Abstract
The induction of apoptosis, a highly regulated and clearly defined mode of cell dying, is a vital tenet of modern cancer therapy. In this review we focus on three aspects of apoptosis research which we believe are the most crucial and most exciting areas currently investigated and that will need to be better understood in order to enhance the efficacy of therapeutic measures. First, we discuss which target to select for cancer therapy and argue that not the cancer cell as such, but its interaction with the microenvironment is a more promising and genetically stable site of attack. Second, the complexity of combination therapy is elucidated using the PI3-K-mediated signaling network as a specific example. Here we show that the current clinical approach to sensitize malignancies to apoptosis by maximal, prolonged inhibition of so-called survival pathways can actually be counter productive. Third, we propose that under certain conditions which will need to be clearly defined in future, chronification of a tumor might be preferable to the attempt at a cure. Finally, we discuss further problems with utilizing apoptosis induction in cancer therapy and propose a novel potential therapeutic approach that combines the previously discussed features.
Keywords: apoptosis, cancer therapy, microenvironment, combination therapy, chronification
1. Introduction
Despite the emergence of alternative paths leading to cell death, such as necroptosis [1] and—more controversially—autophagy [2], apoptosis remains, due to its limited side effects, the major preferred mode of action to eliminate the tumor burden of cancer patients. While we habitually refer to apoptosis as “programmed cell death” or “suicide”, a more correct, although less salient term would be “energy-consuming process that regulates cell dying”, as in terms of therapeutic implications it is more advantageous to think of it as a process initiated by death signals (may they be drugs, radiation or proteins), rather than as the mere consequence of poisoning. Cell dying, from the induction of DNA damage or death receptor activation when the cellular remains are taken up by macrophages [3], can take anywhere from several hours to many days. It is during that period when the most interesting events occur within the cell and when the unique features of cancer counteract and prevent the death signal. Interestingly, Nixon’s “war on cancer” which aimed to find a cure for these malignancies within the next 25 years was declared the year prior to the publication of Kerr, Wyllie and Currie’s seminal paper which first defined apoptosis [4]. In the intervening 40-odd years we have reached a near comprehensive mechanistic understanding of apoptosis (summarized in Figure 1), however the therapeutic successes on the whole have been less forthcoming.
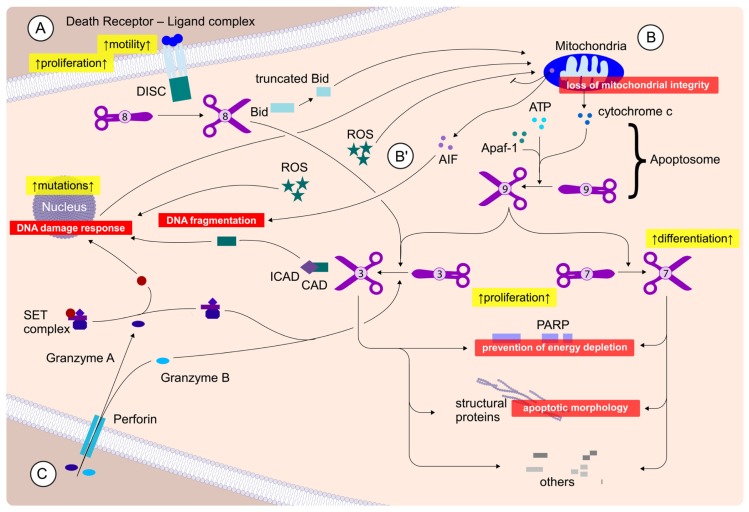
Figure 1.
Apoptosis induction and signaling. Apoptosis can be initiated via three distinct routes: (A) Extrinsic pathway: Ligands bind to death receptors leading to formation of the DISC complex and activation of caspase 8 (depicted as scissors). Caspase 8 either directly activates, via cleavage, caspases 3 and 7, or needs to enhance the “death signal” via Bid cleavage which thus initiates a mitochondrial feedback loop. Whether cells depend on this mitochondrial enhancement is traditionally determined experimentally and dying cells are either referred to as type I (no mitochondrial amplification necessary) or type II (mitochondrial amplification necessary) cells; (B) Intrinsic pathway: Chemotherapy and radiation, among others, cause DNA damage either directly or by producing oxygen radicals (ROS), which can either further impair the nuclear DNA integrity or compromise the mitochondria. This leads to the activation of mitochondrial death signaling, via the release of cytochrome c and other factors into the cytosol. Here, cytochrome c combines with caspase 9, Apaf-1 and ATP to form the Apoptosome which activates caspases 3 and 7. Of note, there is also a caspase-independent form of apoptosis (B′), whereby AIF, which can also function as a ROS scavenger, gets released from the mitochondria and triggers chromatin condensation and DNA fragmentation; (C) CTL (Cytotoxic T lymphocytes), or NK (natural killer) cell-mediated apoptosis: A specialized form of apoptosis whereby the immune system triggers apoptosis by inserting a perforin “tunnel” in its target cell. This leads to granzymes entering the cell and activating caspase 3 and 7. All forms of caspase-dependent apoptosis converge at the level of caspase 3 and 7, the executioner caspases, which then proceed to cause the structural alterations to the cell, either directly or via activation of CAD DNase. Typical cellular responses to apoptosis are depicted in red, while in yellow effects of pathway activation are shown, which are undesirable during cancer therapy.
Two of the three pillars of modern cancer therapy, radio- and chemotherapy, work predominately by inducing apoptosis, the third—surgical resection—is, cases of pre-metastatic cancer aside, seldom fully effective if not augmented by the other two. Apoptosis is a naturally occurring mechanism that plays a vital role during development, immune system priming and in the maintenance of tissue homeostasis [3,5]. The appearance of discontinuous uncontrolled growth, i.e., cancer, means that homeostasis is no longer maintained and a sub-population of cells, which we can now refer to as “malignant”, has escaped the body’s natural surveillance, either by genetic or epigenetic means. This apoptosis resistance is one of the defining hallmarks of cancer [6]. Therapeutic intervention basically tries to restart this intrinsic feature of cells, namely to commit suicide when they become abnormal, but, of course, in cells that have previously already escaped this signal. The consequence is that high doses of radiation or chemotoxic substances are needed to overcome the block in apoptosis, leading to the collateral damage of healthy tissue. To maximize apoptosis of malignant cells, while concurrently minimizing the damage sustained by normal cells, one should consider alternate focuses of attack. We propose three such approaches in this review: (1) only indirectly target the highly resistant cancerous cells and alternatively aim at the tumor’s interaction with its microenvironment; (2) Augment traditional therapeutic approaches with carefully timed additions of pharmacological signaling inhibitors or therapeutic antibodies; (3) Consider an alternative primary aim as desired therapeutic outcome, enhanced and prolonged quality of life versus tumor-free survival.
2. Discussion
2.1. AMARe Mortis
One of the most obvious updates that occurred between the 2000 and 2011 version of Hanahan and Weinberg’s seminal work on the hallmarks of cancer [6,7] is that in the latter the role of non-tumorgenic factors is much more emphasized. No longer is a cancerous growth purely understood in terms of transformed cells only, but the direct surroundings, the so-called microenvironment, is also taken into account. We feel this is an important, long overdue shift in focus, as studies already from the same time period as the earliest apoptosis studies [8,9] indicated that the cellular interaction with its (solid) microenvironment might affect the tumor cell’s ability to survive treatment. Importantly, the limitations of in vivo culture systems, i.e., cell culture in plastic flasks, which lack a physiological microenvironment were also already noted and a “spheroid” model of cell culture to—at least partially—counter the in vitro limitations was developed [10]. However, till recently the role of the microenvironment in cancer was mainly investigated in the context of metastasis and invasion, in particular since anoikis, a specialized form of apoptosis which occurs upon the loss of cell-matrix adhesion, is often seen as restricted to non-transformed cells [11]. It is only relative recently that the concepts of CAM-DR [12–37], cell-adhesion mediated drug resistance, and—probably still to a lesser extent—CAM-RR [9,10,12,38,39], cell-adhesion mediated radiation resistance, have gained interest in the scientific community. There are several different types of interaction (Figure 2), which we previously categorized into three groups [40]:
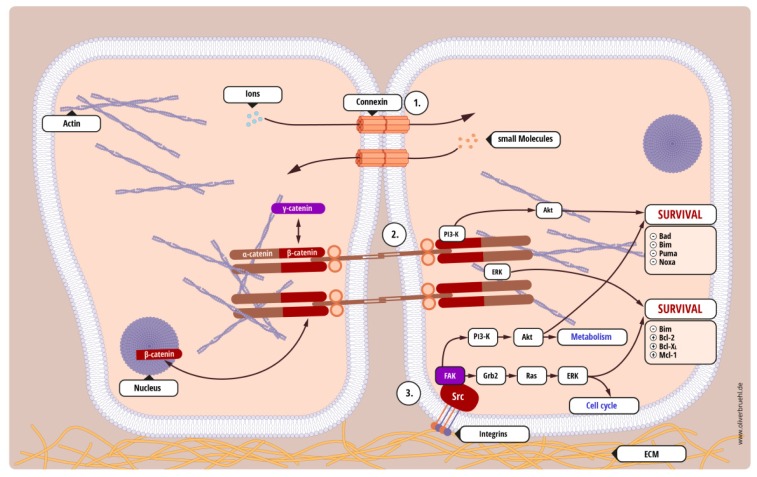
Figure 2.
