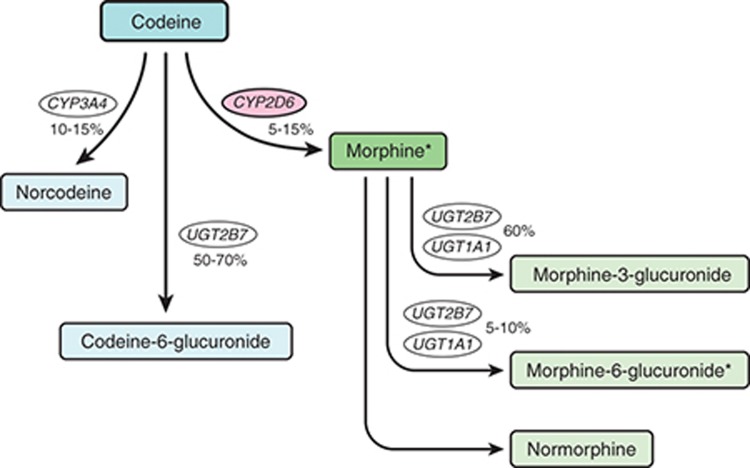
O-Demethylation of codeine into morphine by CYP2D6 represents a minor pathway in extensive metabolizers, accounting for 5–10% of codeine clearance in such individuals, but is essential for its opioid activity (Figure 1). The percentage of codeine converted to morphine can be much higher in ultrarapid metabolizers and can be affected by drug interactions.10 Morphine is further glucuronidated to morphine-3-glucuronide and morphine-6-glucuronide. Morphine-6-glucuronide is known to have analgesic activity in humans, whereas morphine-3-glucuronide is generally not considered to possess analgesic properties. About 80% of an administered dose of codeine is converted to inactive metabolites by (i) glucuronidation to codeine-6-glucuronide via uridine 5′-diphosphate glucuronosyltransferase-2B7 and (ii) N-demethylation to norcodeine via CYP3A4. The analgesic activity of codeine-6-glucuronide in humans is unknown, and norcodeine is thought to have no analgesic properties.9 Common adverse reactions to codeine include nausea, vomiting, drowsiness, light-headedness, dizziness, sedation, shortness of breath, constipation, and itching. Serious adverse reactions include respiratory depression and, rarely, circulatory depression, respiratory arrest, shock, and cardiac arrest.
Figure 1.
Codeine metabolism pathway in an individual with cytochrome P450 2D6 (CYP2D6) extensive metabolism. Asterisks (*) denote active metabolites.
The association of CYP2D6 metabolizer phenotype with the formation of morphine from codeine is well defined. Pharmacokinetic and pharmacodynamic studies show a decrease in morphine levels and a decrease in analgesia in poor metabolizers receiving codeine as compared with extensive metabolizers.11,12 A decreased incidence of gastrointestinal side effects (i.e., constipation) was reported in poor vs. extensive metabolizers,13 whereas a later study by the same group of investigators found that central side effects (e.g., sedation, nausea, and dry mouth) did not differ between poor and extensive metabolizers.11
By contrast, pharmacokinetic studies show increased conversion of codeine to morphine in CYP2D6 ultrarapid vs. extensive metabolizers,14 which can result in toxic systemic concentrations of morphine10 even at low codeine doses. However, it should be noted that there is a large amount of variability within the patients genotyped as extensive metabolizers,14 and it is possible that some of these subjects may develop symptoms similar to those of patients genotyped as ultrarapid metabolizers.15 The genomic or environmental mechanisms causing considerable variation among individuals with the same diplotype are unknown. Case reports detail the occurrence of severe or life-threatening side effects following standard doses of codeine in ultrarapid metabolizers.10,16,17
This guideline recommends using alternative analgesics to codeine in patients who are CYP2D6 poor or ultrarapid metabolizers. It is important to recognize that in addition to codeine, several other opioids are metabolized, at least in part, by CYP2D6. The opioids tramadol, hydrocodone, and oxycodone are O-demethylated by CYP2D6 to O-desmethyltramadol, hydromorphone, and oxymorphone, respectively.18,19
