Abstract
Exposure to Agent Orange (AO) and the contaminating chemical 2,3,7,8-Tetrachlorodibenzodioxin (TCDD) has been associated with the development of chronic lymphocytic leukemia (CLL). Of the195 veterans diagnosed with CLL from 2001–2010 in a retrospective cohort from the Minneapolis VA, 33 (17%) were exposed to AO. Prognostic factors including Rai stage, lymphocyte doubling time and cytogenetics did not differ between exposed and unexposed patients. Exposed patients were younger at diagnosis (61 vs 72 years, p=0.001) and time to CLL treatment was shorter (9.6 vs 30.2 months, p=0.02). Overall survival did not differ between exposed and unexposed patients on Kaplan Meier analysis, but when adjusted for age, AO exposure had a hazard ratio of death of 1.8 compared to non-exposure (95% CI 0.7–4.5 p=0.24). The high estimate of the mortality hazard combined with the relatively low numbers in the exposure group suggests that further examination in a larger patient population is warranted.
Keywords: Chronic lymphocytic leukemia, Agent Orange, prognosis, survival
INTRODUCTION
From 1962–1971, 45 million liters of Agent Orange (AO) and other herbicides were sprayed in South Vietnam and Cambodia to destroy dense jungle and crops used to conceal and feed enemy troops1. AO is a 1:1 mixture of 2 herbicides but was contaminated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) during the manufacturing process2. The National Academy of Science has investigated the health effects of TCDD exposure. Data from animal studies suggest that exposure to TCDD can increase cancer formation and also enhance cancer formation in presence of other carcinogens2. Studies suggests that TCDD binds to aryl hydrocarbon receptor and cause changes in gene transcription that induce cell metabolism and decrease hormone levels3. Alterations in cellular signaling are believed to underlie the association between cancer formation and TCCD.
In 2002, the Department of Veterans Affairs added chronic lymphocytic leukemia (CLL) to the list of diseases with sufficient evidence of an association with Agent Orange exposure. The change in classification was primarily based upon data from agricultural exposure to similar herbicides. A case control study of farmers from Nebraska found statistically significant increased odds of CLL death (OR 1.67) and farmers from counties with increased herbicide use were at the highest risk4. Similar risk was seen in case control comparison between Iowa farmers and residents of surrounding states with suggested increased rates in farmers who handled pesticides5. Studies have also evaluated veterans who sprayed AO and thus were likely to have the highest TCDD exposure. An increased incidence of melanoma and prostate cancer was found in US veterans who sprayed AO compared to veterans serving in the region who did not spray herbicides6. Additionally, the overall risk of cancer was increased in veterans with the highest TCDD exposure. Limited hematologic cancers were found and when examined together, rates comparable to national averages were seen6. Conversely, an increase in CLL incidence was found in Vietnam veterans compared to the Australian public (OR 1.55)7. Overall, epidemiologic studies suggest that exposure to herbicides and AO increases the risk of developing CLL.
There is no data to-date as to whether AO exposure alters features of CLL disease presentation orprognostic features including stage at diagnosis, lymphocyte doubling time or cytogenetics. These prognostic factors are important to understand to determine if the natural history of CLL in exposed patients differs in comparison to unexposed patients. We completed a retrospective cohort study to investigate if Agent Orange exposure was associated with an altered prognosis, time to treatment, or overall survival in veterans with newly diagnosed CLL.
MATERIALS AND METHODS
Patients with CLL were identified in the VAMC (Minneapolis MN) Tumor Registry after IRB approval. Due to availability of computerized medical records, patients diagnosed between 2001–2010 were included. Of the 205 patients identified from the tumor registry, 199 were appropriately classified, but4 patients were excluded due to a lack of clonal lymphocyte population >5.0 × 109/L lymphocytes on flow cytometry or a tissue diagnosis of small lymphocytic lymphoma. Patients’ charts were reviewed for demographic information and laboratory parameters at diagnosis. To assess the impact of AO exposure on CLL prognosis, bone marrow cytogenetics, Rai disease stage and lactate dehydrogenase (LDH) at diagnosis, and lymphocyte doubling time were also determined. Cytogenetics was identified through standard karyotyping or florescent in-situ hybridization. Poor risk cytogenetics included 17p- and 11q-, whereas 13p- was considered good risk cytogenetics. Survival was defined time from diagnosis to death from any cause. Patients were censored at last follow-up if listed as alive. Lastly, to determine if AO exposure affected CLL treatment, medical records were examined for timing, reason, type, and number of chemotherapy treatments received. Patients were excluded from the time to first chemotherapy if chemotherapy was initiated due to an alternate malignancy. In order to limit abstraction bias, AO exposure was identified from the VA tumor registry and medical record independently from the other information. At the Veterans Affairs Medical Center, exposure is classified by Benefits and Compensation officers who have access to service files to determine if a person served on land or inland waters in Vietnam during the appropriate timeframe.