The tumor’s interaction with its solid microenvironment. Three of the five common forms of adhesions are depicted here and their role in cell death prevention is highlighted (expanded from [40]): (1) Gap junctions, aqueous channels consisting of connexins, through which ions and small molecules can be exchanged between adjacent cells. Generally, they are considered more in terms of cell-cell communication rather than cell-cell adhesion, however, we previously showed both that gap junctions contribute to glioblastoma cell-cell adhesion and, by diluting the apoptotic signal, reduce cell death [28]; (2) Adherens junctions are not only contacts between cells, but also anchorage points to the cytoskeleton. They consist of plasma membrane spanning cadherins and adjacent cadherin interactions on the plasma membrane lead to the formation of a zipper-like structure which provides a strong adhesive link between cells. In addition, components of the adherens junctions not only mediate cell-cell adhesions, but are also involved in the regulation of signaling cascades, in particular the Wnt pathway; (3) Focal adhesions are links between the extracellular matrix and the actin cytoskeleton. Their dynamic regulation plays an important role in cell motility, while there are also sites of origin for (a) intracellular survival signals and (b) extracellular cues for microenvironment remodeling. As example, we show in detail two survival pathways that are activated by adherens junctions and focal adhesions, the Raf/MEK/ERK pathway and the PI3-K/Akt signaling cascade (see Figure 3). Note that (−) indicates reduced, while (+) stands for increased protein activity. Not shown are desmosomes, which act as tethering points for intermediate filaments, and tight junctions, which form a boundary between apical and basal domains of the plasma membrane. While the former are usually exclusively discussed in terms of reduced expression during metastasis [45], the role of tight junctions is more controversial, as its components either found to be up- or down-regulated in cancers [46]. Of note, PTEN, the negative regulator of PI3-K/Akt signaling also regulates tight junctions [47].
Homotypic cell-cell interaction—tumor cells interact with other adjacent tumor cells;
Heterotypic cell-cell interaction—tumor cells interact with other non-malignant cells, for example tumor-associated stroma or healthy tissue that is being invaded;
Cell-substrate interaction—tumor cells interact with solid structures, such as the extracellular matrix or the basal membrane.
While restoration of the “normal” unaltered microenvironment can be sufficient to block tumor progression and tumor formation [41,42], it is important to note that these adhesions do not primarily mediate cellular survival. Their absence does not necessarily lead to anoikis, as anchorage-independent growth is also a hallmark of cancer [6], but they form communication points between cells or sites from which pro-survival signals can be mediated upon stress. For example, the presence of the extracellular matrix, through cell-substrate interactions, not only facilitates cellular locomotion and thus invasion [43], but during migration it also enhances the cells’ resistance to apoptosis induction [44].
Furthermore, our own work in glioblastoma has also shown that differences in the microenvironment can significantly alter cellular behavior and—maybe even more importantly—that glioblastoma cells themselves alter their environment depending on their position within the tumor [28,49]. We previously showed that glioblastoma cells have developed different modes of interaction with their microenvironment and that by inhibiting one form of interaction, i.e., blocking cell substrate adhesion, we did not sensitize cells for apoptosis but only drove them to enhanced (gap junction-mediated) cell-cell interactions [28]. Importantly, only by blocking both types of interaction, could we sensitize these cells for therapy-induced apoptosis [28]. Proceeding from this work, we recently were able to add additional pieces to the puzzle by showing that both, in vitro and in vivo, glioblastoma cells alter their microenvironment by secreting fibronectin, in essence creating their own extracellular matrix [49]. Interestingly, in vivo, only the cells either at the invading edge of the tumor or those that have already migrated away from the tumor bulk produced fibronectin, while those within the tumor presumably interact via gap-junctions [49]. Blocking cell-substrate interactions led to reduced invasion and tumor shrinkage [49]. We are currently examining the possibility of blocking, both cell-cell and cell-substrate interactions, thus extending the therapeutic window and sensitizing cells to apoptosis.
This is not an isolated phenomenon; expanding from our previous report, Table 1 lists twenty-seven occurrences of AMAR (adhesion-mediated apoptosis resistance [40]) in a wide range of cancers and identifies the molecular mechanisms if known. It is striking that the molecules involved in any survival strategies mediated by AMAR are identical to those currently being targeted via pharmaceutical inhibitors or therapeutic antibodies, such as the PI3-K signaling cascade or the Bcl-2 family (highlighted in Figure 2). However, why do we believe targeting the interaction of the cancer cell with its microenvironment is more promising than targeting the cancer cell directly? Cancer cells within a population are almost by definition a highly unstable target with high mutation rates [53], thus the risk of allowing a fast proliferating population to escape treatment restrains by genotypic or, indeed, epigenetic means is always high. In contrast, the non-tumorigenic microenvironment is a much more stable target. Indeed, the reversion of tumor-associated cells of the microenvironment back to a normal phenotype has been shown to be readily achieved [54] and Table 1 clearly demonstrates that, aside from the aforementioned possibility where this approach is sufficient to block tumor progression [42], it leads to an apoptosis sensitization in a vast array of tumors. Also as the interaction of tumor cells with their microenvironment is initiated at the cell surface, it is a relative easy target, as pharmacological inhibitors and therapeutic antibodies do not need to be taken up by the cells. Finally, while apoptosis resistance can be mediated almost at any given position within the apoptosis cascade, or by hyperactivation of survival signals, there is a much more limited range of often closely related surface molecules, i.e., less potential targets and less molecules that can compensate for any protein successfully targeted.
Table 1.
AMAR in different malignancies. Instances of AMAR described in the literature, sorted by cancer cell type investigated. Of note, the repeated identification of the PI3K/Akt signaling network as AMAR mediator (see also Section II and Figure 3). Of additional interest is the frequent suggestion of integrin antagonists as potential therapy. Small molecule inhibitors are currently being clinically evaluated and show some promise in phase II trials [50–52]. Dark fields indicate lack of information. Abbreviations: He, Heterotypic cell-cell interaction; ECM, Cell-substrate interaction; Ho, Homotypic cell-cell interaction (modified and expanded from [40]).