Tramadol. Tramadol, in its available racemic form, is extensively metabolized via a number of pathways, including CYP2D6-mediated oxidation to O-desmethyltramadol, which has a 200-fold greater affinity for µ-opioid receptors as compared with the parent drug.8,20 Thus, (+)-O-desmethyltramadol is principally responsible for opioid receptor–mediated analgesia, whereas (+)- and (−)-tramadol contribute to analgesia by inhibiting reuptake of the neurotransmitters serotonin and noradrenaline. CYP2D6 poor metabolizers have been shown to have much lower median plasma areas under the concentration–time curve for the active metabolite after a dose of tramadol as compared with extensive metabolizers.18 In addition, several prospective clinical trials have shown that, as compared with CYP2D6 extensive metabolizers, poor metabolizers more often fail to exhibit analgesia in response to tramadol.18,20,21 Pharmacokinetic studies in ultrarapid metabolizers showed higher peak plasma concentrations of (+)-O-desmethyltramadol after a dose of tramadol, in addition to greater analgesia, stronger miosis, and higher incidence of nausea as compared with extensive metabolizers.22 On the basis of this evidence, it is likely that tramadol may have reduced clinical efficacy in CYP2D6 poor metabolizers.
Hydrocodone. Hydrocodone is biotransformed by CYP2D6 into hydromorphone, which has a 10- to 33-fold greater affinity for μ-opioid receptors as compared with the parent drug. Poor metabolizers as compared with extensive metabolizers have lower peak concentrations of hydromorphone after a dose of hydrocodone;23 however, CYP2D6 metabolizer status does not appear to affect response to hydrocodone.23,24 Currently, there is no information on the pharmacokinetics of hydrocodone in CYP2D6 ultrarapid metabolizers. Thus, there is insufficient evidence to conclude whether poor metabolizers can be expected to have decreased analgesia or whether ultrarapid metabolizers have an increased risk of toxicity with normal doses of hydrocodone.
Oxycodone. Approximately 11% of an oxycodone dose is O-demethylated by CYP2D6 to the minor metabolite oxymorphone, which has a 40-fold higher affinity and 8-fold higher potency for µ-opioid receptors as compared with the parent drug.8,25 CYP2D6 poor metabolizers have been shown to have lower peak concentrations of oxymorphone after a dose of oxycodone as compared with extensive metabolizers.26,27 However, conflicting data exist on the association of CYP2D6 metabolizer phenotype with the analgesic effect and toxicity of oxycodone in prospective clinical studies. Differential analgesic response to experimental pain was observed between extensive metabolizers and poor metabolizers, as well as between ultrarapid metabolizers and extensive and poor metabolizers in two studies in healthy volunteers.28,29 However, clinical studies in postoperative patients and in cancer patients failed to demonstrate a significant difference in analgesia or side effects of oxycodone across CYP2D6 phenotypes.26,27 Physiological alterations (e.g., miosis) after dosing with oxycodone correlate best with exposure to the parent compound.19 Due to these conflicting data, it is difficult to conclude whether CYP2D6 metabolizer phenotype affects oxycodone analgesia or risk of toxicity.
The differences in reported associations of CYP2D6 phenotype with hydrocodone and oxycodone analgesia as compared with that of codeine may be due to differing relative roles of the parent drug and the circulating metabolites in analgesia among these CYP2D6 substrates.19
Linking genetic variability to variability in drug-related phenotypes
There is substantial evidence linking CYP2D6 genotype to variability in codeine efficacy and toxicity (Supplementary Table S6 online). Decreased codeine analgesia has been observed in poor metabolizers, whereas severe or life-threatening toxicity following normal doses of codeine has been documented in ultrarapid metabolizers. This body of evidence, rather than randomized clinical trials involving pharmacogenetic testing, provides the basis for the therapeutic recommendations in Table 2.
