Key Points
A novel inhibitor G-749 is very potent against FLT3 kinase mutants including D835Y and ITD/F691L that confer resistance to PKC412 and AC220.
G-749 shows several desirable characteristics to overcome other drug resistances conferred by patient plasma, FLT3 ligand, and stromal cells.
Abstract
Aberrant activations of Fms-like tyrosine receptor kinase (FLT) 3 are implicated in the pathogenesis of 20% to 30% of patients with acute myeloid leukemia (AML). G-749 is a novel FLT3 inhibitor that showed potent and sustained inhibition of the FLT3 wild type and mutants including FLT3-ITD, FLT3-D835Y, FLT3-ITD/N676D, and FLT3-ITD/F691L in cellular assays. G-749 retained its inhibitory potency in various drug-resistance milieus such as patient plasma, FLT3 ligand surge, and stromal protection. Furthermore, it displayed potent antileukemic activity in bone marrow blasts from AML patients regardless of FLT3 mutation status, including those with little or only minor responses to AC220 or PKC412. Oral administration of G-749 yielded complete tumor regression and increased life span in animal models. Thus, G-749 appears to be a promising next-generation drug candidate for the treatment of relapsed and refractory AML patients with various FLT3-ITD/FLT3-TKD mutants and further shows the ability to overcome drug resistance.
Introduction
Acute myeloid leukemia (AML) is an aggressive hematologic disorder in which the hematopoietic progenitor cells lose their ability to differentiate normally and continue to proliferate. Fms-like tyrosine receptor kinase (FLT) 3 plays an important role in normal hematopoiesis and leukemogenesis and is expressed in most AML blasts.1 In 20% to 25% of AML patients, the FLT3 gene acquires an internal tandem duplication in the juxtamembrane domain of FLT3 (FLT3-ITD), and this is associated with poor prognosis.2,3 FLT3 point mutations within the activation loop of the tyrosine kinase domain (FLT3-TKD) have also been detected in ∼7% of AML patients.4 FLT3-ITD or FLT3-TKD mutants undergo constitutive autophosphorylation of FLT3, causing aberrant signaling activation of several pathways such as Ras/mitogen-activated protein kinase, Janus kinase/signal transducer and activator of transcription (STAT) 5, and protein kinase B (AKT).5-7 Activated FLT3-kinase mutations eventually induce transformation and tumorigenesis in hematopoietic cells and suppress normal myeloid differentiation and therefore are attractive therapeutic targets to treat AML.
A number of receptor tyrosine kinase inhibitors (TKIs) targeting FLT3 have been developed and clinically examined in AML patients8,9: midostaurin (PKC412), the multiple kinase inhibitor in phase 3 trials in combinational chemotherapy10; sorafenib in phase 1 trials in relapsed/refractory AML patients11; and quizartinib (AC220), a selective FLT3 inhibitor in phase 2 trials.12 PKC412 induced a 92% complete remission (CR) rate in FLT3-mutated AML in combinational chemotherapy,13 sorafenib achieved a 23% CR in 65 FLT3-ITD AML patients after chemotherapy or allogeneic hematopoietic stem cell transplantation,11 and AC220 led to a 3% to 6% CR and 46% to 54% composite CR rate in 190 relapsed/refractory FLT3-ITD–positive AML patients.14,15
The development of drug resistance during the treatment of hematologic malignance has been a challenging issue for TKIs. Recent evidence suggests that the majority of patients treated with a single FLT3 inhibitor experienced only transient and partial response because of the development of drug resistance, which hinders treatment with FLT3-TKIs.16-18 Various factors including point mutations, plasma inhibitory activity (PIA), protective effect by bone marrow stromal cells, and high levels of FLT3 ligand (FL) have been identified to confer FLT3 inhibitor drug resistance. Point mutations within the kinase domain of FLT3-ITD especially at the positions of N676, F691, and D835 lead to substantial resistance to AC220 and PKC412.16,19 Additional resistance mutations to AC220 have also been described using in vitro models.20 Drug resistance comes from PIA where the inhibition of PKC412 and CEP701 against FLT3 autophosphorylation was dramatically decreased in human plasma milieu.21 The bone marrow microenvironment also contributes to the reduction of drug sensitivity in vivo. It was shown that bone marrow stromal cells support the survival of neighboring blast cells, resulting in the long-term survival and growth of leukemia cells.22-26 The increased secretion of FL after induction therapy of cytarabine was also known to attenuate efficacy of FLT3 inhibitors.27 Therefore, there is an unmet need for the next-generation FLT3 inhibitor to overcome drug resistance.
Here, we report that a novel inhibitor, G-749, with a unique kinase inhibition profile is very potent against FLT3 kinase and provides sustained inhibition of FLT3 phosphorylation and downstream effectors in FLT3-ITD–expressing cell lines. Using BaF3 model cells, we demonstrate that G-749 is highly potent against clinically known FLT3 mutants including gatekeeper and TKD that confer resistance to PKC412 and AC220. Notably, in comparison with PKC412 and AC220, G-749 shows several desirable characteristics to overcome other known drug resistances conferred by patient plasma, FL surge, and protection by stromal cells. Oral dosing of G-749 leads to complete tumor regression without relapse in the mouse xenograft model and increases survival in the bone marrow engraftment model. Furthermore, G-749 shows potent antileukemic activity in patient blasts harboring FLT3-ITD, FLT3-TKD, and FLT3-ITD/TKD mutations through inhibition of phosphorylated (p) FLT3 and p–extracellular signal-regulated kinase (ERK) 1/2, including those with little or only minor response to AC220 and/or PKC412. Thus, we believe that G-749 is a promising drug candidate with strong therapeutic potential to overcome drug resistance for AML treatment.
Methods
Kinase assay, cell culture, and FLT3 inhibitors
Biochemical assay for FLT3 wild type (WT) and FLT-D835Y was performed according to Perkin-Elmer time-resolved fluorescence resonance energy transfer protocol using suggested experimental materials. The detailed methods are described in the supplemental Methods (available on the Blood Web site). Human leukemia cell line Molm-14 was kindly provided by Dr S. Kang, Emory School of Medicine; MV4-11, RS4-11, K562, HEL, and HS-5 were obtained from ATCC. The 6 different BaF3 cell lines were kindly provided by Dr J. Cools.20,28 Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum or calf bovine serum, 2 mM of l-glutamine, and 1% of the antibiotics penicillin/streptomycin and cultured in a humidified atmosphere at 37°C with 5% CO2. G-749 was synthesized at Genosco (Cambridge, MA). Its synthetic process is described in supplemental Figure 1 and the supplemental Methods. The FLT3 inhibitors AC220 and PKC412 were purchased from LC Laboratories.