| Cancer | Adhesion type | Apoptosis inducing agents investigated | Molecular basis for AMAR | Therapeutic suggestion | Reference |
|---|---|---|---|---|---|
| Multiple myeloma | He, ECM | Melphalan | enhanced HSP70 expression | no concrete suggestion | [17] |
| Multiple myeloma | ECM | Doxorubicin, etoposide | not determined | no concrete suggestion | [23] |
| Multiple myeloma | ECM | CD95 | increased soluble c-Flip | no concrete suggestion | [29] |
| Multiple myeloma | ECM | Doxorubicin, melphalan | not determined | no concrete suggestion | [27] |
| Multiple myeloma | ECM | Bortezomib | not determined | no concrete suggestion | [19] |
| Multiple myeloma | He | Doxorubicin | not determined | Integrin antagonists | [22] |
| Multiple myeloma | He, ECM | Lenalidomide | not determined | All-trans retinoic acid | [25] |
| Multiple myeloma | ECM | Doxorubicin, mitoxantrone | Up-regulation of p27Kip1 | no concrete suggestion | [26] |
| Multiple myeloma | He | Melphalan, treosulfan, doxorubicin, dexamethasone, bortezomib | Rho/Rho-Kinase signaling | Statins | [13] |
| Multiple myeloma, acute myeloid leukemia | ECM | Tipifarnib | not determined | combine with bortezomib | [15] |
| Burkett’s lymphoma | ECM | Etoposide | Bcl-2 family | no concrete suggestion | [30] |
| B-cell lymphoma | He | Rituximab | not determined | Integrin antagonists | [21] |
| Histiocytic lymphoma | ECM | Mitroxantrone | not determined | no concrete suggestion | [14] |
| Lymphoma | He | Mitroxantrone | IAPs via NF-kappaB | no concrete suggestion | [31] |
| Lymphoblastic leukemia | He | Cytarabine, etoposide | not determined | no concrete suggestion | [32] |
| Chronic lymphocytic leukemia | He | Fludarabine | PI3K/Akt mediated signaling | PI3K inhibitor | [33] |
| Acute myeloid leukemia | ECM | Cytarabine | Bcl-2 via PI3K/Akt | Integrin antagonists | [16] |
| Acute myeloid leukemia | ECM | Daunorubicin | XIAP via PI3K/Akt | no concrete suggestion | [18] |
| Acute myeloid leukemia | ECM, He | Rutin, etoposide | GSK3beta | Rutin | [20] |
| Chronic myeloid leukemia | ECM | Melphalan, cytarabin, radiation | not determined | Integrin antagonists | [12] |
| Breast cancer | ECM | Paclitaxel, vincristine | PI3K/Akt mediated signaling | no concrete suggestion | [34] |
| Breast cancer | Ho | Staurosporine | Bcl-2 family | no concrete suggestion | [37] |
| Hepatocellular carcinoma | ECM | Paclitaxel | not determined | Integrin antagonists | [24] |
| Small cell lung cancer | ECM | Doxorubicin, etoposide | Tyrosine phosphorylation | Integrin antagonists | [35] |
| Glioblastoma | ECM | Etoposide | Bcl-2 family | no concrete suggestion | [36] |
| Glioblastoma | ECM | Radiation | not determined | no concrete suggestion | [38] |
| Glioblastoma | Ho, ECM | TRAIL | not determined | Carbenoxolone | [28] |
There are two major caveats frequently expressed with regards to this strategy. First, reducing cellular adherence might enhance metastasis. While obviously more work in this area needs to be done, it is worth bearing in mind, that metastasis is the cumulative effect of increased motility and invasion of the surrounding tissue. Motility is not achieved by reduced adhesion to surrounding cells, but by dynamic regulation of those adhesions. Indeed we could show for glioblastoma cells that less adhesive cells are actually less invasive [49]. Indeed it has even been suggested that under certain circumstances microenvironmental changes at a future site of metastasis precede the establishing of secondary tumors. This formation of a premetastatic niche [55,56] is mediated by a process called dynamic reciprocity and blocking the environmental interaction of cancer cells and niche should reduce metastatic spread and increase the likelihood of circulating tumor cells to undergo anoikis. Therefore, one of the side effects of targeting the tumor cells’ adhesions can actually be a reduction in size and distance from tumor bulk of metastasis [49], a more localized disease and therefore an increase of the therapeutic window; Second, it might provide a far less comfortable therapeutic window than targeting survival signals within the cancer cell to which it is inferred to be addicted. However, it should be pointed out that there have been undisputed successes in targeting the interaction of tumor cells with their surroundings (summarized in Table 1) and that several survival signals that are enhanced by AMAR are also considered key signaling cascades in cancer progression, such as, for example, PI3-K mediated signaling (Figures 2 and 3). Furthermore, the aforementioned dynamic reciprocity, which is best understood as the continuous bidirectional interaction between tumor cells and their surrounding cellular and extracellular microenvironment [57] also needs to be considered here. The microenvironment not only provides an anchorage point for genetically unstable cancer cells that mediates AMAR, signals from the tumor cells also alter the microenvironment turning it into a specialized tissue distinct from healthy, “normal” tissue without influencing its genetic make-up.
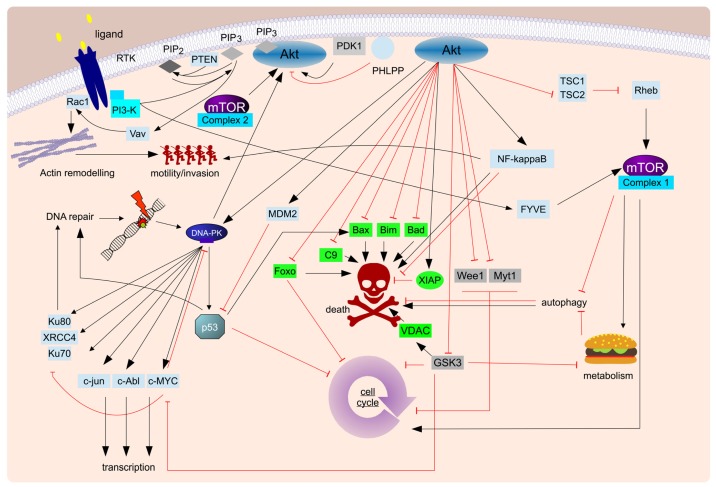
Figure 3.
The so-called PI3-K/Akt/mTOR survival pathway. The complex signaling cascades initiated by the near-apical PI3-K are usually referred to as the PI3-K/Akt/mTOR survival pathway. Shown here is a highly simplified diagram of the non-controversial interactions that have been described in the literature, with particular emphasis on the potential therapeutic targets within this signaling complex, PI3-K, mTOR, Akt and DNA-PK, while the pro-apoptic molecules that can be blocked by activation of the PI3-K/Akt/mTOR pathway are highlighted in green. Of interest are two particular aspects: (1) The complexity of interactions which—depending on cellular context, outside influences and time points investigated—can lead, in extreme cases, to diametrically opposite outcomes. For the sake of clarity the interaction and crosstalk with other equally complex signaling cascades, most notably MAP kinase signaling [48], is not shown; (2) While cellular survival is indisputably a key aspect regulated by PI3-K, other cellular aspects of equal interest to cancer research are also affected by PI3-K signaling, such as motility, metabolism, cell cycle progression and DNA damage repair (underscored). Considering both of these points it becomes apparent that effective modulation of this cascade in order to enhance therapeutic efficacy cannot be simply reduced to a strong, prolonged inhibition of the most apical proteins.
In summary, using one of the most enduring cancer models, Paget’s “Soil and Seed” hypothesis [58], we propose to target the soil and not the seed, thus more effectively preventing cancer dissemination and propagation.
2.2. Tempus Occidendi et Tempus Sanandi
Critics often unfairly refer to the current cancer treatments collectively as “cut, poison and burn” (see, for example, http://cutpoisonburn.com), there is—due to the very nature of what cancer is—a grain of truth in that statement: Successful cancer therapy will always be a balancing act between killing as many cancer cells as possible on the one hand and minimizing the side effects for the patients’ healthy tissue on the other. Aside from rare exceptions where we have a target specific to cancer, for example in the case of novel fusion proteins such as BCR-ABL [59], anything that induces apoptosis in cancer cells will also potentially induce apoptosis in healthy cells. The current trend in treatment of cancer is moving away from the so-called high dose density paradigm, administering chemotherapeutic doses at or near the limit of tolerable doses, towards the metronomic therapy [60]. The latter approach administers lower doses of drugs continuously or at frequent intervals, to reduce cytotoxic side-effects and increase treatment efficacy [61]. A strategy currently employed to tilt the scales in the patients’ favor—that is to say to further reduce the side-effects, while maintaining efficacy against the tumor—is to minimize the cancer cells’ resistance towards therapy, in essence sensitizing the tumor for apoptosis induction, allowing equal or better outcome with less side-effects. This approach is based on the alterations in signaling pathways that lead to the specific hallmarks of cancer [6].
The more essential such a pathway is and the more commonly it is altered in different cancers, the more promising it is as a potential co-target in therapy. One of the most frequently activated signaling cascade in cancer is the PI3-K/Akt/mTOR pathway (Figure 3) [62], which is not only involved in cell survival and invasion [63], but also in proliferation, DNA damage repair and cell growth/metabolic regulation [64,65]. The PTEN gene, the gene product of which is a negative regulator of PI3-K/Akt/mTOR signaling, and PIK3CA, which encodes a catalytic subunit of PI3-K, are frequently mutated in, depending on the specific cancer, around a third of all tumors [66], loss of PTEN heterozygozity is described in 59% and 76% of glioblastoma and uveal melanoma, respectively [67]. Indeed, it is often cited as the second most frequent mutated gene in cancer (for example [68]). There is also compelling preclinical evidence that inhibition of this pathway restores sensitivity to therapy in breast cancer, non-small-cell lung cancer and glioblastoma [69], while additional cancer entities, such as renal cell carcinoma, neuroblastoma, pancreatic neuroendocrine tumors and leukemia [70–73] are currently being further evaluated. At least twenty pharmacological inhibitors targeting various (isoform-) specific targets of this signaling cascade are currently at various stages of development [67]. Several clinical trials, phase I–III, suggest that most inhibitors of PI3-K are well-tolerated and have little side effects [69,74].