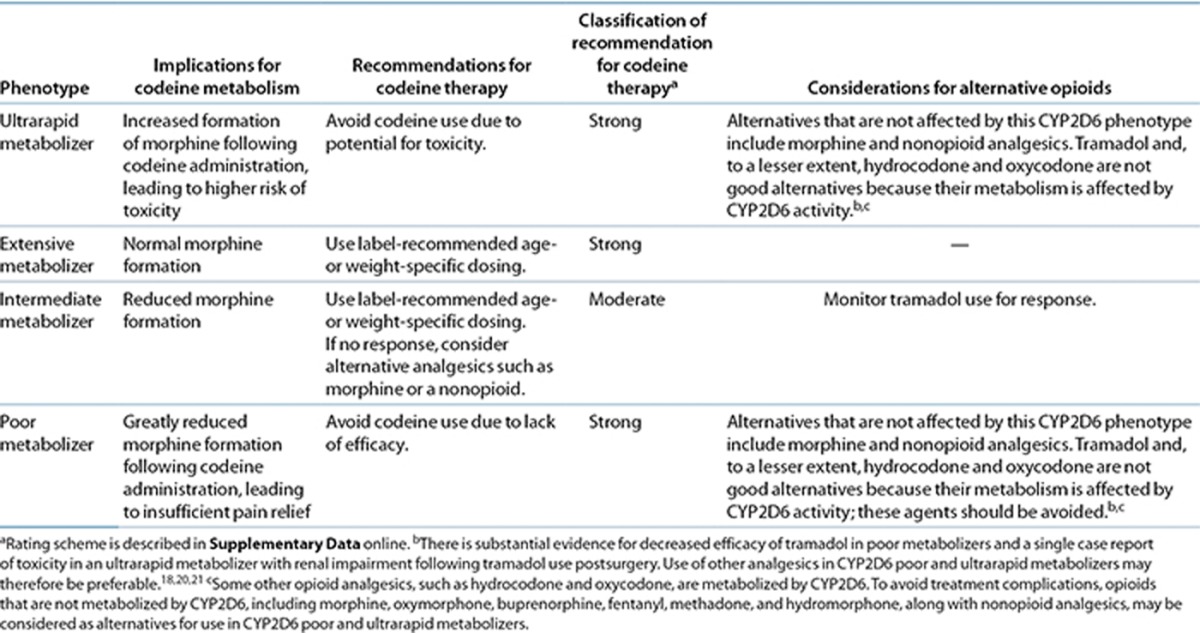
Table 2. Codeine therapy recommendations based on cytochrome P450 2D6 (CYP2D6) phenotype.
Therapeutic recommendation
Table 2 summarizes the therapeutic recommendations for codeine based on CYP2D6 phenotype. A standard starting dose of codeine, as recommended in the product label, is warranted in patients with an extensive metabolizer phenotype (i.e., a CYP2D6 activity score of 1.0–2.0). Likewise, a standard starting dose of codeine is warranted in patients with an intermediate metabolizer phenotype (i.e., activity score of 0.5); these patients should be monitored closely for less-than-optimal response and should be offered an alternative analgesic if warranted. If the CYP2D6 substrate tramadol is selected as alternative therapy in intermediate metabolizers, therapy should be monitored closely due to the possibility of poor response.
If clinical genotyping identifies a patient as a CYP2D6 poor metabolizer (i.e., activity score of 0), current evidence supports the avoidance of codeine and the use of an alternative analgesic due to the possibility of lack of effect. Use of an analgesic other than the CYP2D6 substrates tramadol, hydrocodone, or oxycodone in poor metabolizers may be preferable. There is insufficient evidence in the literature to recommend a higher dose of codeine in poor metabolizers, especially considering the evidence that select adverse effects do not differ between poor and extensive metabolizers.11 In a patient identified as a CYP2D6 ultrarapid metabolizer (i.e., activity score of >2.0), the choice of another analgesic should be made to avoid the risk of severe toxicity with a “normal” dose of codeine.