Statistical Analysis
Baseline labs, lymphocyte doubling time and time to initial CLL treatment were compared between exposed and unexposed patients using Student’s t-test. Fisher’s exact test was used to compare categorical variables. Kaplan Meier analysis compared overall survival between Agent Orange-exposed and unexposed patients. A multivariable Cox regression model was used to determine the effect of age on the survival analysis.
RESULTS
Of the195 confirmed CLL cases, 33 patients (16.9%) had Agent Orange exposure. Median follow-up time was 40.7 (range, 0.1–123) months. The baseline demographics and laboratory parameters are summarized in Table 1. Patients with Agent Orange exposure were younger at diagnosis (61 vs. 72 years, p=0.001). White blood cell count, absolute lymphocyte count, hemoglobin and platelet count at diagnosis were similar between the groups. Therefore, other than age, the exposed and unexposed groups were comparable.
TABLE I.
Baseline demographics and laboratory parameters of patients with CLL who were exposed and unexposed to Agent Orange.
| Agent Orange | |||
|---|---|---|---|
| Exposed (n=33) | Unexposed (n=162) | p-value | |
| Age at diagnosis (years) | 61.2 ± 0.6 | 72.5 ± 0.8 | <0.0001 |
| Male n (%) | 33 (100%) | 160 (98%) | 1.0 |
| Caucasian n (%) | 29 (100%) | 105 (96%) | 0.6 |
| WBC at diagnosis (x109/L) | 17.4 ± 1.5 | 19.6 ± 2.0 | 0.5 |
| ALC at diagnosis (x109/L) | 11.8 ± 1.6 | 13.6 ± 1.3 | 0.5 |
| Hemoglobin at diagnosis (g/dl) | 14.5 ± 0.4 | 13.8 ± 0.2 | 0.08 |
| Platelet count at diagnosis (x109/L) | 206 ± 11 | 194 ± 5 | 0.3 |
Mean ± SE presented unless noted. T-tests comparing continuous variables; Fisher’s exact test comparison is between categorical variables. WBC=white blood count. ALC=Absolute lymphocyte count.
The impact of AO exposure on CLL prognosis was determined through comparison of recognized prognostic indicators among exposed and unexposed patients. The most significant prognostic factor, Rai stage at diagnosis, did not differ between exposed and unexposed patients (Figure 1, Chi-square p=0.34). CT or abdominal ultrasound was completed in 33% of patients at diagnosis (60 CT and 4 patients with ultrasound). Imaging results increased the Rai Stage in 9% (6/64 patients) who had imaging completed. Completion of imaging at diagnosis was not associated with AO exposure (Chi-square 0.38). Lymphocyte doubling time was also comparable in exposed and unexposed patients (27 vs. 23 months, respectively) (p=0.6). Twenty five percent of exposed patients had a lymphocyte doubling time less than one year compared to 30% of unexposed patients (p=0.7). LDH values were available in 68% of patients and an elevated LDH was comparably present in exposed and unexposed patients at diagnosis (11% vs 10%, respectively, p=0.9). Beta-2-microglobulin levels at diagnosis were only available in 6.7% (13/195) and thus were not evaluated. Cytogenetic analysis was limited as only 24% of patients underwent a bone marrow biopsy. Poor risk cytogenetics (17p-, 11q-) were found in 1 of 10 (10%)patients with AO exposure and 3 of 37 (8%)unexposed patients. Overall, prognostic factors did not differ based upon AO exposure.
FIGURE 1.