Interestingly, the current clinical paradigm regarding combination therapy seems to use a constant high level of a pharmacological inhibitor followed by addition of conventional chemotherapy, based on the assumption that first the survival signal has to be blocked than the drug can exert a more potent effect. However, recently, we and others have shown that timing and length of inhibition play a crucial and occasionally counter-intuitive role [75,76]. Lee and colleagues showed that maximal sensitization to doxorubicin-induced apoptosis could be achieved for triple-negative breast cancer cells by 24 h (but not 48 h) prestimulation with elotinib [75]. This sensitizing effect was not seen during co- or poststimulation with elotinib and was independent of cell cycle progression/proliferation and initial DNA damage and “dynamic rewiring of oncogenic signaling” has been proposed as cause [75]. Our own work with doxorubicin independently confirmed a similar effect in neuroblastoma (and to a limited extent glioblastoma also): Here we could show that posttreatment with NVP-BEZ235, a dual PI3-K/mTOR inhibitor, sensitizes cancer cells for doxorubicin-induced apoptosis to a far greater extent than co- or pretreatment [76]. Interestingly, pretreatment even desensitizes neuroblastoma cells for doxorubicin-induced cell death, which has lead us to propose the following model: The exposure to doxorubicin probably induces death signaling at several different sites within the cell, all of which cumulate at the mitochondria to induce cell death via the intrinsic apoptosis pathway. This death signal is not transduced further, unless inhibition of PI3-K-activated Akt leads, via GSK-3β, to Porin modulation and loss of mitochondrial membrane potential, cytochrome c release and caspase activation [76]. Therefore the role of PI3-K-mediated signaling in the context of neuroblastoma cell survival after doxorubicin exposure is at the level of mitochondria. Prolonged exposure to the inhibitor used also leads to a cell cycle arrest and the induction of autophagy [76], in essence altering the metabolism of the cell, changing the energy priorities and therefore the role of the mitochondria. Optimal sensitization is only achieved, when PI3-K-dependent signaling at the mitochondria is inhibited, but prior to metabolic alterations in the cells that occur as a consequence of prolonged inhibition of the PI3-K/Akt/mTOR network. The future challenges will lie in how to translate these findings [75,76] into a clinically relevant setting. Furthermore, algorithms and databases need to be developed to understand how each combination, which in future will be likely to grow more complex than the one inducer, one sensitizer combinations currently investigated, affects which tumor (sub)type under which conditions. This is a daunting task, but computational power and high throughput assays have finally reached a stage where it becomes a feasible and, in our opinion, promising undertaking.
In conclusion, we believe it is wrong to view most pathways as linear entities with one defined function, i.e., “the PI3-K/Akt/mTOR survival pathway”. These signaling cascades are networks with multiple often independent functions. To further improve combination therapy we need to understand not only which molecules we wish to target within a pathway, but when and for how long to target them to optimize the sensitizing effect. Furthermore, we must be aware that optimal sensitization for apoptosis is not necessarily equivalent to maximal inhibition of the targeted “survival pathway”.
2.3. Caedite eos?
Traditionally, the medical profession has always seen it as their aim to heal the patient. However, one has to consider whether this approach maximizes the patients’ chances for a long and unaffected life. As we have alluded to in Section 1, a recent trend is emerging using population genetics, and in essence treats cancerous tumors as fast evolving populations in a relatively stable environment (the human body, the microenvironment, see above). This trend is fairly new and still in its infancy, as data on the composition of tumors, the presence of subclones and the extreme heterogeneity, only now become available as large scale sequencing approaches become more common. However the strong parallels between a given population of organisms interacting with an environment and cancer cells with the tumor environment/microenvironment are hard to ignore: Any successful attempt at treating the malignancy is an environmental chance that leads to evolutionary pressure, natural selection and the emergence of a new population that is better adapted to the novel environmental conditions, or in essence a therapy-resistant subclone becomes the dominant clone. Importantly, current data indicate that said subclones are actually often already present at diagnosis, within a given tumor a large cellular heterogenity exists, reflected in altered protein expression [77]. These subpopulations are kept in check by other better adapted populations of cancer cells [78,79]. This means that the fitness landscape of a tumor is to be considered fairly rugged. Combined with recent data on the additional epigenetic landscape Huang, for example, concludes “Darwinian selection (within a tumor) is not operating at maximum efficacy” [80]. If the tumor responds to treatment, basically the induction of selective pressure, this induces an extinction event and cancer relapse is then dependent on a combination of initial population size and mutational—and presumably also epigenetic [81]—fitness distribution [82]. Basically the dynamic mechanisms at play that led to the extinction of the dinosaurs and the rise of our mammalian ancestors (who were competing for similar ecological niches) are the same that lead to the emergence of a treatment-resistant tumor after massive apoptosis induction (Figure 4). Based on this model a more flexible approach, adaptive therapy, has been proposed [83]: Using the relatively crude measurement of tumor size as determinant, the authors of this study compared an adaptive therapy, where the drug is only given when tumor size surpasses a critical volume, with a standard fixed regime. The adaptive therapy led to an overall reduced tumor burden and a prolonged chemosensitivity of the malignancy [83]. Further analysis of these findings confirm the above described model, the fitter, chemosensitive cells (i.e., the dominant subclone) suppress the growth of the less fit, but resistant cells [83]. Additional, surprising findings of these experiments have so far not been explained and need further investigation, in particular that the author needed progressively lower drug doses and longer time intervals between administrations to achieve tumor growth control. The authors of the above quoted study will no doubt be the first to acknowledge that the adaptive study performed is rather limited, tumor size is an unreliable readout that, for example, ignores the problem of metastasis, and a truly adaptive study would have used different drugs with different mechanisms of action. Nevertheless the findings are a potent proof of principle that indicates that therapies with an alternative end point, not focused on using the maximal tolerated dose of therapy to fully eradicate the tumor, can be in the long run more beneficial for the patient.
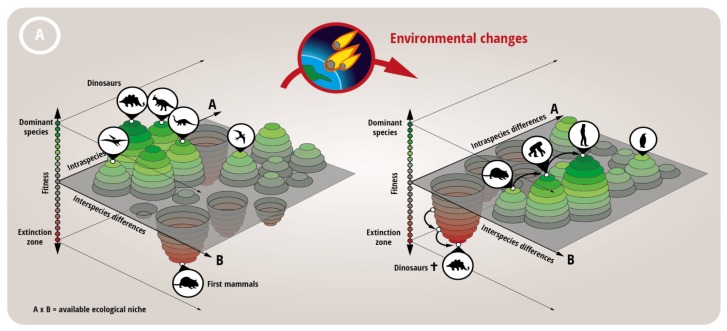
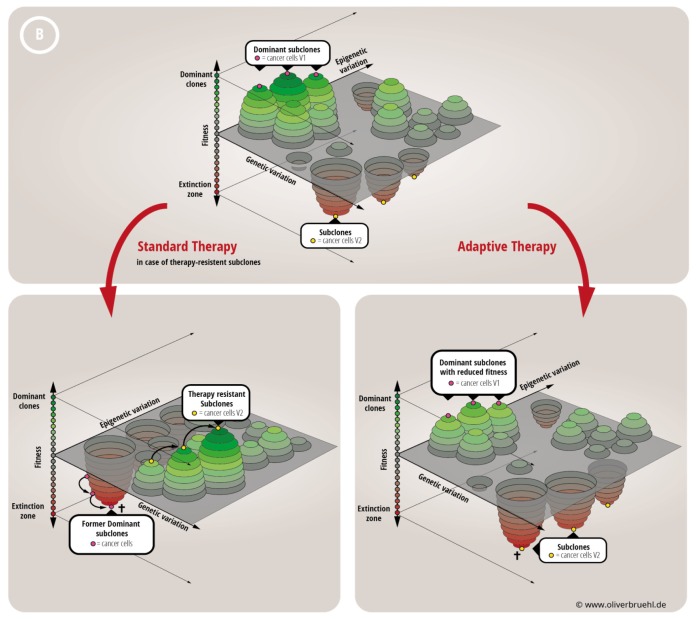
Figure 4.