Cell proliferation, apoptosis, and fluorescence-activated cell sorter analysis
Cells were seeded at a density of 2 × 104 cells per well and treated with the indicated concentrations of test inhibitor for 72 hours at 37°C. The conditioned medium (CM) was prepared from HS-5 cell culture for 5 days under routine culture conditions, clarified by centrifugation, and used immediately. The CM was added to complete medium at a final concentration of 35%. In coculture experiments, 5 × 104 AML blast cells were plated in 24-well plates containing 1 × 104 HS-5 monolayers and then cultured for at least 48 hours before the exposure of inhibitors. Cell viability was determined by an ATPLite assay (Perkin-Elmer). Caspase-3/7 activity was measured by using the Caspase-Glo 3/7 assay (Promega).
For flow cytometry analysis experiments, MV4-11 cells were washed and resuspended in binding buffer containing fluorescein isothiocyanate–conjugated anti–annexin V antibody (Roche Diagnostics). Cells were stained with allophycocyanin-conjugated anti-CD45 antibody (Becton Dickinson) to exclude contamination of HS-5 stromal cells. CD45-positive cells were gated and analyzed by flow cytometry using a FACSCalibur (Becton Dickinson).
Western blot analysis and enzyme-linked immunosorbent assay
The p-FLT3 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Cell Signaling Technology and performed according to the manufacturer’s protocol. The detail protocol of western analysis is described in the supplemental Methods.
Patient samples
In accordance with the Declaration of Helsinki, bone marrow mononuclear cells from AML patients were collected with informed consent. Institutional Review Board approval was obtained from Chonnam National University Hospital (Gwangju, South Korea) and Seoul St. Mary’s Hospital (Seoul, South Korea) for this study. The FLT3 mutation status was determined by the diagnosis protocol of each hospital organization. Mutations of the FLT3 gene were examined as previously reported.4 Cryopreserved samples were thawed and incubated with culture medium enriched to 20% fetal bovine serum for 90 minutes before experimental procedures. Only samples with >70% viability were used for proceeding with tests. For blast viability, the blasts were treated with FLT3 inhibitors for 72 hours and supplemented with 50 ng/mL interleukin 3 and 50 ng/mL stem cell factor. Their viability was determined by an ATPLite assay.
In vivo mouse models
The subcutaneous MV4-11 tumor model and the bone marrow–engrafted model are described in the supplemental Methods. For statistical analysis, analysis of variance (ANOVA) was performed using Prism 5.0 to examine statistical differences (details in the supplemental Methods).
Results
Biochemical properties and selectivity of G-749
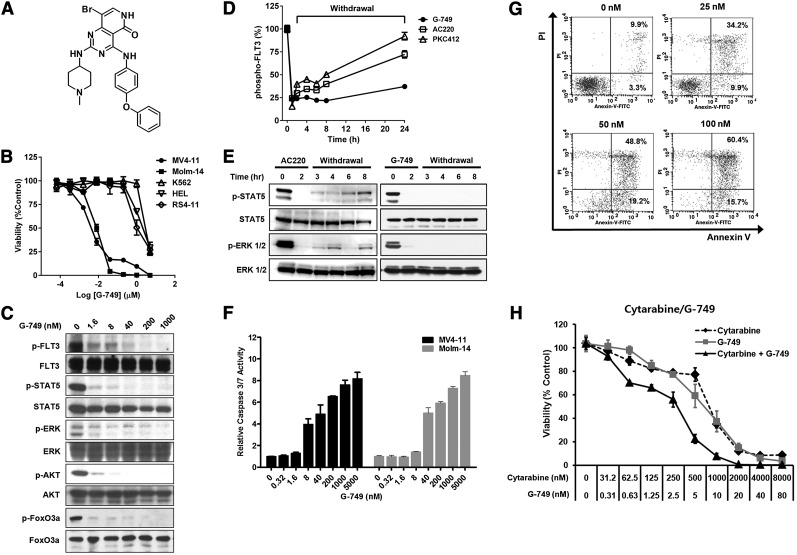
A novel small-molecule FLT3 kinase inhibitor, G-749, was designed and synthesized using a structure-based drug design approach (Figure 1A and supplemental Figure 1). It displayed potent inhibition of FLT3-WT and FLT3-D835Y mutants with biochemical IC50 values of 0.4 and 0.6 nM, respectively (Table 1). Even when the adenosine triphosphate (ATP) concentration was gradually increased up to ≥1 mM (supplemental Figure 2), G-749 still retained strong inhibitory activity with a low nanomolar IC50 value (0.4 to 2.7 nM). These data demonstrate that G-749 is ATP competitive and shows sustained binding to the ATP binding pocket. Potent inhibition of FLT3 was confirmed in leukemia cells where G-749 inhibited autophosphorylation of FLT3 with an IC50 value of ≤8 nM in FLT3-WT bearing RS4-11 and in FLT3-ITD harboring MV4-11 and Molm-14 cells (Figure 1C and supplemental Figure 3A-B).
Figure 1.
A novel small-molecule FLT3 inhibitor. (A) Chemical structure of G-749. (B) Five human leukemia cell lines were incubated with increasing concentrations of G-749 for 72 hours. Cell viability was determined using ATPLite assay. G-749 inhibited the proliferation of MV4-11 and Molm-14 with 50% inhibition concentration (IC50) values of 3.5 and 7.5 nM, respectively. The IC50 values were calculated using nonlinear regression. (C) Molm-14 cells were incubated with the indicated concentrations of G-749 for 2 hours. The phosphorylation levels of FLT3 (Tyr 591), STAT5 (Tyr 694), ERK1/2 (Tyr 204), AKT (Ser 473), and FoxO3a (Thr 32) were detected by western blot. Each total protein was used as a loading control. (D) MV4-11 cells were incubated with 100 nM inhibitors for 2 hours in 10% serum and then washed with fresh medium. The autophosphorylation levels of FLT3 were determined for 24 hours by p-FLT3 ELISA. (E) After washout as in panel D, the phosphorylation levels of STAT5 and ERK1/2 were monitored for 8 hours with treatment of G-749 or AC220. (F) After 18 hours, caspase-3/7 activities were measured in MV4-11 and Molm-14 cells treated with the indicated concentration of G-749. (G) MV4-11 cells were treated with the indicated concentrations of G-749 for 36 hours. Cells were stained with propidium iodide/annexin V and then analyzed by flow cytometry. The percentages of early and late apoptotic cells were indicated in the right lower and right upper quadrants, respectively. (H) MV4-11 cells were treated with cytarabine, G-749, or a combination of cytarabine and G-749 (ratio 100:1), and cell viability was measured. Error bars show standard deviation (SD).
Table 1.