To avoid treatment complications, opioids that are not metabolized by CYP2D6, including morphine, oxymorphone, buprenorphine, fentanyl, methadone, and hydromorphone,30 along with nonopioid analgesics, may be considered as alternatives for use in CYP2D6 poor metabolizers and ultrarapid metabolizers based on the type, severity, and chronicity of the pain being treated.
Other considerations
Other genes affecting codeine metabolism and response. Other genes affect the metabolism and response to codeine. See Supplementary Data online for more information.
Pediatrics. Functional CYP2D6 activity is not appreciably expressed in fetal liver but increases rapidly after birth.31 These in vitro data, together with in vivo data obtained from a longitudinal phenotyping study conducted in the first year of life,32 reveal considerable interindividual variability in CYP2D6 activity within the first 2–4 weeks of life. In the neonatal setting, both ontogeny and genetic variation contribute to interindividual variability in the disposition of CYP2D6 substrates and are consistent with functional CYP2D6 activity being acquired concurrently with the maturation of other systems, such as renal function.33 Overall, available data are consistent with genetic variation being more important than ontogeny as a determinant of variability in CYP2D6 activity beyond the first month of postnatal life. Therefore, CYP2D6 genotype is expected to be equally reliable for inferring phenotype from genotype in children as in adults. Codeine is not recommended in children less than 2 years of age but presumably would carry additional dangers in neonates and young children who are ultrarapid metabolizers.17
Breastfed infants. Codeine and its metabolites, including morphine, are secreted into human breast milk, but the amount is typically low and dose dependent. However, breastfeeding women with an ultrarapid metabolizer phenotype may achieve high serum concentrations of morphine on standard codeine therapy.34 This may lead to high levels of morphine in breast milk and dangerously high morphine exposure in their breastfed infants.35 Notably, a fatal opioid poisoning in a breastfed neonate from an ultrarapid metabolizer mother receiving codeine has been described.36 Case reports such as this prompted the FDA and regulatory agencies in the United Kingdom and Canada to change the codeine product label to address the increased risk of morphine overdose in breastfed infants whose mothers are taking codeine and are ultrarapid metabolizers. However, a recent study demonstrated that the use of postpartum safety guidelines can improve the safety of codeine exposure in breastfed infants regardless of their CYP2D6 genotype.37 Nevertheless, caution should be used when prescribing codeine to a breastfeeding woman with an ultrarapid metabolizer status.
Use of codeine after tonsillectomy with or without adenoidectomy. In August 2012, the FDA38 distributed a safety announcement that warned about codeine use in children, particularly following tonsillectomy with or without adenoidectomy for obstructive sleep apnea. The announcement followed reports of codeine-related deaths15,17 and serious adverse drug reactions39 after tonsillectomy in young children and specified that the risk for breathing problems and death may be increased in children who are ultrarapid metabolizers. In February 2013, the FDA announced its strongest and new black box warning against codeine use to manage postoperative pain in children following tonsillectomy with or without adenoidectomy.40 This warning was in response to further FDA review of the codeine-related deaths and serious adverse drug reactions. The FDA warning is applicable to all children undergoing tonsillectomy with or without adenoidectomy irrespective of their obstructive sleep apnea status or CYP2D6 genotype/phenotype.40
Potential Benefits and Risks for the Patient
The potential benefit of CYP2D6 genotype testing is that patients with genotypes that confer a higher risk of ineffective analgesia or of an adverse event may be identified and an alternative analgesic may be administered. CYP2D6 genotyping is reliable when performed in qualified laboratories. However, as with any laboratory test, a possible risk to the patient is an error in genotyping that could have long-term adverse health implications for the patient.