Tumor cell population dynamics and the potential of chronifying treatment strategies. In recent years the subclonal and microenvironment-dependent composition of tumor populations has become apparent. However, lessons learned from population genetics and ecology have only just begun to be applied to the design of novel treatment strategies (for example [81,82,84,85]). (A) Probably the best known example of what can happen to species (simplified here as various dinosaurs and a proto-mammal) competing for a single ecological niche (Earth) upon a drastic change in the environment (a comet hitting the planet) is the extinction of the dinosaurs and rise of the mammals during Cretaceous-Paleogene extinction event (better known as K-T extinction). The alterations in the environment (which include the absence of the hitherto dominant species) allow a minor species to repopulate the niche and evolve to new dominance; (B) The dynamics described above can also be recapitulated for the ecological niche in which a tumor grows. Therapy causes drastic alterations in the tumor microenvironment (which include the absence of the hitherto dominant subclone), which can allow a minor, therapy-resistant subclone to repopulate the niche and evolve to new dominance (lower left). Traditionally alternative therapeutic approaches or dose escalation are then attempted to control the malignancy. An alternative approach, as suggested by [83] and shown in the lower right, is to reduce the overall fitness of the tumor by reducing the fitness of the dominant clone, but keep the dominance intact. This can result in a stable microenvironment and a disease stasis [83].
These ideas can also be successfully translated into a clinical setting, as our recent work on the so-called RIST therapy demonstrates [86]. We found that a therapy consisting of inhibitors targeting aberrant signaling in cancer cells (Rapamycin and Sunitinib) combined with chemotherapy (Irinotecan and Temozolomide) enhances apoptosis and reduces proliferation of glioblastoma cells compared to single agents alone, both in vitro and in vivo [86]. Importantly, as part of an experimental approach in compassionate use setting, meaning only where all convenient treatment options had already failed, children with recurrent solid tumors, particularly with glioblastoma, were treated with the RIST therapy, a metronomically designed protocol. Overall, we found that for many patients survival time was prolonged, but the tumor burden could not be completely diminished. Two index patients with glioblastoma were treated with RIST therapy after surgical resection and treatment with temozolomide and irradiation resulting in a stable tumor burden over a long period of time, up to 66 month [86]. After cessation of treatment tumor progress was noted, but, importantly, after resumption of therapy a stable state could be regained.
Interestingly, at least for a subgroup of cancers that mediate their treatment resistance not by genetic but epigenetic means (see for example [87,88], as well as our own work [89]), this approach could be particularly advantageous. Recent modeling suggests that populations consisting of individuals with high phenotypic plasticity, often exhibit—in the context of a multipeaked fitness landscape—an enhanced evolutionary rate, but at the price of decreased average fitness [90]. If these findings can indeed be translated into a tumor setting, this might indicate that tumors that quickly develop resistance to a given therapy are also the tumors which are best suited to a chronification approach.
3. Conclusions
While apoptosis is still considered the preferable mode of killing cancer cells, as healthy tissue and therefore the whole patient are less negatively affected by it than by necrosis [102], recent data also suggest an undesirable effect on surviving tumor cells [103]. A “Phoenix Rising” pathway has been proposed, whereby classical caspase-dependent apoptosis induces potent growth-stimulating signals that can induce repopulation of the tumor via Prostaglandin E2 secretion [103]. Indeed, the unsuccessful induction of apoptosis can also have several undesirable side effects, such as increased caspase-mediated tumor cell migration and metastasis [104,105], or induction of therapy resistance via damage to the tumor microenvironment [106]. In addition caspase activity has also been shown to play a key role in differentiation of stem cells [107,108] and it remains to be elucidated how this affects tumor progression. In summary, there are several aspects concerning apoptosis outside the scope of this review that need to be addressed to further improve therapeutic outcome. Nevertheless, should the points and hypotheses raised here be validated in a large-scale clinical setting, we would propose following an approach to enhance apoptosis induction during cancer therapy and thus extend the patients’ quality and quantity of life.
Inhibit cancer cell/microenvironment interaction and adhesion, thus priming the cells for apoptosis while concurrently extending the therapeutic window by blocking invasion and metastasis. Then combine conventional therapy (surgery, radio- and chemotherapy) with pharmaceutical inhibitors and therapeutic antibodies in the sense of a complex combination therapy to reduce the tumor burden. Finally, and there we see the greatest need for an open and frank debate regarding the ethical consequences, decide whether a maximal reduction in tumor burden, i.e., a cure, should be attempted, or whether a chronification of the malignancy, i.e., keeping tumor size constant at a level that minimizes the growth’s effect on the patient, would be more desirable.
It is important to point out that we do not propose to chronify all tumors and avoid any attempt of cure. There are several types of cancer where the mortality rate decreased drastically over the last decades, for example breast, prostate colon and cervical malignancies, where we see a decrease in mortality from 1975 to 2010 between 30 and almost 60 percent [109]. However, survival rates for other tumors have remained unaltered or even worsened, for example glioblastoma or melanoma respectively [109,110]. Therefore, we believe that the next major challenge in apoptosis therapy is to identify those high risk patients, not just by cancer entity but also those which harbor a particular subtype of malignancy, that are best served by the treatment schedule proposed here (Figure 5).
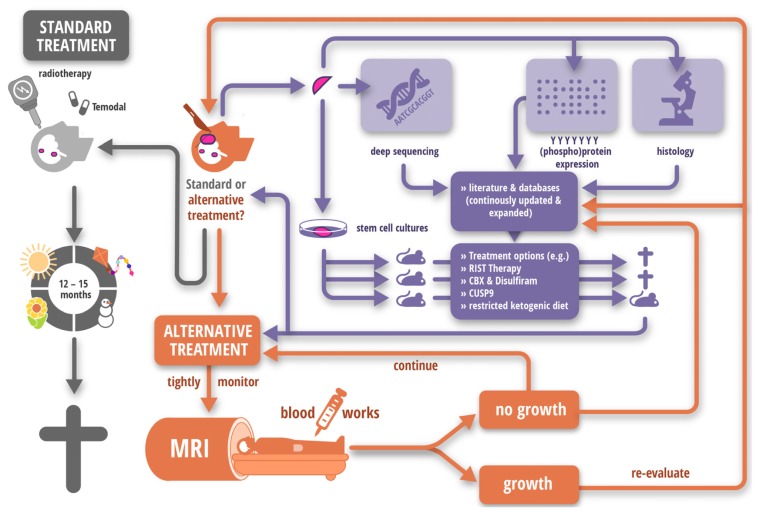
Figure 5.
Example of a hypothetical treatment strategy for Glioblastoma. Glioblastoma is the most common primary brain tumor with an average patient’s life expectancy of approximately 12–15 month post-diagnosis [91,92]. Current standard of care is maximal surgical resection, followed by radiotherapy and several courses of chemotherapy in form of Temodal [91], as shown on the left. However, another future possibility could be to use the ever increasing information obtained by new sequencing techniques [93,94], proteomic profiling [95,96] and molecular histology [97] to devise an alternative treatment strategy. Of course, the feasibility and the risks would need to be discussed with the patient and discussions should be rendered on an individual basis. Furthermore, since culturing primary patient material as spheroids stably preserves the expression patterns [98] and these cells are also able to recapitulate the patients’ tumor in a murine environment [99], using a xenotransplant as a surrogate readout for patients’ responses to various treatment options could also be feasible. Combining these data sets, an alternative treatment strategy can then be devised. Important aspects that should be considered regarding the patient’s quality of life are: (1) Chemotherapeutic components should be included at metronomic doses to minimize adverse reactions/side effects; (2) The components should be easily administered to avoid repeated hospital stays, i.e., oral administration is preferable to IV infusions. We feel that several approaches might fulfill these criteria with regards to glioblastoma, e.g., restricted ketogenic diet [100], CUSP9 [101], or our own approaches, the RIST protocol [86] or treatment with CBX [28] and Disulfiram [49]. The success of all of these approaches have been shown to varying degrees to be dependent on the AMAR, sequential dosing and chronification. Importantly, tumor progression needs to be tightly monitored, as well as patient’s general well-being, and should the tumor size not remain constant, therapy needs to be adapted accordingly. To avoid the emergence of therapy-resistant clones, consequently the treatment needs to be adapted when the tumor responds to treatment too well, and shrinks.
Acknowledgments
We would like to thank Angelika Vollmer for critically reading the first draft of the manuscript, Katia La Ferla-Brühl for her scientific input and all our students, past and present, whose enthusiastic feedback after our lecture courses in Medicine and Molecular Medicine helped us to sharpen and redefine many of the arguments mentioned herein.