Potency comparison of FLT3 inhibitors in biochemical and cellular assays
| Compound | FLT3 activity (IC50, nM) | Human leukemia cell line (IC50, nM) | BaF3 cells with FLT3 mutants (IC50, nM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | D835Y | MV4-11 | Molm-14 | WT | ITD | ITD/N676D | ITD/F691L | D835Y | D835Y/N676D | |
| G-749 | 0.4 ± 0.2 | 0.6 ± 0.2 | 3.5 ± 0.9 | 7.5 ± 0.3 | 6.1 ± 0.4 | 11.8 ± 3.7 | 21.4 ± 1.1 | 38.1 ± 6.6 | 3.1 ± 0.5 | 2.1 ± 0.7 |
| AC220 | 8.8 ± 1.4 | 28.2 ± 2.1 | 1.1 ± 0.4 | 1.3 ± 0.5 | 9.0 ± 1.8 | 3.7 ± 0.8 | 36.2 ± 4.0 | 194.2 ± 28 | 52.4 ± 4.7 | 149.7 ± 27.0 |
| PKC412 | 15.4 ± 3.5 | 24.2 ± 3.1 | 18.5 ± 2.8 | 21.5 ± 1.5 | 28.9 ± 3.0 | 21.6 ± 1.1 | 128.7 ± 29 | 16.1 ± 4.7 | 11.4 ± 1 | 17.3 ± 3.7 |
Biochemical kinase assay for FLT3-WT and FLT3-D835Y obtained from Carna Bioscience Inc. was performed in time-resolved fluorescence resonance energy transfer in series concentration of G-749, AC220, and PKC412, and their IC50 values were calculated. For the determination of IC50 values in human leukemia cell lines, the MV4-11 and Molm-14 cells were treated with test inhibitors, and cell viability was measured. The BaF3 model cell expressing double FLT3 mutants, FLT3-ITD/N676D, FLT3-ITD/D835Y, and FLT3-D835Y/N676D, and single mutants, FLT3-ITD and FLT3-D835Y, were treated with test inhibitors, and cell viability was measured.
To evaluate its kinase selectivity, 100 nM G-749 was initially challenged against 282 kinases using the Millipore Kinase Profiler (supplemental Table 2). Major kinases inhibited by G-749 were further selected to determine their IC50 potency (supplemental Table 1). This assay revealed that G-749 displayed a unique inhibition pattern. It was highly potent against FLT3, FLT3-D835Y, and Mer (1 nM of each kinase IC50). The receptor tyrosine kinases (Ret, FLT1, Axl, Fms, fibroblast growth factor receptor [FGFR]-1, and FGFR3) and serine/threonine kinases (Aurora B and C) were also inhibited with IC50 values of 9-30 and 6-24 nM, respectively. Other kinases including c-kit receptor tyrosine kinase, platelet-derived growth factor receptors, and epidermal growth factor receptor were not potently inhibited (IC50 value of >300 nM); however, their mutants were significantly inhibited. Collectively, G-749 was identified as a novel and potent FLT3 inhibitor with a unique kinase inhibitory profile.
Antiproliferative activity on leukemia cells
The antiproliferative activity of G-749 was assessed in several leukemia cell lines. G-749 showed strong antiproliferation of leukemia cells addicted to FLT3-ITD (MV4-11 and Molm-14) in a dose-dependent manner, whereas it did not potently inhibit proliferation of leukemia cells without FLT3 expression (HEL and K562) or of non–FLT3-addicted cells with FLT3-WT (RS4-11) (Figure 1B and Table 1). The selective and potent antiproliferative activity of G-749 was found to come from the inhibition of FLT3-ITD because antiproliferation was rescued in FLT3-ITD–addicted BaF3 cells in the presence of interleukin 3 (supplemental Figure 5). The phosphorylation of downstream effectors in the FLT3 signaling pathway, such as p-STAT5, p-AKT, p-ERK1/2, and p-FoxO3a, was also potently inhibited by G-749 (Figure 1C). Similar inhibition of downstream effectors was seen in MV4-11 cells at levels comparable to AC220 and PKC412 (supplemental Figure 3C). G-749 treatment led to significantly increased active caspase-3/7 level and cleaved poly ADP ribose polymerase in a dose-dependent manner in both cell lines (Figure 1F and supplemental Figure 3D). Fluorescence-activated cell sorter analysis revealed that G-749 treatment increased apoptosis of MV4-11 cells in a dose-dependent manner (Figure 1G). Taken together, it causes antiproliferative activity through apoptosis.
We next investigated the sustained potency of FLT3 inhibitors. After washout, G-749 sustained strong inhibition of p-FLT3, p-ERK and p-STAT5 in a time-dependent manner, whereas in comparison, AC220 and PKC412 gradually lost their inhibitory activity over 24 hours (Figure 1D-E). Remarkably, the potent inhibition of G-749 against FLT3 pathways sustained after washout was found to reflect prolonged antiproliferation in comparison with AC220 and PKC412 (supplemental Figure 5).
It was found that the antiproliferation effect of G-749 also increased synergistically when used in combination with cytarabine (Figure 1H and supplemental Figure 6). Combination index calculated by CalcuSyn analysis showed good synergistic cell death in all tested concentrations (50% effective dose, 0.58264; 75% effective dose, 0.50659; and 90% effective dose, 0.44793), suggesting that G-749 is suitable for combinatorial therapy.
Potent inhibition of G-749 against FLT3 mutants
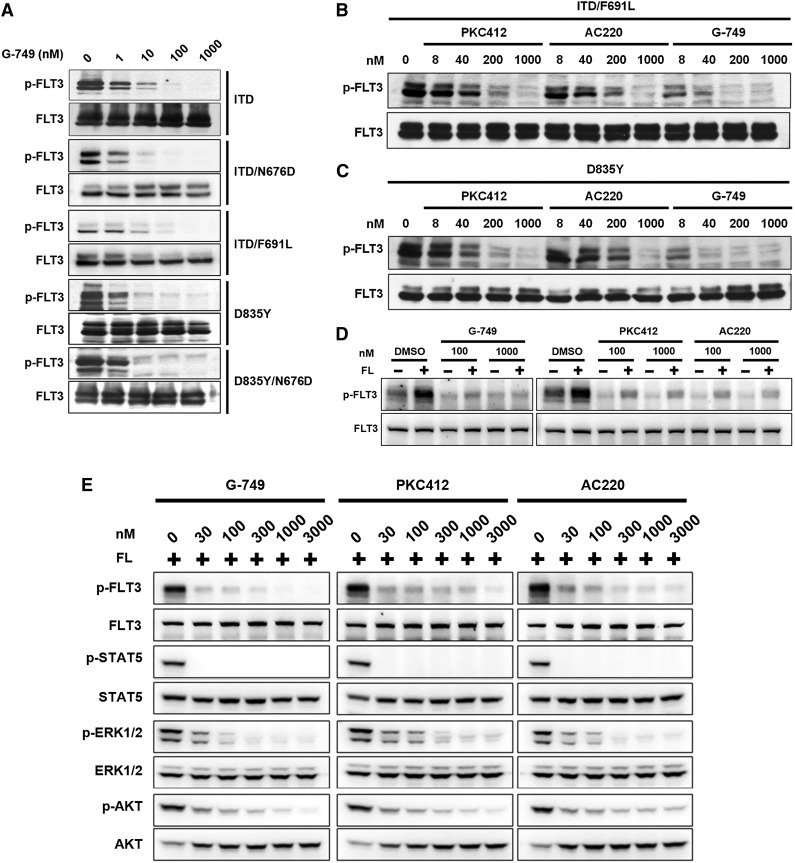
To evaluate whether G-749 could inhibit several FLT3 mutants causing drug resistance, we examined its potency against BaF3 cell lines that stably express FLT3-ITD/N676D (ITD and ATP-binding domain mutation), FLT3-ITD/F691L (ITD and gatekeeper mutation), FLT3-D835Y (activation loop mutation), or FLT3-D835Y/N676D (activation loop and ATP-binding mutation).19,20,29 Although PKC412 was less potent against BaF3 cells expressing FLT3-ITD/N676D (128 nM in IC50) than those expressing FLT3-ITD/F691L or FLT3-D835Y, AC220 showed strong potency against cells expressing FLT3-ITD (3.7 nM in IC50) but significantly decreased potency against those expressing FLT3-ITD/N676D, FLT3-ITD/F691L, FLT3-D835Y, and FLT3-D835Y/N676D. In comparison, G-749 showed strong potency against autophosphorylation of all tested FLT3 mutants with IC50 of <10 nM (Figure 2A), whose inhibition level was closely correlated with antiproliferation activity (Table 1). Additionally, the direct comparison of phosphorylation levels using western blotting revealed that G-749 more potently inhibited the autophosphorylation of the FLT3-ITD/F691L and FLT3-D835Y with IC50 of <8 nM than PKC412 and AC220 with IC50 of 40-200 nM, respectively (Figure 2B-C).
