Abstract
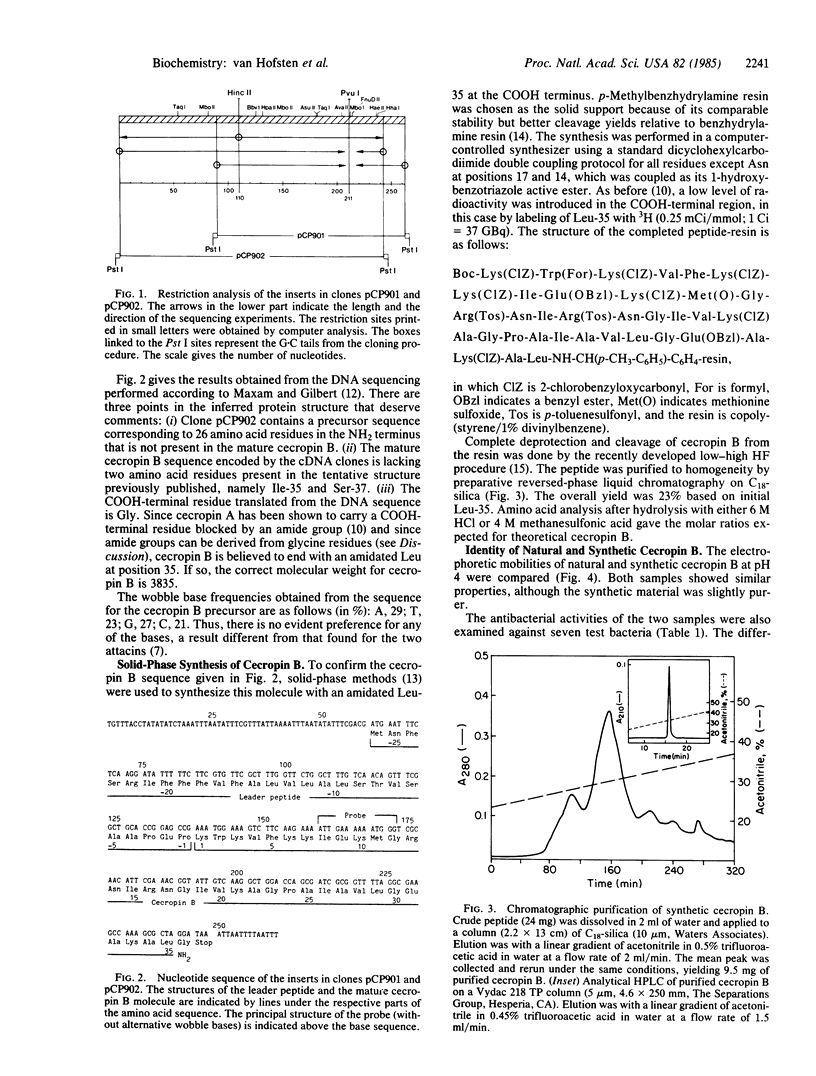
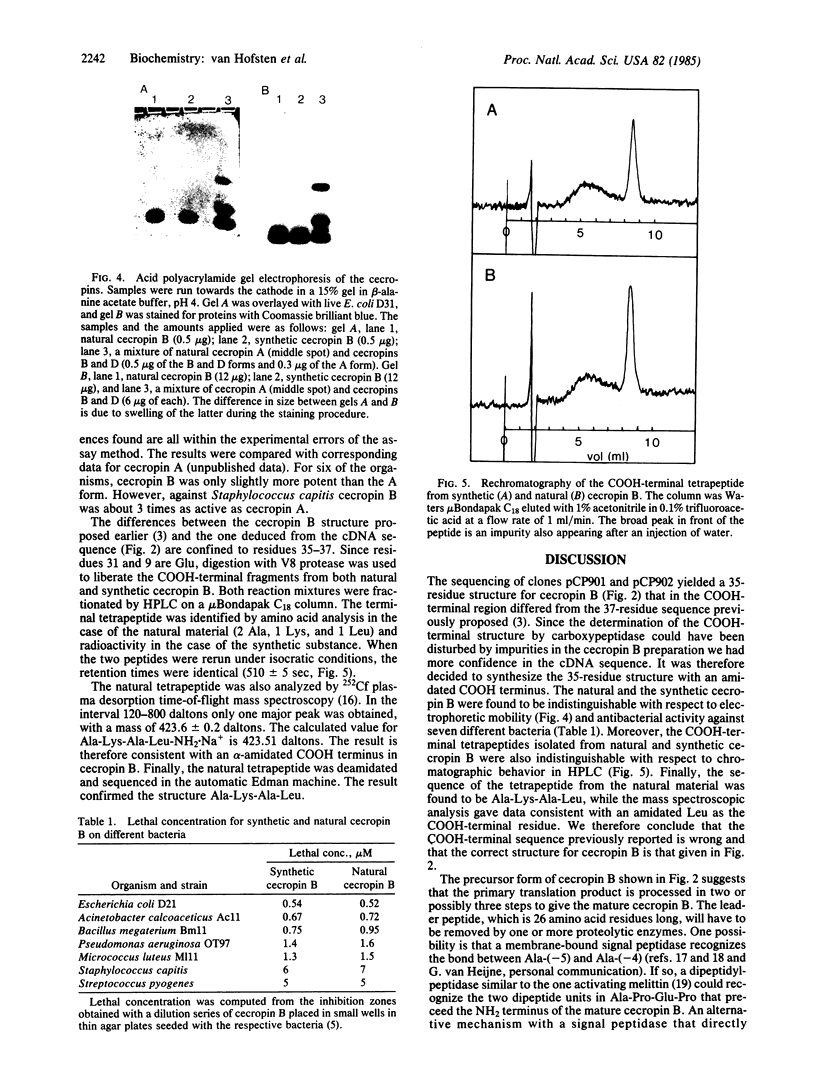
Two cDNA clones containing coding information for cecropin B from the Cecropia moth (Hyalophora cecropia) were identified by means of a synthetic probe. Sequencing of the two inserts showed that cecropin B is processed from a 62-amino acid residue precursor molecule including a 26-residue leader peptide and a COOH-terminal glycine residue. The latter presumably donates the nitrogen of the amide group present on the COOH-terminal leucine residue of the mature cecropin B. The sequence deduced for the mature cecropin B differed in the COOH-terminal region from the tentative structure previously determined by carboxypeptidase digestion. To settle the discrepancy, cecropin B was synthesized according to the cDNA sequence with an amidated COOH-terminal leucine. Natural and synthetic cecropin B were found to be indistinguishable with respect to electrophoretic mobility and antibacterial activity against seven different bacteria. The COOH-terminal tetrapeptides were isolated from both natural and synthetic cecropin B and found to be indistinguishable. The correct sequence for cecropin B is (formula; see text).
Full text
PDF

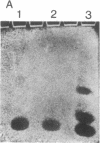
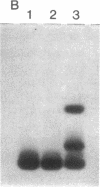
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu D., Merrifield R. B., Steiner H., Boman H. G. Solid-phase synthesis of cecropin A and related peptides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6475–6479. doi: 10.1073/pnas.80.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Steiner H. Humoral immunity in Cecropia pupae. Curr Top Microbiol Immunol. 1981;94-95:75–91. doi: 10.1007/978-3-642-68120-2_2. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Glembotski C. C. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström A., Engström P., Tao Z. J., Carlsson A., Bennich H. Insect immunity. The primary structure of the antibacterial protein attacin F and its relation to two native attacins from Hyalophora cecropia. EMBO J. 1984 Sep;3(9):2065–2070. doi: 10.1002/j.1460-2075.1984.tb02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Andersson K., Steiner H., Bennich H., Boman H. G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983;2(4):571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Bennich H., Kapur R., Boman H. G. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur J Biochem. 1982 Sep;127(1):207–217. doi: 10.1111/j.1432-1033.1982.tb06857.x. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Kockum K., Faye I., Hofsten P. V., Lee J. Y., Xanthopoulos K. G., Boman H. G. Insect immunity. Isolation and sequence of two cDNA clones corresponding to acidic and basic attacins from Hyalophora cecropia. EMBO J. 1984 Sep;3(9):2071–2075. doi: 10.1002/j.1460-2075.1984.tb02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G., Haiml L., Suchanek G. Stepwise cleavage of the pro part of promelittin by dipeptidylpeptidase IV. Evidence for a new type of precursor--product conversion. Eur J Biochem. 1980 Oct;111(1):49–58. doi: 10.1111/j.1432-1033.1980.tb06073.x. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Edlund T., Ny T., Faye I., Boman H. G. Insect immunity. Isolation of cDNA clones corresponding to attacins and immune protein P4 from Hyalophora cecropia. EMBO J. 1983;2(4):577–581. doi: 10.1002/j.1460-2075.1983.tb01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsueda G. R., Stewart J. M. A p-methylbenzhydrylamine resin for improved solid-phase synthesis of peptide amides. Peptides. 1981 Spring;2(1):45–50. doi: 10.1016/s0196-9781(81)80010-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Steiner H., Hultmark D., Engström A., Bennich H., Boman H. G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- Sundqvist B., Kamensky I., Håkansson P., Kjellberg J., Salehpour M., Widdiyasekera S., Fohlman J., Peterson P. A., Roepstorff P. Californium-252 plasma desorption time of flight mass spectroscopy of proteins. Biomed Mass Spectrom. 1984 May;11(5):242–257. doi: 10.1002/bms.1200110509. [DOI] [PubMed] [Google Scholar]
- Vlasak R., Unger-Ullmann C., Kreil G., Frischauf A. M. Nucleotide sequence of cloned cDNA coding for honeybee prepromelittin. Eur J Biochem. 1983 Sep 1;135(1):123–126. doi: 10.1111/j.1432-1033.1983.tb07626.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]