Finally, we would like to dedicate this work to Fiona Jardine whose untimely death during the writing of this manuscript served as a potent reminder that we have still a long way to go, while her courage is an inspiration for us all.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kaczmarek A., Vandenabeele P., Krysko D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Levine B., Yuan J. Autophagy in cell death: An innocent convict? J. Clin. Investig. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maghsoudi N., Zakeri Z., Lockshin R.A. Programmed cell death and apoptosis—Where it came from and where it is going: From elie metchnikoff to the control of caspases. Exp. Oncol. 2012;34:146–152. [PubMed] [Google Scholar]
- 6.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland R.M., Durand R.E. Radiosensitization by nifuroxime of the hypoxic cells in an in vitro tumour model. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1972;22:613–618. doi: 10.1080/09553007214551511. [DOI] [PubMed] [Google Scholar]
- 9.Durand R.E., Sutherland R.M. Radiation-resistant tumor cells may be more sensitive in vitro. Cancer Res. 1972;32:2587–2588. [PubMed] [Google Scholar]
- 10.Sutherland R.M., Durand R.E. Cell contact as a possible contribution to radiation resistance of some tumours. Br. J. Radiol. 1972;45:788–789. doi: 10.1259/0007-1285-45-538-788. [DOI] [PubMed] [Google Scholar]
- 11.Chiarugi P., Giannoni E. Anoikis: A necessary death program for anchorage-dependent cells. Biochem. Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Damiano J.S., Hazlehurst L.A., Dalton W.S. Cell adhesion-mediated drug resistance (cam-dr) protects the k562 chronic myelogenous leukemia cell line from apoptosis induced by bcr/abl inhibition cytotoxic drugs and gamma-irradiation. Leukemia. 2001;15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- 13.Schmidmaier R., Baumann P., Simsek M., Dayyani F., Emmerich B., Meinhardt G. The hmg-coa reductase inhibitor simvastatin overcomes cell adhesion-mediated drug resistance in multiple myeloma by geranylgeranylation of rho protein and activation of rho kinase. Blood. 2004;104:1825–1832. doi: 10.1182/blood-2003-12-4218. [DOI] [PubMed] [Google Scholar]
- 14.Hazlehurst L.A., Argilagos R.F., Emmons M., Boulware D., Beam C.A., Sullivan D.M., Dalton W.S. Cell adhesion to fibronectin (cam-dr) influences acquired mitoxantrone resistance in u937 cells. Cancer Res. 2006;66:2338–2345. doi: 10.1158/0008-5472.CAN-05-3256. [DOI] [PubMed] [Google Scholar]
- 15.Yanamandra N., Colaco N.M., Parquet N.A., Buzzeo R.W., Boulware D., Wright G., Perez L.E., Dalton W.S., Beaupre D.M. Tipifarnib and bortezomib are synergistic and overcome cell adhesion-mediated drug resistance in multiple myeloma and acute myeloid leukemia. Clin. Cancer Res. 2006;12:591–599. doi: 10.1158/1078-0432.CCR-05-1792. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga T., Fukai F., Miura S., Nakane Y., Owaki T., Kodama H., Tanaka M., Nagaya T., Takimoto R., Takayama T., et al. Combination therapy of an anticancer drug with the fniii14 peptide of fibronectin effectively overcomes cell adhesion-mediated drug resistance of acute myelogenous leukemia. Leukemia. 2008;22:353–360. doi: 10.1038/sj.leu.2405017. [DOI] [PubMed] [Google Scholar]
- 17.Nimmanapalli R., Gerbino E., Dalton W.S., Gandhi V., Alsina M. Hsp70 inhibition reverses cell adhesion mediated and acquired drug resistance in multiple myeloma. Br. J. Haematol. 2008;142:551–561. doi: 10.1111/j.1365-2141.2008.07217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X., Wang C., Qin Y.W., Yan S.K., Gao Y.R. The association of up-regulation of x-linked inhibitor of apoptosis protein with cell adhesion-mediated drug resistance in u937 cells. Hematol. Oncol. 2008;26:21–26. doi: 10.1002/hon.828. [DOI] [PubMed] [Google Scholar]
- 19.Noborio-Hatano K., Kikuchi J., Takatoku M., Shimizu R., Wada T., Ueda M., Nobuyoshi M., Oh I., Sato K., Suzuki T., et al. Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of vla-4 expression in multiple myeloma. Oncogene. 2009;28:231–242. doi: 10.1038/onc.2008.385. [DOI] [PubMed] [Google Scholar]
- 20.Bourogaa E., Bertrand J., Despeaux M., Jarraya R., Fabre N., Payrastre L., Demur C., Fournie J.J., Damak M., Feki A.E., et al. Hammada scoparia flavonoids and rutin kill adherent and chemoresistant leukemic cells. Leuk. Res. 2011;35:1093–1101. doi: 10.1016/j.leukres.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Mraz M., Zent C.S., Church A.K., Jelinek D.F., Wu X., Pospisilova S., Ansell S.M., Novak A.J., Kay N.E., Witzig T.E., et al. Bone marrow stromal cells protect lymphoma b-cells from rituximab-induced apoptosis and targeting integrin α-4-β-1 (vla-4) with natalizumab can overcome this resistance. Br. J. Haematol. 2011;155:53–64. doi: 10.1111/j.1365-2141.2011.08794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiziltepe T., Ashley J.D., Stefanick J.F., Qi Y.M., Alves N.J., Handlogten M.W., Suckow M.A., Navari R.M., Bilgicer B. Rationally engineered nanoparticles target multiple myeloma cells overcome cell-adhesion-mediated drug resistance and show enhanced efficacy in vivo. Blood Cancer J. 2012;2:e64. doi: 10.1038/bcj.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hazlehurst L.A., Valkov N., Wisner L., Storey J.A., Boulware D., Sullivan D.M., Dalton W.S. Reduction in drug-induced DNA double-strand breaks associated with β1 integrin-mediated adhesion correlates with drug resistance in u937 cells. Blood. 2001;98:1897–1903. doi: 10.1182/blood.v98.6.1897. [DOI] [PubMed] [Google Scholar]
- 24.Zhu B., Zhao L., Zhu L., Wang H., Sha Y., Yao J., Li Z., You Q., Guo Q. Oroxylin a reverses cam-dr of hepg2 cells by suppressing integrinβ1 and its related pathway. Toxicol. Appl. Pharmacol. 2012;259:387–394. doi: 10.1016/j.taap.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Bjorklund C.C., Baladandayuthapani V., Lin H.Y., Jones R.J., Kuiatse I., Wang H., Yang J., Shah J.J., Thomas S.K., Wang M., et al. Evidence of a role for cd44 and cell adhesion in mediating resistance to lenalidomide in multiple myeloma: Therapeutic implications. Leukemia. 2014;28:373–383. doi: 10.1038/leu.2013.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fei M., Hang Q., Hou S., Ruan C. Cell adhesion to fibronectin down-regulates the expression of spy1 and contributes to drug resistance in multiple myeloma cells. Int. J. Hematol. 2013;98:446–455. doi: 10.1007/s12185-013-1435-4. [DOI] [PubMed] [Google Scholar]
- 27.Damiano J.S., Cress A.E., Hazlehurst L.A., Shtil A.A., Dalton W.S. Cell adhesion mediated drug resistance (cam-dr): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 28.Westhoff M.A., Zhou S., Bachem M.G., Debatin K.M., Fulda S. Identification of a novel switch in the dominant forms of cell adhesion-mediated drug resistance in glioblastoma cells. Oncogene. 2008;27:5169–5181. doi: 10.1038/onc.2008.148. [DOI] [PubMed] [Google Scholar]
- 29.Shain K.H., Landowski T.H., Dalton W.S. Adhesion-mediated intracellular redistribution of c-fas-associated death domain-like il-1-converting enzyme-like inhibitory protein-long confers resistance to cd95-induced apoptosis in hematopoietic cancer cell lines. J. Immunol. 2002;168:2544–2553. doi: 10.4049/jimmunol.168.5.2544. [DOI] [PubMed] [Google Scholar]
- 30.Taylor S.T., Hickman J.A., Dive C. Epigenetic determinants of resistance to etoposide regulation of bcl-x(l) and bax by tumor microenvironmental factors. J. Natl. Cancer Inst. 2000;92:18–23. doi: 10.1093/jnci/92.1.18. [DOI] [PubMed] [Google Scholar]
- 31.Lwin T., Hazlehurst L.A., Li Z., Dessureault S., Sotomayor E., Moscinski L.C., Dalton W.S., Tao J. Bone marrow stromal cells prevent apoptosis of lymphoma cells by upregulation of anti-apoptotic proteins associated with activation of NF-κB (relb/p52) in non-hodgkin’s lymphoma cells. Leukemia. 2007;21:1521–1531. doi: 10.1038/sj.leu.2404723. [DOI] [PubMed] [Google Scholar]
- 32.Fortney J.E., Zhao W., Wenger S.L., Gibson L.F. Bone marrow stromal cells regulate caspase 3 activity in leukemic cells during chemotherapy. Leuk. Res. 2001;25:901–907. doi: 10.1016/s0145-2126(01)00051-0. [DOI] [PubMed] [Google Scholar]