Figure 2.
Potent inhibition of FLT3 in various mutant cells. (A) The BaF3 cells expressing the indicated FLT3 mutation were incubated for 2 hours with the indicated concentrations of G-749, and (B-C) for direct comparison with 3 inhibitors, the BaF3 cells expressing FLT3-ITD/F691L (B) or FLT3-D835Y (C) were incubated with the indicated concentrations of G-749, AC220, and PKC412 for 2 hours. The autophosphorylation level of FLT3 was visualized and compared by western blotting analysis. (D) Molm-14 cells were treated with FLT3 inhibitors in the presence of 5 ng/mL of FL for 2 hours. The autophosphorylation level of FLT3 was then analyzed. (E) For direct comparison, the phosphorylation levels of FLT3, STAT5, ERK1/2, and AKT were analyzed by immunoblotting as in panel D. Total protein of each protein was used as a loading control, otherwise specified.
Effective inhibition of FLT3 pathways in high-FL milieu
We examined the potency of G-749 in high concentrations of FL milieu. Exogenous FL addition led to elevated p-FLT3 in a dose/time-dependent manner in BaF3 cells expressing FLT3-WT (supplemental Figure 7A-B) and in RS4-11 (supplemental Figure 3B). The increasing FL concentration in Molm-14 cells led to decreased cell death by AC220 up to ∼2.5-fold and by G-749 up to ∼1.9-fold (supplemental Figure 8). These data are consistent with the hypothesis that increased FL levels could impede the efficacy of FLT3 inhibitors to some degree.
However, even at a high FL level, G-749 showed more potent inhibition of p-FLT3, p-ERK1/2, and p-AKT than AC220 and PKC412 (Figure 2D-E). Notably, all tested FLT3 inhibitors potently and equally inhibited p-STAT5 in spite of high FL addition in Molm-14. FL addition did not activate p-STAT5 but slightly increased p-ERK and p-AKT levels in the BaF3 cells expressing FLT3-WT (supplemental Figure 7B), suggesting that the FL-activating p-ARK and p-ERK are responsible for slightly reducing efficacy of AC220 and G-749. Nevertheless, G-749 shows significant potency against p-FLT3, p-ERK1/2, and p-AKT in high-FL milieu.
Potent inhibitory activity in normal and patient human plasma milieu
We examined the potency of G-749 in normal and patient plasma milieu to assess PIA. G-749 and AC220 equally inhibited p-FLT3 in culture medium and normal human plasma milieu in a dose-dependent manner, but PKC412 did not inhibit p-FLT3 in plasma milieu even at the high concentration of 1 μM (Figure 3A, top and middle), which was further confirmed by ELISA analysis (supplemental Figure 9).
Figure 3.
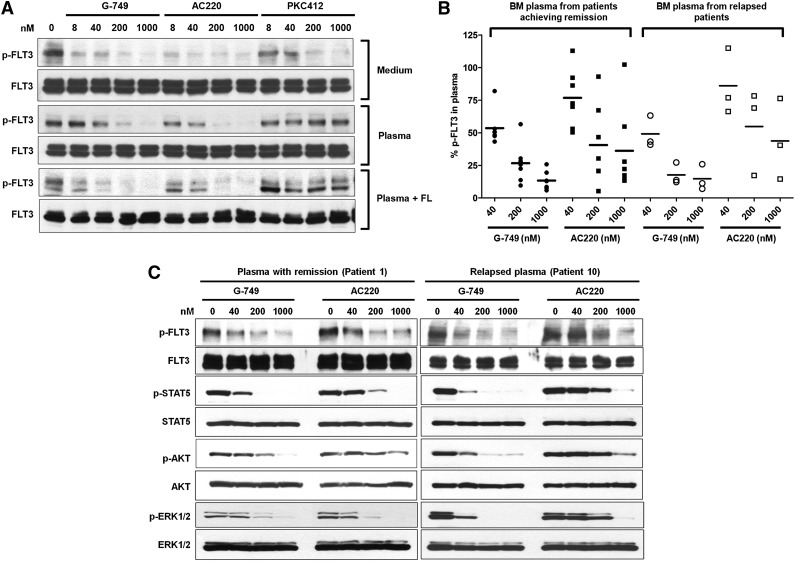
Potent inhibition of G-749 in AML patient plasma milieu. (A) Molm-14 cells were treated with increasing concentrations of indicated inhibitors in normal culture medium (top), normal human plasma from healthy donor (middle), or normal plasma supplemented with 5 ng/mL of FL (bottom) for 2 hours. The autophosphorylation level of FLT3 was then evaluated in western blot. (B) The bone marrow (BM) plasmas were obtained from AML patients who achieved CR (n = 7) or those with relapse (n = 3) after the induction therapy of cytarabine. Molm-14 cells were treated with the indicated concentrations of G-749 or AC220 in relapsed or CR patient plasma milieu for 2 hours. The p-FLT3 was then analyzed by densitometry and plotted as the percentage over dimethylsulfoxide (DMSO) control to display distribution of data. ANOVA followed by Newman-Keuls multiple comparison test was performed to examine the inhibition level of p-FLT3. P < .05 was calculated between G-749 and AC220 in each concentration for pairwise comparison. Noticeably, even groups treated with 1000 nM AC220 showed greater deviation for inhibition levels of p-FLT3 from 10% to 100% residual p-FLT3 in both relapsed and CR plasma. G-749 showed less deviation for this than AC220 in all tested concentrations. (C) The phosphorylation levels of FLT3, STAT5, ERK1/2, and AKT were further evaluated in patient plasma milieu from the patient achieving remission (patient 1, left-side blot) and the relapsed patient (patient 10, right-side blot). Noticeably, G-749 potently inhibited FLT3 and downstream pathways in both remission and relapsed plasma, whereas AC220 significantly lost its potency against them in relapsed plasma.