Caveats: Appropriate Use and/or Potential Misuse of Genetic Tests
Rare CYP2D6 variants may not be included in the genotype test used, and patients with rare variants may be assigned a “wild-type” (CYP2D6*1) genotype by default. Thus, an assigned “wild-type” allele may, in rare cases, harbor a loss-of-function variant resulting in inadequate pain response to codeine. Like all diagnostic tests, that for CYP2D6 genotype is one of multiple pieces of information that clinicians should consider in guiding their therapeutic choice for each patient. Furthermore, there are several other factors that cause potential uncertainty in the genotyping results and phenotype predictions. These are discussed in detail in the Supplementary Data online.
Disclaimer
Clinical Pharmacogenetics Implementation Consortium guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision making and to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variations among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health-care provider to determine the best course of treatment for a patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application to be made solely by the clinician and the patient. CPIC assumes no responsibility for any injury to persons or damage to persons or property arising out of or related to any use of CPIC's guidelines, or for any errors or omissions.
Acknowledgments
We acknowledge the critical input of M. Relling and members of the Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network, funded by the National Institutes of Health (NIH). This work was supported by NIH U01 GM092666, U01 GM061373, R01 GM088076, R01 HD058556, R01-DA14211, and R01 DA25931; Pharmacogenomics Knowledgebase (R24-GM61374); American Lebanese Syrian Associated Charities; and the Agency for Healthcare Research and Quality R01 HS19818-01. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
A.G. and K.R.C. each received compensation for services as an expert witness on a legal case involving codeine. The other authors declared no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Supplementary Material
References
- Crews K.R., et al. Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin. Pharmacol. Ther. 2012;91:321–326. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I., Lundqvist E., Bertilsson L., Dahl M.L., Sjöqvist F., Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc. Natl. Acad. Sci. USA. 1993;90:11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A., Simon S.D., Pearce R.E., Bradford L.D., Kennedy M.J., Leeder J.S. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther. 2008;83:234–242. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- Borges S., et al. Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J. Clin. Pharmacol. 2010;50:450–458. doi: 10.1177/0091270009359182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A., Gotschall R.R., Forbes N.S., Simon S.D., Kearns G.L., Leeder J.S. Optimization of cytochrome P4502D6 (CYP2D6) phenotype assignment using a genotyping algorithm based on allele frequency data. Pharmacogenetics. 1999;9:669–682. doi: 10.1097/01213011-199912000-00002. [DOI] [PubMed] [Google Scholar]
- Peñas-Lledó E.M., Blasco-Fontecilla H., Dorado P., Vaquero-Lorenzo C., Baca-García E., Llerena A. CYP2D6 and the severity of suicide attempts. Pharmacogenomics. 2012;13:179–184. doi: 10.2217/pgs.11.146. [DOI] [PubMed] [Google Scholar]
- Zhou L.P., Luan H., Dong X.H., Jin G.J., Man D.L., Shang H. Genetic variants of CYP2D6 gene and cancer risk: a HuGE systematic review and meta-analysis. Asian Pac. J. Cancer Prev. 2012;13:3165–3172. doi: 10.7314/apjcp.2012.13.7.3165. [DOI] [PubMed] [Google Scholar]