- 33.Niedermeier M., Hennessy B.T., Knight Z.A., Henneberg M., Hu J., Kurtova A.V., Wierda W.G., Keating M.J., Shokat K.M., Burger J.A. Isoform-selective phosphoinositide 3′-kinase inhibitors inhibit cxcr4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: A novel therapeutic approach. Blood. 2009;113:5549–5557. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoudjit F., Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 35.Sethi T., Rintoul R.C., Moore S.M., MacKinnon A.C., Salter D., Choo C., Chilvers E.R., Dransfield I., Donnelly S.C., Strieter R., et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: A mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 36.Uhm J.H., Dooley N.P., Kyritsis A.P., Rao J.S., Gladson C.L. Vitronectin a glioma-derived extracellular matrix protein protects tumor cells from apoptotic death. Clin. Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- 37.Wang L., Li Z., Wang C., Yang Y., Sun L., Yao W., Cai X., Wu G., Zhou F., Zha X. E-cadherin decreased human breast cancer cells sensitivity to staurosporine by up-regulating bcl-2 expression. Arch. Biochem. Biophys. 2009;481:116–122. doi: 10.1016/j.abb.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Cordes N., Meineke V. Cell adhesion-mediated radioresistance (cam-rr) Extracellular matrix-dependent improvement of cell survival in human tumor and normal cells in vitro. Strahlenther. Onkol. 2003;179:337–344. doi: 10.1007/s00066-003-1074-4. [DOI] [PubMed] [Google Scholar]
- 39.Sandfort V., Koch U., Cordes N. Cell adhesion-mediated radioresistance revisited. Int. J. Radiat. Biol. 2007;83:727–732. doi: 10.1080/09553000701694335. [DOI] [PubMed] [Google Scholar]
- 40.Westhoff M.A., Fulda S. Adhesion-mediated apoptosis resistance in cancer. Drug Resist. Updat. 2009;12:127–136. doi: 10.1016/j.drup.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Dolberg D.S., Bissell M.J. Inability of rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 42.Bissell M.J., Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoelzinger D.B., Demuth T., Berens M.E. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J. Natl. Cancer Inst. 2007;99:1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 44.Pullan S., Wilson J., Metcalfe A., Edwards G.M., Goberdhan N., Tilly J., Hickman J.A., Dive C., Streuli C.H. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J. Cell. Sci. 1996;109:631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- 45.Chidgey M., Dawson C. Desmosomes: A role in cancer? Br. J. Cancer. 2007;96:1783–1787. doi: 10.1038/sj.bjc.6603808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morin P.J. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005;65:9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 47.Langlois M.J., Bergeron S., Bernatchez G., Boudreau F., Saucier C., Perreault N., Carrier J.C., Rivard N. The pten phosphatase controls intestinal epithelial cell polarity and barrier function: Role in colorectal cancer progression. PLoS One. 2010;5:e15742. doi: 10.1371/journal.pone.0015742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendoza M.C., Er E.E., Blenis J. The ras-erk and pi3k-mtor pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westhoff M.A., Zhou S., Nonnenmacher L., Karpel-Massler G., Jennewein C., Schneider M., Halatsch M.E., Carragher N.O., Baumann B., Krause A., et al. Inhibition of NF-κB signaling ablates the invasive phenotype of glioblastoma. Mol. Cancer Res. 2013;11:1611–1623. doi: 10.1158/1541-7786.MCR-13-0435-T. [DOI] [PubMed] [Google Scholar]
- 50.Paolillo M., Russo M.A., Serra M., Colombo L., Schinelli S. Small molecule integrin antagonists in cancer therapy. Mini Rev. Med. Chem. 2009;9:1439–1446. doi: 10.2174/138955709789957404. [DOI] [PubMed] [Google Scholar]
- 51.Mas-Moruno C., Rechenmacher F., Kessler H. Cilengitide: The first anti-angiogenic small molecule drug candidate design synthesis and clinical evaluation. Anticancer Agents Med. Chem. 2010;10:753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller D.H., Weber T., Grove R., Wardell C., Horrigan J., Graff O., Atkinson G., Dua P., Yousry T., Macmanus D., et al. Firategrast for relapsing remitting multiple sclerosis: A phase 2 randomised double-blind placebo-controlled trial. Lancet Neurol. 2012;11:131–139. doi: 10.1016/S1474-4422(11)70299-X. [DOI] [PubMed] [Google Scholar]
- 53.Tomlinson I.P., Novelli M.R., Bodmer W.F. The mutation rate and cancer. Proc. Natl. Acad. Sci. USA. 1996;93:14800–14803. doi: 10.1073/pnas.93.25.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu M., Polyak K. Microenvironmental regulation of cancer development. Curr. Opin. Genet. Dev. 2008;18:27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaplan R.N., Rafii S., Lyden D. Preparing the “Soil”: The premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sceneay J., Smyth M.J., Moller A. The pre-metastatic niche: Finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 57.Schultz G.S., Davidson J.M., Kirsner R.S., Bornstein P., Herman I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paget S. The distribution of secondary growths in cancer of the breast 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 59.An X., Tiwari A.K., Sun Y., Ding P.R., Ashby C.R., Jr, Chen Z.S. Bcr-abl tyrosine kinase inhibitors in the treatment of philadelphia chromosome positive chronic myeloid leukemia: A review. Leuk. Res. 2010;34:1255–1268. doi: 10.1016/j.leukres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Gately S., Kerbel R. Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J. 2001;7:427–436. [PubMed] [Google Scholar]
- 61.Kerbel R., Folkman J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 62.Yuan T.L., Cantley L.C. Pi3k pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cassinelli G., Zuco V., Gatti L., Lanzi C., Zaffaroni N., Colombo D., Perego P. Targeting the akt kinase to modulate survival invasiveness and drug resistance of cancer cells. Curr. Med. Chem. 2013;20:1923–1945. doi: 10.2174/09298673113209990106. [DOI] [PubMed] [Google Scholar]
- 64.Franke T.F. Pi3k/akt: Getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 65.Hemmings B.A., Restuccia D.F. Pi3k-pkb/akt pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chalhoub N., Baker S.J. Pten and the pi3-kinase pathway in cancer. Annu. Rev. Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brana I., Siu L.L. Clinical development of phosphatidylinositol 3-kinase inhibitors for cancer treatment. BMC Med. 2012;10:161. doi: 10.1186/1741-7015-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin Y., Shen W.H. Pten: A new guardian of the genome. Oncogene. 2008;27:5443–5453. doi: 10.1038/onc.2008.241. [DOI] [PubMed] [Google Scholar]
- 69.Burris H.A., 3rd Overcoming acquired resistance to anticancer therapy: Focus on the pi3k/akt/mtor pathway. Cancer Chemother. Pharmacol. 2013;71:829–842. doi: 10.1007/s00280-012-2043-3. [DOI] [PubMed] [Google Scholar]
- 70.Figlin R.A., Kaufmann I., Brechbiel J. Targeting pi3k and mtorc2 in metastatic renal cell carcinoma: New strategies for overcoming resistance to vegfr and mtorc1 inhibitors. Int. J. Cancer. 2013;133:788–796. doi: 10.1002/ijc.28023. [DOI] [PubMed] [Google Scholar]
- 71.Fulda S. The pi3k/akt/mtor pathway as therapeutic target in neuroblastoma. Curr. Cancer Drug Targets. 2009;9:729–737. doi: 10.2174/156800909789271521. [DOI] [PubMed] [Google Scholar]