We further examined the potency of G-749 and AC220 by using the bone marrow plasma from 10 AML patients who achieved CR (n = 7) or relapsed (n = 3) after induction therapy with cytarabine (supplemental Table 3). Western blot analysis revealed that G-749 consistently inhibited p-FLT3 in all 10 patient plasma milieus, but AC220 significantly lost its potency with big variations even at 1 μM (P < .05 compared with 1 μM G-749 group, n = 10) (Figure 3B), suggesting that the inhibition degree of AC220 highly varies from patient to patient. Of particular interest was the inhibition of FLT3 pathways in relapsed patient plasma milieu (patient 10). G-749 fully inhibited p-FLT3, p-STAT5, p-AKT, and p-ERK1/2, whereas AC220 could not inhibit them even at high concentrations (Figure 3C).
To address the lost potency of AC220 in the relapsed patient milieu, we examined changes in p-FLT3 in normal plasma supplemented with FL and found that AC220 showed potent inhibition of p-FLT3 (Figure 3A, bottom); thus FL in plasma does not contribute to the lost potency. In addition, the plasma protein binding of AC220 and G-749 is ∼98% and 99%, respectively, so that the free form of the compound is not responsible for the reduction in potency. Therefore, it is reasonable that other unknown factors in patient plasma may be involved in impeding the efficacy of FLT3 inhibitor including AC220 but excluding G-749 and remain to be addressed. Collectively, these PIA studies clearly suggest that G-749 effectively and potently inhibits FLT3 and downstream effectors in relapsed patient plasma.
Effective inhibition in stroma-protecting milieu
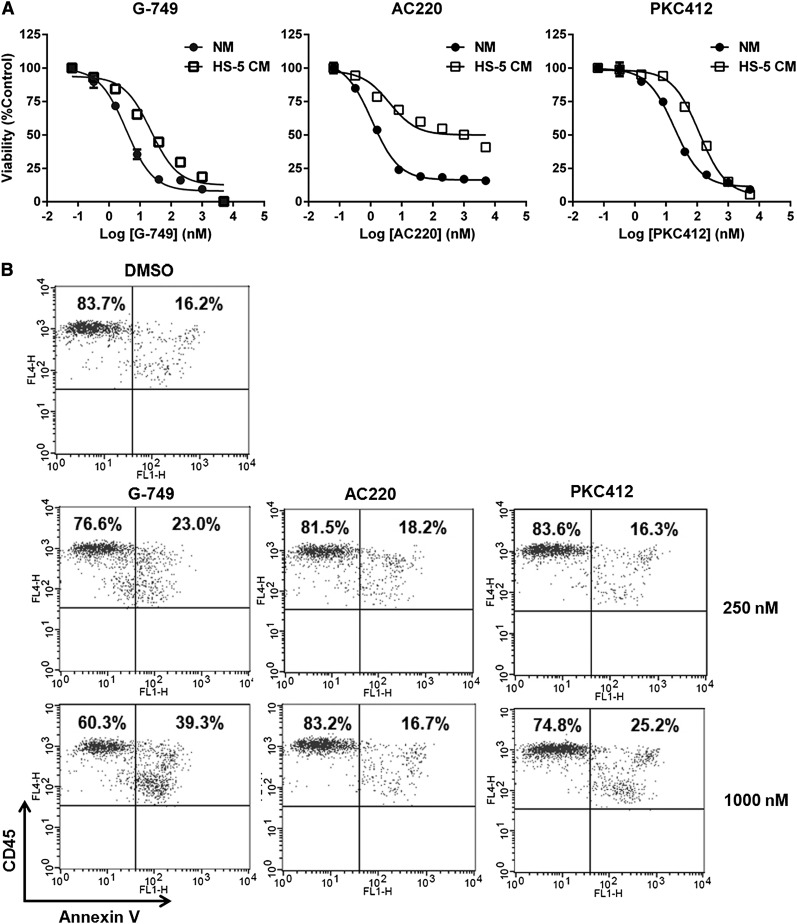
We investigated the antileukemic activity of FLT3 inhibitors in drug-resistant milieu provided by bone marrow stromal cells known to promote leukemia cell survival.22-25 Although AC220 caused only 50% cell death of MV4-11 even at the high concentration of 5 μM in CM, G-749 and PKC412 similarly caused 90% cell death at the same concentration (Figure 4A). To determine if cell death caused by FLT3 inhibitor could be attenuated in the direct contact environment of stromal cell HS-5, we cocultured MV4-11 or patient blast of genotype FLT3-ITD (patient 12) with the HS-5 cell and then compared the potency of FLT3 inhibitors. In the case of MV4-11/HS-5 coculture (supplemental Figure 10), the treatment of 1000 nM G-749 and PKC412 caused 93.8% and 84.5% cell death, respectively, whereas that of 1000 nM AC220 caused 60.0%. Furthermore, in coculture of patient blast with HS-5 (Figure 4B), the treatment of 1000 nM G-749 and PKC412 caused 39.3% and 25.2% cell death, respectively, whereas that of 1000 nM AC220 showed 16.7%, similar to the DMSO control. These data strongly demonstrate that G-749 shows effective potent antileukemic activity even in the stroma-protecting milieu.
Figure 4.
Stromal protective effect on cell death by FLT3 inhibitors. (A) MV4-11 cells were incubated for 72 hours with the indicated concentrations of G-749, AC220, and PKC412 in CM supplemented with 35% HS-5–derived medium or normal culture medium (NM). Cell viabilities were then determined by test inhibitors and plotted for comparison between NM and CM. (B) AML blasts (patient 12, supplementary Table 4) were cultured with HS-5 monolayer for 48 hours with each test inhibitor at 250 and 1000 nM. All cells were stained with annexin V (x-axis) and CD45 (y-axis) and then gated for CD45 positivity. The percentages of live and apoptotic AML blasts were indicated in the upper left and upper right, respectively. (C) Molm-14 cells were incubated for 6 hours in CM with the indicated concentrations of G-749 or AC220. The phosphorylation levels of FLT3, STAT5, AKT, and ERK1/2 were detected by immunoblotting. Noticeable differential response is that G-749 potently inhibited p-ERK1/2, but AC220 did not.
To better understand how FLT3 inhibitors differentially respond to the stroma-protective milieu, we monitored changes in the FLT3 pathway in CM milieu. G-749 and AC220 similarly inhibited p-FLT3 and p-STAT5 in CM milieu. Of particular interest was the differential inhibition of p-ERK1/2. Unlike AC220, G-749 could potently inhibit p-ERK1/2 in Molm-14 and MV4-11 (Figure 4C and supplemental Figure 11), and its inhibition level against p-FLT3 in CM was found to be very similar to that in normal medium. G-749 showed potent inhibition against p-ERK1/2 in Molm-14 and against p-ERK1/2 and p-AKT in MV4-11, whereas AC220 showed little or no inhibition against p-ERK1/2 and/or p-AKT in both cells. Thus, the synergistically reduced inhibitory activity against p-ERK1/2 and/or p-AKT could be responsible for reduced antiproliferative activity in MV4-11 cells. Irrespective of influential and complex genetic backgrounds and protective bypass signals provided by the CM, G-749 persistently displayed potent inhibition against p-FLT3, p-ERK, and p-AKT.