- Volpe D.A., et al. Uniform assessment and ranking of opioid µ receptor binding constants for selected opioid drugs. Regul. Toxicol. Pharmacol. 2011;59:385–390. doi: 10.1016/j.yrtph.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Thorn C.F., Klein T.E., Altman R.B. Codeine and morphine pathway. Pharmacogenet. Genomics. 2009;19:556–558. doi: 10.1097/FPC.0b013e32832e0eac. [DOI] [PubMed] [Google Scholar]
- Gasche Y., et al. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N. Engl. J. Med. 2004;351:2827–2831. doi: 10.1056/NEJMoa041888. [DOI] [PubMed] [Google Scholar]
- Eckhardt K., Li S., Ammon S., Schänzle G., Mikus G., Eichelbaum M. Same incidence of adverse drug events after codeine administration irrespective of the genetically determined differences in morphine formation. Pain. 1998;76:27–33. doi: 10.1016/s0304-3959(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Lötsch J., Rohrbacher M., Schmidt H., Doehring A., Brockmöller J., Geisslinger G. Can extremely low or high morphine formation from codeine be predicted prior to therapy initiation. Pain. 2009;144:119–124. doi: 10.1016/j.pain.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Mikus G., et al. Effect of codeine on gastrointestinal motility in relation to CYP2D6 phenotype. Clin. Pharmacol. Ther. 1997;61:459–466. doi: 10.1016/S0009-9236(97)90196-X. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J., et al. Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J. 2007;7:257–265. doi: 10.1038/sj.tpj.6500406. [DOI] [PubMed] [Google Scholar]
- Kelly L.E., et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129:e1343–e1347. doi: 10.1542/peds.2011-2538. [DOI] [PubMed] [Google Scholar]
- Dalén P., Frengell C., Dahl M.L., Sjöqvist F. Quick onset of severe abdominal pain after codeine in an ultrarapid metabolizer of debrisoquine. Ther. Drug Monit. 1997;19:543–544. doi: 10.1097/00007691-199710000-00011. [DOI] [PubMed] [Google Scholar]
- Ciszkowski C., Madadi P., Phillips M.S., Lauwers A.E., Koren G. Codeine, ultrarapid-metabolism genotype, and postoperative death. N. Engl. J. Med. 2009;361:827–828. doi: 10.1056/NEJMc0904266. [DOI] [PubMed] [Google Scholar]
- Stamer U.M., Musshoff F., Kobilay M., Madea B., Hoeft A., Stuber F. Concentrations of tramadol and O-desmethyltramadol enantiomers in different CYP2D6 genotypes. Clin. Pharmacol. Ther. 2007;82:41–47. doi: 10.1038/sj.clpt.6100152. [DOI] [PubMed] [Google Scholar]
- Lalovic B., Kharasch E., Hoffer C., Risler L., Liu-Chen L.Y., Shen D.D. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin. Pharmacol. Ther. 2006;79:461–479. doi: 10.1016/j.clpt.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Poulsen L., Arendt-Nielsen L., Brøsen K., Sindrup S.H. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin. Pharmacol. Ther. 1996;60:636–644. doi: 10.1016/S0009-9236(96)90211-8. [DOI] [PubMed] [Google Scholar]
- Stamer U.M., et al. Impact of CYP2D6 genotype on postoperative tramadol analgesia. Pain. 2003;105:231–238. doi: 10.1016/s0304-3959(03)00212-4. [DOI] [PubMed] [Google Scholar]
- Kirchheiner J., Keulen J.T., Bauer S., Roots I., Brockmöller J. Effects of the CYP2D6 gene duplication on the pharmacokinetics and pharmacodynamics of tramadol. J. Clin. Psychopharmacol. 2008;28:78–83. doi: 10.1097/JCP.0b013e318160f827. [DOI] [PubMed] [Google Scholar]
- Otton S.V., Schadel M., Cheung S.W., Kaplan H.L., Busto U.E., Sellers E.M. CYP2D6 phenotype determines the metabolic conversion of hydrocodone to hydromorphone. Clin. Pharmacol. Ther. 1993;54:463–472. doi: 10.1038/clpt.1993.177. [DOI] [PubMed] [Google Scholar]
- Kaplan H.L., et al. Inhibition of cytochrome P450 2D6 metabolism of hydrocodone to hydromorphone does not importantly affect abuse liability. J. Pharmacol. Exp. Ther. 1997;281:103–108. [PubMed] [Google Scholar]