- 72.Wolin E.M. Pi3k/akt/mtor pathway inhibitors in the therapy of pancreatic neuroendocrine tumors. Cancer Lett. 2013;335:1–8. doi: 10.1016/j.canlet.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 73.Okumura N., Yoshida H., Kitagishi Y., Murakami M., Nishimura Y., Matsuda S. Pi3k/akt/pten signaling as a molecular target in leukemia angiogenesis. Adv. Hematol. 2012;2012:843085. doi: 10.1155/2012/843085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen P.Y., Lee E.Q., Reardon D.A., Ligon K.L., Alfred Yung W.K. Current clinical development of pi3k pathway inhibitors in glioblastoma. Neuro Oncol. 2012;14:819–829. doi: 10.1093/neuonc/nos117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee M.J., Ye A.S., Gardino A.K., Heijink A.M., Sorger P.K., MacBeath G., Yaffe M.B. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149:780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Westhoff M.A., Faham N., Marx D., Nonnenmacher L., Jennewein C., Enzenmüller S., Gonzalez P., Fulda S., Debatin K.M. Sequential dosing in chemosensitization: Targeting the PI3K/Akt/mTOR pathway in neuroblastoma. PLoS One. 2013;8:e83128. doi: 10.1371/journal.pone.0083128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoelzinger D.B., Mariani L., Weis J., Woyke T., Berens T.J., McDonough W.S., Sloan A., Coons S.W., Berens M.E. Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia. 2005;7:7–16. doi: 10.1593/neo.04535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mullighan C.G., Phillips L.A., Su X., Ma J., Miller C.B., Shurtleff S.A., Downing J.R. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322:1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Denison T.A., Bae Y.H. Tumor heterogeneity and its implication for drug delivery. J. Control. Release. 2012;164:187–191. doi: 10.1016/j.jconrel.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang S. Genetic and non-genetic instability in tumor progression: Link between the fitness landscape and the epigenetic landscape of cancer cells. Cancer Metastasis Rev. 2013;32:423–448. doi: 10.1007/s10555-013-9435-7. [DOI] [PubMed] [Google Scholar]
- 81.Brock A., Chang H., Huang S. Non-genetic heterogeneity—A mutation-independent driving force for the somatic evolution of tumours. Nat. Rev. Genet. 2009;10:336–342. doi: 10.1038/nrg2556. [DOI] [PubMed] [Google Scholar]
- 82.Foo J., Leder K., Mumenthaler S.M. Cancer as a moving target: Understanding the composition and rebound growth kinetics of recurrent tumors. Evol. Appl. 2013;6:54–69. doi: 10.1111/eva.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gatenby R.A., Silva A.S., Gillies R.J., Frieden B.R. Adaptive therapy. Cancer Res. 2009;69:4894–4903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gatenby R.A., Brown J., Vincent T. Lessons from applied ecology: Cancer control using an evolutionary double bind. Cancer Res. 2009;69:7499–7502. doi: 10.1158/0008-5472.CAN-09-1354. [DOI] [PubMed] [Google Scholar]
- 85.Durrett R. Population genetics of neutral mutations in exponentially growing cancer cell populations. Ann. Appl. Probab. 2013;23:230–250. doi: 10.1214/11-aap824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nonnenmacher L., Westhoff M.A., Fulda S., Karpel-Massler G., Halatsch M., Engelke J., Simmet T., Gorbacioglu S., Debatin K.M. RIST: A potent new combination therapy for glioblastoma. Clin. Cancer Res. 2014 doi: 10.1002/ijc.29138. to be submitted. [DOI] [PubMed] [Google Scholar]
- 87.Zhou J., Bi C., Janakakumara J.V., Liu S.C., Chng W.J., Tay K.G., Poon L.F., Xie Z., Palaniyandi S., Yu H., et al. Enhanced activation of stat pathways and overexpression of survivin confer resistance to flt3 inhibitors and could be therapeutic targets in aml. Blood. 2009;113:4052–4062. doi: 10.1182/blood-2008-05-156422. [DOI] [PubMed] [Google Scholar]
- 88.Stolzel F., Steudel C., Oelschlagel U., Mohr B., Koch S., Ehninger G., Thiede C. Mechanisms of resistance against pkc412 in resistant flt3-itd positive human acute myeloid leukemia cells. Ann. Hematol. 2010;89:653–662. doi: 10.1007/s00277-009-0889-1. [DOI] [PubMed] [Google Scholar]
- 89.Herrmann M.D., Lennerz J.K., Bullinger L., Bartholomae S., Holzmann K., Westhoff M.A., Corbacioglu S., Debatin K.M. Transitory dasatinib-resistant states in kitmut t(8;21) acute myeloid leukemia cells correlate with altered kit expression. Exp. Hematol. 2014;42:90–100. doi: 10.1016/j.exphem.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 90.Saito N., Ishihara S., Kaneko K. Baldwin effect under multipeaked fitness landscapes: Phenotypic fluctuation accelerates evolutionary rate. Phys. Rev. E. 2013;87:052701. doi: 10.1103/PhysRevE.87.052701. [DOI] [PubMed] [Google Scholar]
- 91.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 92.Johnson D.R., O’Neill B.P. Glioblastoma survival in the united states before and during the temozolomide era. J. Neurooncol. 2011;107:359–364. doi: 10.1007/s11060-011-0749-4. [DOI] [PubMed] [Google Scholar]
- 93.Kim M., Lee K.H., Yoon S.W., Kim B.S., Chun J., Yi H. Analytical tools and databases for metagenomics in the next-generation sequencing era. Genomics Inform. 2013;11:102–113. doi: 10.5808/GI.2013.11.3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee S.H., Sim S.H., Kim J.Y., Cha S., Song A. Application of cancer genomics to solve unmet clinical needs. Genomics Inform. 2013;11:174–179. doi: 10.5808/GI.2013.11.4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Van Oostrum J., Calonder C., Rechsteiner D., Ehrat M., Mestan J., Fabbro D., Voshol H. Tracing pathway activities with kinase inhibitors and reverse phase protein arrays. Proteomics Clin. Appl. 2009;3:412–422. doi: 10.1002/prca.200800070. [DOI] [PubMed] [Google Scholar]
- 96.Carragher N.O., Unciti-Broceta A., Cameron D.A. Advancing cancer drug discovery towards more agile development of targeted combination therapies. Future Med. Chem. 2012;4:87–105. doi: 10.4155/fmc.11.169. [DOI] [PubMed] [Google Scholar]
- 97.Huse J.T., Holland E.C. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer. 2010;10:319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 98.De Witt Hamer P.C., van Tilborg A.A., Eijk P.P., Sminia P., Troost D., van Noorden C.J., Ylstra B., Leenstra S. The genomic profile of human malignant glioma is altered early in primary cell culture and preserved in spheroids. Oncogene. 2008;27:2091–2096. doi: 10.1038/sj.onc.1210850. [DOI] [PubMed] [Google Scholar]
- 99.Pollard S.M., Yoshikawa K., Clarke I.D., Danovi D., Stricker S., Russell R., Bayani J., Head R., Lee M., Bernstein M., et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 100.Seyfried T.N., Marsh J., Shelton L.M., Huysentruyt L.C., Mukherjee P. Is the restricted ketogenic diet a viable alternative to the standard of care for managing malignant brain cancer? Epilepsy Res. 2011;100:310–326. doi: 10.1016/j.eplepsyres.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 101.Kast R.E., Boockvar J.A., Bruning A., Cappello F., Chang W.W., Cvek B., Dou Q.P., Duenas-Gonzalez A., Efferth T., Focosi D., et al. A conceptually new treatment approach for relapsed glioblastoma: Coordinated undermining of survival paths with nine repurposed drugs (cusp9) by the international initiative for accelerated improvement of glioblastoma care. Oncotarget. 2013;4:502–530. doi: 10.18632/oncotarget.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amaravadi R.K., Thompson C.B. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin. Cancer Res. 2007;13:7271–7279. doi: 10.1158/1078-0432.CCR-07-1595. [DOI] [PubMed] [Google Scholar]
- 103.Huang Q., Li F., Liu X., Li W., Shi W., Liu F.F., O’Sullivan B., He Z., Peng Y., Tan A.C., et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barbero S., Mielgo A., Torres V., Teitz T., Shields D.J., Mikolon D., Bogyo M., Barila D., Lahti J.M., Schlaepfer D., et al. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009;69:3755–3763. doi: 10.1158/0008-5472.CAN-08-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gdynia G., Grund K., Eckert A., Bock B.C., Funke B., Macher-Goeppinger S., Sieber S., Herold-Mende C., Wiestler B., Wiestler O.D., et al. Basal caspase activity promotes migration and invasiveness in glioblastoma cells. Mol. Cancer Res. 2007;5:1232–1240. doi: 10.1158/1541-7786.MCR-07-0343. [DOI] [PubMed] [Google Scholar]
- 106.Sun Y., Campisi J., Higano C., Beer T.M., Porter P., Coleman I., True L., Nelson P.S. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through wnt16b. Nat. Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Basu S., Rajakaruna S., Menko A.S. Insulin-like growth factor receptor-1 and nuclear factor kappab are crucial survival signals that regulate caspase-3-mediated lens epithelial cell differentiation initiation. J. Biol. Chem. 2012;287:8384–8397. doi: 10.1074/jbc.M112.341586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fernando P., Brunette S., Megeney L.A. Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J. 2005;19:1671–1673. doi: 10.1096/fj.04-2981fje. [DOI] [PubMed] [Google Scholar]
- 109.Esserman L.J., Thompson I.M., Jr, Reid B. Overdiagnosis and overtreatment in cancer: An opportunity for improvement. JAMA. 2013;310:797–798. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 110.Wen P.Y., Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]