Effective antitumor activity in mouse models
To assess the in vivo pharmacodynamic effect of G-749, a single dose of G-749 HCl salt (10 mg/kg) was administered orally to subcutaneous MV4-11 xenograft mice and showed sustained inhibition of p-FLT3, p-STAT5, and p-ERK1/2 (Figure 5A-B). These data indicate that G-749 effectively inhibits the FLT3 pathway and that its inhibition lasts for 24 hours. Therefore, once-a-day oral dosing was expected to be sufficient for in vivo efficacy in the mouse model.
Figure 5.
In vivo antitumor activity of G-749 in xenograft and engrafted mouse model. (A-B) Pharmacodynamic analysis of G-749 in a subcutaneous MV4-11 xenograft model. (A) MV4-11 tumor-bearing mice received a single oral dose of G-749 HCl salt (10 mg/kg). Mice were euthanized at the indicated time point. From homogenized tumor tissues, the levels of p-FLT3 were measured by p-FLT3 ELISA in comparison with the vehicle-treated control group. The data from 3 mice at each point are presented (±SD). (B) The p-STAT5 and p-ERK1/2 in the same tumor tissue as in panel A were determined. (C) When the tumor size reached ∼450-600 mm3 in volume, mice (n = 9) received daily oral administration of vehicle or G-749 HCl salt (3, 10, and 30 mg/kg per day) for 28 days. The group of mice treated with 30 mg/kg per day of G-749 was subsequently monitored for an additional 28 days to examine tumor regrowth. ANOVA with Dunnett’s posttest was performed, ***P < .0001 at day 28. Significant inhibition of tumor growth and tumor regression was observed from 4 days onward (P < .05) (D) in vivo antitumor activity in an engrafted tumor model. The disseminated NOD/SCID mice were intravenously inoculated with Molm-14 cells. From 7 days after inoculation, mice received daily oral administration of G-749 (10 or 20 mg/kg per day) (n = 7) for 28 days. The log-rank test was made to compare survival curves between the vehicle-treated group and the 10 mg/kg per day (χ2 = 13.7, df = 1, P = .0002) or 20 mg/kg per day group (χ2 = 13.28, df = 1, P = .0003). The gray bars indicate the G-749 dosing period.
To examine its antitumor efficacy, G-749 HCl salt was administered orally every day for 28 days to the MV4-11 xenograft mice. Significant inhibition of tumor growth was observed in the 3 mg/kg per day dosing group from 4 days onward, and apparent tumor regression was seen in the 10 and 30 mg/kg per day dosing group (Figure 5C). The mice dosed with 30 mg/kg per day were subsequently monitored for an additional 28 days after dosing stopped to examine if the tumor grows back. There was no tumor regrowth up to 28 days suggesting complete tumor regression. Additionally, in this study, weight loss and signs of gross toxicity were not observed in any dose group (supplemental Figure 12).
We confirmed antitumor efficacy in an orthogonal model of bone marrow engraftment using Molm-14 cells, which physiologically differs from the subcutaneous MV4-11 xenograft model. In engrafted NOD/SCID mice, vehicle-treated mice showed expected clinical symptoms such as hind-limb paralysis, rough coat, and decreased activity and died within 9 days. But both 10 and 20 mg/kg per day dosing groups showed increased survival in a dose-dependent manner (Figure 5D). Collectively, G-749 yields effective in vivo antitumor activity in 2 different leukemia animal models and is expected to yield great efficacy in clinical trials.
Potent antileukemia activity in blasts of AML patients
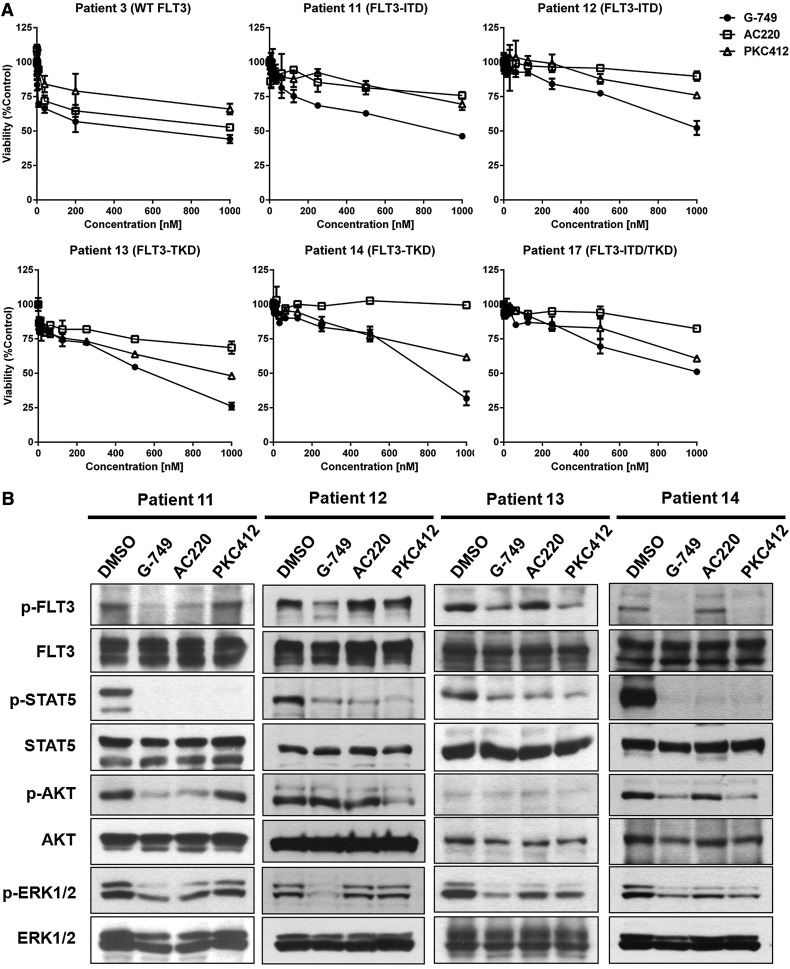
To evaluate the antileukemia activity in primary cells, bone marrow blasts were obtained from 16 AML patients diagnosed as FLT3-WT, FLT3-ITD, FLT3-D835Y, and FLT3-ITD/D835Y (supplemental Table 4). The overall magnitude of antileukemic activity of G-749 was more effective than that of AC220 and PKC412 (Figure 6A and supplemental Figure 13). Remarkably, the antileukemic response of G-749 was ∼75% to the blasts with FLT3-D835Y and ∼50% to those with FLT3-ITD/D835Y that showed no or little response to AC220. These data are consistent with the findings from the BaF3 model cells.