- Davis M.P., Varga J., Dickerson D., Walsh D., LeGrand S.B., Lagman R. Normal-release and controlled-release oxycodone: pharmacokinetics, pharmacodynamics, and controversy. Support. Care Cancer. 2003;11:84–92. doi: 10.1007/s00520-002-0385-9. [DOI] [PubMed] [Google Scholar]
- Zwisler S.T., Enggaard T.P., Mikkelsen S., Brosen K., Sindrup S.H. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol. Scand. 2010;54:232–240. doi: 10.1111/j.1399-6576.2009.02104.x. [DOI] [PubMed] [Google Scholar]
- Andreassen T.N., et al. Do CYP2D6 genotypes reflect oxycodone requirements for cancer patients treated for cancer pain? A cross-sectional multicentre study. Eur. J. Clin. Pharmacol. 2012;68:55–64. doi: 10.1007/s00228-011-1093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwisler S.T., et al. The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism. Basic Clin. Pharmacol. Toxicol. 2009;104:335–344. doi: 10.1111/j.1742-7843.2009.00378.x. [DOI] [PubMed] [Google Scholar]
- Samer C.F., et al. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br. J. Pharmacol. 2010;160:919–930. doi: 10.1111/j.1476-5381.2010.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollason V., Samer C., Piguet V., Dayer P., Desmeules J. Pharmacogenetics of analgesics: toward the individualization of prescription. Pharmacogenomics. 2008;9:905–933. doi: 10.2217/14622416.9.7.905. [DOI] [PubMed] [Google Scholar]
- Stevens J.C., et al. Developmental changes in human liver CYP2D6 expression. Drug Metab. Dispos. 2008;36:1587–1593. doi: 10.1124/dmd.108.021873. [DOI] [PubMed] [Google Scholar]
- Blake M.J., et al. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin. Pharmacol. Ther. 2007;81:510–516. doi: 10.1038/sj.clpt.6100101. [DOI] [PubMed] [Google Scholar]
- Allegaert K., Rochette A., Veyckemans F. Developmental pharmacology of tramadol during infancy: ontogeny, pharmacogenetics and elimination clearance. Paediatr. Anaesth. 2011;21:266–273. doi: 10.1111/j.1460-9592.2010.03389.x. [DOI] [PubMed] [Google Scholar]
- Willmann S., Edginton A.N., Coboeken K., Ahr G., Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin. Pharmacol. Ther. 2009;86:634–643. doi: 10.1038/clpt.2009.151. [DOI] [PubMed] [Google Scholar]
- Madadi P., et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin. Pharmacol. Ther. 2009;85:31–35. doi: 10.1038/clpt.2008.157. [DOI] [PubMed] [Google Scholar]
- Koren G., Cairns J., Chitayat D., Gaedigk A., Leeder S.J. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368:704. doi: 10.1016/S0140-6736(06)69255-6. [DOI] [PubMed] [Google Scholar]
- Kelly L.E., et al. A clinical tool for reducing central nervous system depression among neonates exposed to codeine through breast milk. PLoS ONE. 2013;8:e70073. doi: 10.1371/journal.pone.0070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration. FDA Drug Safety Communication: Codeine use in certain children after tonsillectomy and/or adenoidectomy may lead to rare, but life-threatening adverse events or death . < http://www.fda.gov/Drugs/DrugSafety/ucm313631.htm > ( 2012
- Voronov P., Przybylo H.J., Jagannathan N. Apnea in a child after oral codeine: a genetic variant - an ultra-rapid metabolizer. Paediatr. Anaesth. 2007;17:684–687. doi: 10.1111/j.1460-9592.2006.02182.x. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Safety review update of codeine use in children; new Boxed Warning and Contraindication on use after tonsillectomy and/or adenoidectomy FDA Drug Safety Communication. < http://www.fda.gov/drugs/drugsafety/ucm339112.htm > ( 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.