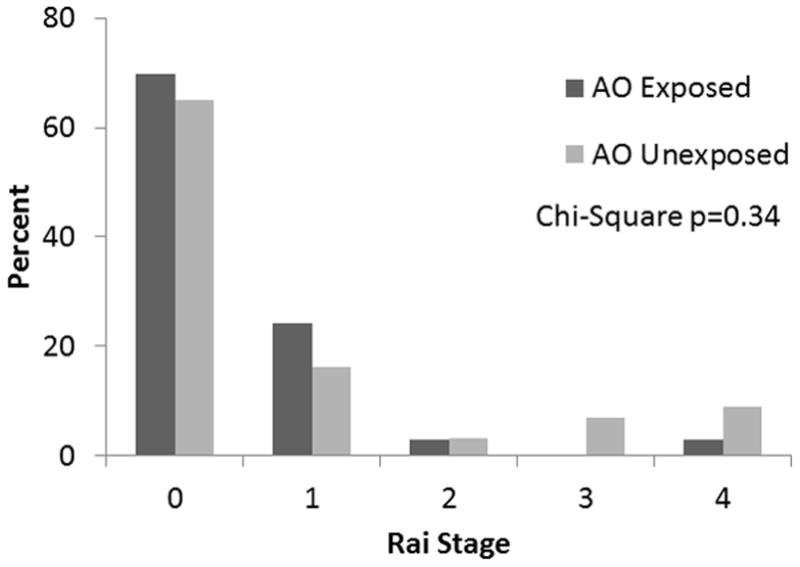
Rai stage at diagnosis of CLL patients who were exposed and unexposed to Agent Orange (AO)
As AO exposure is known to providers, we evaluated if exposure category impacted timing or type of CLL therapy. Therapy over the follow-up period was required by 15% of exposed patients and 17% of unexposed patients required therapy over the follow-up period. Seventy five percent (24/33) had imaging completed prior to treatment. Time to first CLL treatment was significantly shorter in patients with AO exposure (9.6 vs. 30.2 months, respectively; p=0.02) suggesting a more aggressive course. No significant difference in reason for treatment initiation was found between the groups (Table III), but the power to detect differences will be limited due to sample size. First line fludarabine therapy was used more often in exposed than unexposed patients, which may have been due to their younger age at diagnosis (100% AO exposed vs 36% AO unexposed, Fisher’s Exact p=0.01). On average, exposed patients received 1.8 ± 0.6 lines of chemotherapy compared to 1.7 ± 0.2lines in unexposed patients (p=0.9). Treatment for other hematologic malignancies that could have represented Richter’s transformation of the CLL was found in 6% of exposed patients and 3.7% of unexposed patients (p=0.6). Despite AO exposed patients needing therapy sooner than unexposed patients, the number of chemotherapy lines and rate of transformation did not differ between the groups.
TABLE III.
Reasons for initiation of CLL directed therapy
| Reason for Treatment | Agent Orange Exposed (N=5) | Agent Orange Unexposed (N=28) |
|---|---|---|
| Cytopenias | 2 (40%) | 11 (39%) |
| Lymphadenopathy | 0 | 7 (25%) |
| Leukocytosis | 0 | 6 (21%) |
| Multiple organ involvement | 1 (20%) | 2 (7%) |
| B-symptoms | 1 (20%) | 1 (4%) |
| Clinical trial | 1 (20%) | 1 (4%) |
Fisher’s Exact p=0.15
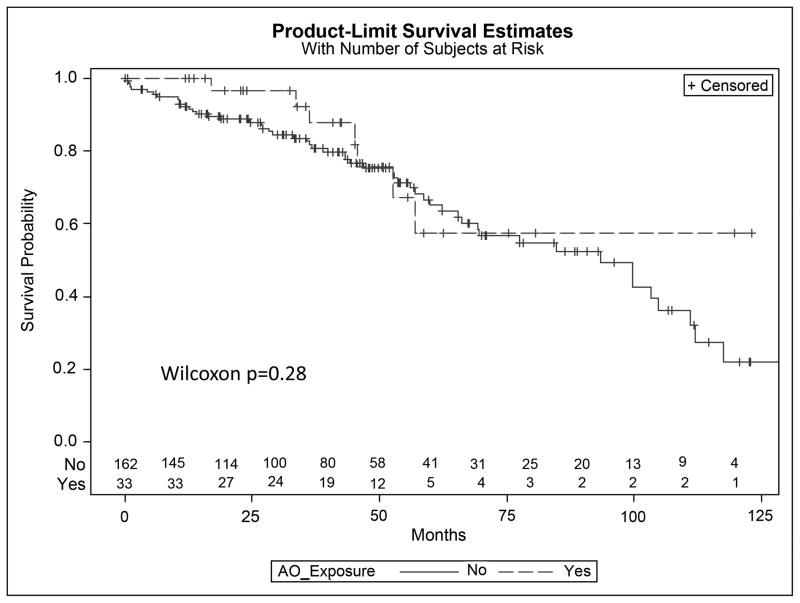
Lastly, we evaluated the impact of AO exposure on overall survival. Kaplan Meier analysis showed no difference in survival between exposed and unexposed patients (Figure 2, Wilcoxon p=0.28). Due to the significant difference in age at diagnosis among exposed and unexposed patients, a multivariable Cox regression model was performed to adjust for age. Agent Orange exposure had a hazard ratio of death of 1.8 compared to non-exposure in CLL patients (95% CI: 0.7–4.5, p = 0.24). The wide confidence interval is secondary to small sample size and thus warrants evaluation in future analysis.
FIGURE 2.
Kaplan Meier curves of survival of CLL patients who were exposed and unexposed to Agent Orange (AO)
DISCUSSION