Figure 6.
Potent inhibition of G-749 against patient blasts harboring FLT3-ITD or FLT3-D835Y. (A) AML patient blasts expressing FLT3-WT (patient 3), FLT3-ITD (patients 11 and 12), FLT3-D835Y (patients 13 and 14), or FLT3-ITD/D835Y (patient 16) were incubated for 72 hours with the indicated concentrations of G-749, AC220, or PKC412, and their viability was then determined. For each FLT3 inhibitor, the percentage over DMSO control was presented as a mean value, with error bars representing ±SD. (B) Inhibition of the FLT3 signal pathway. The blasts harboring FLT3-ITD (patients 11 and 12) or FLT3-D835Y (patients 13 and 14) were incubated with 100 nM of FLT3 inhibitors for 2 hours (in the case of patient 12, with 250 nM), and then the phosphorylation levels of FLT3, STAT5, AKT, and ERK1/2 were analyzed. Each total protein was used as a loading control.
We further determined whether the antileukemic activity of G-749 couples with the inhibition of FLT3 pathways in patient blasts. In the patient 11 and 12 blasts with FLT3-ITD, G-749 showed more potent inhibition of p-FLT3 and p-ERK1/2 than AC220 and PKC412. In the patient 13 and 14 blasts with FLT3-D835Y, G-749 also showed more potent inhibition against p-FLT3 than AC220. Both G-749 and PKC412 effectively inhibited p-ERK1/2 in patient 13 and 14 blasts. Remarkably, in cases where they did not inhibit the p-FLT3 (patient 12), AC220 and PKC412 potently inhibited p-STAT5 (Figure 6B). These data suggest that G-749 shows inhibition of p-FLT3 and downstream effectors in patient blasts, regardless of FLT3 mutation status, eventually resulting in efficient antileukemic activity against blasts of AML patients.
Discussion
We report the development of G-749 as a novel FLT3 inhibitor with potent activity against the FLT3-TKD mutants, and its sustained inhibition against FLT3 disease pathways was responsible for potent antileukemic activity via apoptosis in AML cells and patient blasts representing different FLT3 mutation status and even in drug-resistant environments such as high-FL milieu, stroma-protecting milieu, and relapsed patient plasma. Because it has been proposed that complete and enduring inhibition of FLT3 is critical for achieving clinical efficacy and may contribute to persistence of leukemic progenitors after treatment of FLT3 inhibitors,8,9 the prolonged and potent inhibitory activity of G-749 against FLT3 mutants and aberrant downstream pathways could be very effective in treating relapsed and refractory AML patients.
Kinase profiling revealed the potent inhibition of Mer and Aurora B by G-749. Mer kinase is known to be crucial in maintaining cell function, but its aberrant levels of Tyro3/Axl/Mer receptors and their ligands have been reported in numerous cancers including acute lymphoblastic leukemia.30-32 Another emerging target for AML treatment is Aurora B, which plays an essential role in regulating chromosome segregation and cytokinesis. The inhibition of aurora B, more than aurora A, showed an antiproliferative and proapoptotic effect on in vitro and in vivo acute lymphoblastic leukemia and AML models.33,34 Therefore, the inhibition of aurora B and Mer in addition to inhibition of activated FLT3 disease pathways may contribute to a significant antileukemic effect in AML therapy. Other kinases such as FLT1, FMS, and AXL are also known to play a role in the proliferation of hematologic malignant tumors,35,36 and further studies with them and other possible targets if any remain to be addressed.
FLT3-TKD mutants especially at N676, F691, and D835 were identified to confer resistance to PKC412 and AC220.16,19 In this study, we demonstrated potent inhibitory activity of G-749 against FLT3-ITD/N676D, FLT3-ITD/F691L, and FLT3-D835Y mutants. Considering that model AML cell lines may have limitations in predicting the clinical response of FLT3 inhibitors, we used patient bone marrow blasts. G-749 showed a dose-dependent cytotoxic response in all leukemic blasts harboring FLT3-WT, FLT3-ITD, FLT3-D835Y, and FLT3-ITD/D835Y. In patient blasts, this cytotoxic activity was well correlated with inhibition of G-749 against p-FLT3 regardless of FLT3 mutant status, including those with no or little response to AC220 and PKC412. Therefore, G-749 is expected to potently inhibit FLT3 mutants conferring resistance to other TKIs in clinical study.
The PIA was proposed to be a useful surrogate assay for the determination of FLT3 inhibition in patients receiving oral FLT3 inhibitor because plasma protein binding, which varies between patients, can influence the free drug level necessary for pharmacologic activity.37-39 In this study, we demonstrated that G-749 showed significant inhibition of FLT3 and downstream pathways even in relapsed AML patient plasma; this caused significant loss of potency of AC220. We demonstrated that the high FL levels and freely available drug in plasma were not mainly responsible for the differential response of G-749 and AC220 in patient plasma. Rather, this differential could render the hypothesis that the plasma status of patients after the cytarabine treatment could significantly influence the efficacy of some TKIs targeting FLT3 leading to drug resistance; this remains to be addressed.
A number of studies have reported that TKIs can rapidly eliminate circulating blasts in peripheral blood. However, this efficacy was restricted to leukemic blasts at the bone marrow probably because of the ability of the bone marrow stroma cells to protect hematologic malignancy.40,41 In coculture milieu where bone marrow stroma HS-5 protected patient blasts or MV4-11 cells from dying, G-749 displayed antileukemic activity. In the CM from HS-5 where various hematopoietic growth factors42 significantly altered FLT3 downstream pathways, it also showed potent inhibition of ERK1/2 and AKT activations via bypass signals that AC220 did not inhibit. These findings suggest that inhibition of the FLT3-ERK1/2 axis is indispensable to overcoming drug resistance. By overriding the survival bypass signals of leukemic cells provided by the stromal cells, G-749 is expected to yield strong antileukemic activity even in the complex bone marrow microenvironment.
Conclusively, a novel FLT3 inhibitor, G-749, shows potent antileukemic activity in various drug-resistant conditions and could be the next-generation drug candidate for the treatment of relapsed and refractory AML.
Supplementary Material
Acknowledgments
We thank Drs Hyung-Jun Kim, Yeo-Kyeoung Kim, Sung-Soo Yoon, and Hee-Je Kim for collecting clinical samples.
This work was supported by the National OncoVenture/Korea National Cancer Center and Dr. Moon Hwan Kim for the animal studies and preclinical study of G-749.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.K.L. designed and conducted experiments and analyzed data; H.W.K. designed and synthesized FLT3 inhibitors for optimization to yield G-749; Jungmi L. conducted kinase assays; I.Y.L. performed the cell-based assay; Jaekyoo L. synthesized chemicals for optimization; D.S.J., S.Y.L., S.H.P., and H.H. conducted animal and pharmacokinetic studies; J.-S.C. and J.-H.K. provided input into compound synthesis; S.W.K., J.K.K., and J.C. provided input into analysis of experiments and revised the manuscript; J.S.K. directed chemical synthesis of the FLT3 inhibitor project; and H.-J.S. directed the FLT3 biology project and wrote the manuscript.
Conflict-of-interest disclosure: All authors except for J.C. are current or former employees of Genosco and Oscotec Inc. The remaining author declares no competing financial interests.
Correspondence: Ho-Juhn Song, Genosco, 767C Concord Ave, Cambridge, MA 02138; e-mail: hsong@genosco.com or songhojuhn@gmail.com.
References
- 1.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3(9):650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 2.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10(12):1911–1918. [PubMed] [Google Scholar]
- 3.Horiike S, Yokota S, Nakao M, et al. Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leukemia. 1997;11(9):1442–1446. doi: 10.1038/sj.leu.2400770. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 5.Kornblau SM, Womble M, Qiu YH, et al. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108(7):2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandts CH, Sargin B, Rode M, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65(21):9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- 7.Rocnik JL, Okabe R, Yu JC, et al. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood. 2006;108(4):1339–1345. doi: 10.1182/blood-2005-11-011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kindler T, Lipka DB, Fischer T. FLT3 as a therapeutic target in AML: still challenging after all these years. Blood. 2010;116(24):5089–5102. doi: 10.1182/blood-2010-04-261867. [DOI] [PubMed] [Google Scholar]
- 9.Sternberg DW, Licht JD. Therapeutic intervention in leukemias that express the activated fms-like tyrosine kinase 3 (FLT3): opportunities and challenges. Curr Opin Hematol. 2005;12(1):7–13. doi: 10.1097/01.moh.0000147891.06584.d7. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1(5):433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 11.Metzelder SK, Schroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353–2359. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 12.Zarrinkar PP, Gunawardane RN, Cramer MD, et al. AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood. 2009;114(14):2984–2992. doi: 10.1182/blood-2009-05-222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105(1):54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 14.Cortes JE, Perl AE, Dombret H, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients ≥ 60 years of age with FLT3 ITD positive or negative relapsed/refractory acute myeloid leukemia [abstract]. Blood. 2012;120(21) Abstract 48. [Google Scholar]
- 15.Levis MJ, Perl AE, Dombret H, et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation [abstract]. Blood. 2012;120(21) Abstract 673. [Google Scholar]
- 16.Smith CC, Wang Q, Chin CS, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kancha RK, Grundler R, Peschel C, Duyster J. Sensitivity toward sorafenib and sunitinib varies between different activating and drug-resistant FLT3-ITD mutations. Exp Hematol. 2007;35(10):1522–1526. doi: 10.1016/j.exphem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107(1):293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 20.Pauwels D, Sweron B, Cools J. The N676D and G697R mutations in the kinase domain of FLT3 confer resistance to the inhibitor AC220. Haematologica. 2012;97(11):1773–1774. doi: 10.3324/haematol.2012.069781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): a pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108(10):3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg E, Liu Q, Nelson E, et al. Using combination therapy to override stromal-mediated chemoresistance in mutant FLT3-positive AML: synergism between FLT3 inhibitors, dasatinib/multi-targeted inhibitors and JAK inhibitors. Leukemia. 2012;26(10):2233–2244. doi: 10.1038/leu.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16(9):1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 24.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5). Exp Hematol. 2001;29(4):448–457. doi: 10.1016/s0301-472x(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 25.Parmar A, Marz S, Rushton S, et al. Stromal niche cells protect early leukemic FLT3-ITD+ progenitor cells against first-generation FLT3 tyrosine kinase inhibitors. Cancer Res. 2011;71(13):4696–4706. doi: 10.1158/0008-5472.CAN-10-4136. [DOI] [PubMed] [Google Scholar]
- 26.Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD. FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat. 2009;12(3):81–89. doi: 10.1016/j.drup.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato T, Yang X, Knapper S, et al. FLT3 ligand impedes the efficacy of FLT3 inhibitors in vitro and in vivo. Blood. 2011;117(12):3286–3293. doi: 10.1182/blood-2010-01-266742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lierman E, Lahortiga I, Van Miegroet H, Mentens N, Marynen P, Cools J. The ability of sorafenib to inhibit oncogenic PDGFRbeta and FLT3 mutants and overcome resistance to other small molecule inhibitors. Haematologica. 2007;92(1):27–34. doi: 10.3324/haematol.10692. [DOI] [PubMed] [Google Scholar]
- 29.Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2010;115(7):1425–1432. doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keating AK, Salzberg DB, Sather S, et al. Lymphoblastic leukemia/lymphoma in mice overexpressing the Mer (MerTK) receptor tyrosine kinase. Oncogene. 2006;25(45):6092–6100. doi: 10.1038/sj.onc.1209633. [DOI] [PubMed] [Google Scholar]
- 31.Keating AK, Kim GK, Jones AE, et al. Inhibition of Mer and Axl receptor tyrosine kinases in astrocytoma cells leads to increased apoptosis and improved chemosensitivity. Mol Cancer Ther. 2010;9(5):1298–1307. doi: 10.1158/1535-7163.MCT-09-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oke A, Pearce D, Wilkinson RW, et al. AZD1152 rapidly and negatively affects the growth and survival of human acute myeloid leukemia cells in vitro and in vivo. Cancer Res. 2009;69(10):4150–4158. doi: 10.1158/0008-5472.CAN-08-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartsink-Segers SA, Zwaan CM, Exalto C, et al. Aurora kinases in childhood acute leukemia: the promise of aurora B as therapeutic target. Leukemia. 2013;27(3):560–568. doi: 10.1038/leu.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu TL, Mercher T, Tyner JW, et al. A novel fusion of RBM6 to CSF1R in acute megakaryoblastic leukemia. Blood. 2007;110(1):323–333. doi: 10.1182/blood-2006-10-052282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011;10(10):1763–1773. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 37.Gambacorti-Passerini C, Barni R, le Coutre P, et al. Role of alpha1 acid glycoprotein in the in vivo resistance of human BCR-ABL(+) leukemic cells to the abl inhibitor STI571. J Natl Cancer Inst. 2000;92(20):1641–1650. doi: 10.1093/jnci/92.20.1641. [DOI] [PubMed] [Google Scholar]
- 38.Kremer JM, Wilting J, Janssen LH. Drug binding to human alpha-1-acid glycoprotein in health and disease. Pharmacol Rev. 1988;40(1):1–47. [PubMed] [Google Scholar]
- 39.Weisberg E, Sattler M, Ray A, Griffin JD. Drug resistance in mutant FLT3-positive AML. Oncogene. 2010;29(37):5120–5134. doi: 10.1038/onc.2010.273. [DOI] [PubMed] [Google Scholar]
- 40.Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17(6):1175–1182. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt T, Kharabi Masouleh B, Loges S, et al. Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell. 2011;19(6):740–753. doi: 10.1016/j.ccr.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Roecklein BA, Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood. 1995;85(4):997–1005. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.