We present the first study of prognosis and treatment in CLL patients with Agent Orange exposure. Conventional prognostic factors such as Rai stage, lymphocyte doubling time and LDH did not differ among exposed and unexposed patients. Veterans exposed to AO had a shorter time to first treatment, which would suggest a more aggressive course especially since stage at diagnosis did not differ. Patients exposed to AO were more likely to be initially treated with fludarabine-based regimens, which have been associated with higher and more durable responses8. The duration of response to fludarabine is likely why an equal number of chemotherapy lines were needed in exposed and unexposed patients. The preferential use of fludarabine in AO exposed patients was probably due to their younger age at diagnosis, but may reflect providers concerns about the natural history of the disease in exposed patients.
Previous epidemiologic studies have suggested an increased risk of CLL death in farmers exposed to herbicides. The odds of death from CLL were 67% higher in Nebraskan farmers compared to non-farmers4. A case control study of Iowa farmers using death certificates also suggested an increased odds of dying from CLL (OR 1.7, 1.2–2.4)9. Our study found a similar risk of death when adjusted for age (HR 1.8). The increase in risk of death may have been secondary to clinical variables or molecular prognostic markers that could not be adequately assessed in a retrospective study. Although our hazard ratio result was not statistically significant, the high estimate of the mortality hazard combined with the relatively low numbers in the exposure group suggest that further examination of this issue in a larger patient population is warranted.
Due to the retrospective nature of the study, there are potential limitations secondary to bias. The use of cytogenetics as a prognostic marker was limited because only a quarter of patients underwent bone marrow biopsy. Approximately equal percentages of exposed and unexposed patients underwent biopsy, though, so information bias is not likely. CD38, ZAP70, and immunoglobulin heavy chain variable gene mutational status was not assayed as part of routine clinical practice at our site and thus was not available in our retrospective cohort. Molecular prognostic information could further categorize patients into risk categories and should be evaluated in prospective research protocols in this area. Screening recommendations do not differ for people exposed to AO and thus a lead time bias is not likely. However, patients with exposure to AO may have a heighted awareness of medical concerns and seek evaluation sooner than other people. Additionally, a diagnostic bias could be present if providers knowledgeable of the association of AO with CLL incidence checked blood work more often in patients with AO exposure. Our study did show that exposed patients were younger at diagnosis; however, they were not diagnosed at an earlier stage suggesting that diagnostic suspicion bias was not present. However, since patients can remain at lower Rai stages for a period of time, we cannot definitely eliminate the possibility of diagnostic suspicion bias. Despite the potential biases of a retrospective study, impactful information has been revealed.
Overall, our findings show that prognostic factors in patients exposed to AO are similar to unexposed patients, but AO-exposed patients required earlier initiation of treatment. Future studies should examine on a larger scale whether AO exposed patients have a more aggressive clinical course and if mortality is indeed higher.
TABLE II.
Prognostic Factors of patients with CLL who were exposed and unexposed to Agent Orange
| Agent Orange | |||
|---|---|---|---|
| Exposed n=33 | Unexposed n=162 | p-value | |
| Doubling of lymphocyte count (n, %) | 12 (36%) | 57 (35%) | |
| Lymphocyte doubling time (months) | 27.3 ± 6.6 | 23.2 ± 2.8 | 0.6 |
| Doubling time <1 year | 3 (25%) | 17 (30%) | 0.7 |
| Elevated LDH (n=136) | 3 (11%) | 11 (10%) | 0.9 |
| Cytogenetics or FISH | 10 (30%) | 37 (23%) | |
| Poor risk cytogenetics (17p-, 11q-) | 1 (10%) | 3 (8%) | 0.8 |
| Good risk cytogenetics (13p-) | 3 (30%) | 3 (8%) | 0.13 |
Acknowledgments
We would like to acknowledge the NIH T32 training grant (5T32HL00706) for support of the above project. The funding source did not have any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Additionally we would like to thank Ryan Shanley for biostatistical assistance.
Footnotes
DECLARATION OF INTERESTS
Gobind Tarchand and Vicki Morrison are employed by the Veterans Affairs Medical Center (Minneapolis MN). Vicki Morrison has been a member of a Celgene Data Monitoring Committee and is on the speakers bureau for Amgen, Celgene, and Genentech. Lisa Baumann Kreuziger’s time is supported by an NIH T32 Training grant.
Contributor Information
Lisa M. Baumann Kreuziger, Email: Bauma260@umn.edu, University of Minnesota, Mayo Mail Code 480, 420 Delaware St. S.E., Minneapolis, MN, USA 55455, Phone: (612) 624-0123, Fax: (612) 625-6919
Gobind Tarchand, Email: Gobind.tarchand@va.gov, Veterans Affairs Medical Center, One Veterans Drive, Minneapolis, MN 55417, 612-467-4135.
Vicki A. Morrison, Email: morri002@umn.edu, University of Minnesota and Veterans Affairs Medical Center, One Veterans Drive, Minneapolis, MN 55417, 612-467-4123
References
- 1.Stellman J, Stellman S, Christian R, Weber T, Tomasallo C. The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature. 2003;422(6933):681–687. doi: 10.1038/nature01537. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Anonymous Veterans and Agen Orange: Update 2002. Washington, DC: National Academy Press; 2003. Chronic Lymphocytic Leukemia; p. 334. [Google Scholar]
- 3.Evans MV, Andersen ME. Sensitivity analysis of a physiological model for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): assessing the impact of specific model parameters on sequestration in liver and fat in the rat. Toxicological sciences. 2000;54(1):71–80. doi: 10.1093/toxsci/54.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Blair A, White DW. Leukemia cell types and agricultural practices in Nebraska. Arch Environ Health. 1985;40(4):211–214. doi: 10.1080/00039896.1985.10545920. [DOI] [PubMed] [Google Scholar]
- 5.Brown LM, Blair A, Gibson R, et al. Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res. 1990;50(20):6585–6591. [PubMed] [Google Scholar]
- 6.Akhtar F, Garabrant D, Ketchum N, Michalek J. Cancer in US Air Force veterans of the Vietnam War. Journal of occupational and environmental medicine. 2004;46(2):123–136. doi: 10.1097/01.jom.0000111603.84316.0f. [DOI] [PubMed] [Google Scholar]
- 7.Australia Department of Veterans’ Affairs. Cancer Incidence in Australiam Vietnam Veteran Study 2005. Canberra, Australia: Department of Veterans’ Affiaris; 2005. [Google Scholar]
- 8.Rai KR, Peterson BL, Appelbaum FR, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343(24):1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- 9.Burmeister LF, Van Lier SF, Isacson P. Leukemia and farm practices in Iowa. Am J Epidemiol. 1982;115(5):720–728. doi: 10.1093/oxfordjournals.aje.a113354. [DOI] [PubMed] [Google Scholar]