Abstract
A series of new 2-aminobenzamide derivatives (1–10) has been synthesized in good to excellent yields by adopting both conventional and/or a time-efficient microwave assisted methodologies starting from isatoic anhydride (ISA) and characterized on the basis of their physical, spectral and microanalytical data. Selected compounds of this series were then tested against various bacterial (Bacillus subtilis (RCMB 000107) and Staphylococcus aureus (RCMB 000106). Pseudomonas aeruginosa (RCMB 000102) and Escherichia coli (RCMB 000103) and fungal strains (Saccharomyces cerevisiae (RCMB 006002), Aspergillus fumigatus (RCMB 002003) and Candida albicans (RCMB 005002) to explore their potential as antimicrobial agents. Compound 5 was found to be the most active compound among those tested, which showed excellent antifungal activity against Aspergillus fumigatus (RCMB 002003) more potent than standard Clotrimazole, and moderate to good antibacterial and antifungal activity against most of the other strains of bacteria and fungi. Furthermore, potential pharmacophore sites were identified and their activity was related with the structures in the solution.
Keywords: isatoic anhydride, conventional method, microwave method, 2-aminobenzamide, antimicrobial activity
1. Introduction
Isatoic anhydride (2H 3,1–benzoxacin-2,4 (1H)-dione, ISA) is a special compound in organic synthesis, first discovered by Friedländer and Wleügel [1] due to its physicochemical properties and special reactivity features which have been very well exploited in the recent past. Its tremendous importance can be exemplified by the presence of a great number of research papers and patents in the chemical literature, especially in the industrial field where it has been applied for manufacturing agrochemicals, desiccants, paints, fragrances and drug products [2,3]. Recently, this compound has been used as an agent for clinical diagnosis in biological research [4] and as starting material for the synthesis of various antimicrobial agents [5]. Most importantly, isatoic anhydrides serves the role of intermediate in the synthesis of a variety of heterocyclic compounds, such as quinazolinones, quinazolones, benzimidazolones, phthalimides, pyrroloquinazolones, quinazolinediones, in addition to its use in the fluorescent labeling of mRNA and tRNA [6–9]. Among the various heterocycles derived from ISA, quinazolones are the most prominent due to their various biological effects including antihypertensive [10], antimicrobial [11–13], antihyperlipidemic [14,15], antioxidant [16], anti-inflammatory [17–19], anticonvulsant [20,21], and anticancer activities [22–25].
Owing to the remarkable applications of isatoic anhydride in organic synthesis and medicinal chemistry, we decided to undertake this study. Herein, we report the synthesis of some novel 2-aminobenzamide derivatives by two different methods in good to excellent yields starting from ISA (Scheme 1) and their antimicrobial activity. We found that conventional method (simply heating respective amine with ISA in a suitable solvent) is better than the time-efficient microwave assisted method for the synthesis of such compounds. In antimicrobial screening, compound 5 showed excellent antimicrobial potential, whereas other compounds exhibited low to moderate antimicrobial activity. The factors which affect the activity of these compounds were also identified and reasons for low or moderate activities of certain compounds were explored.
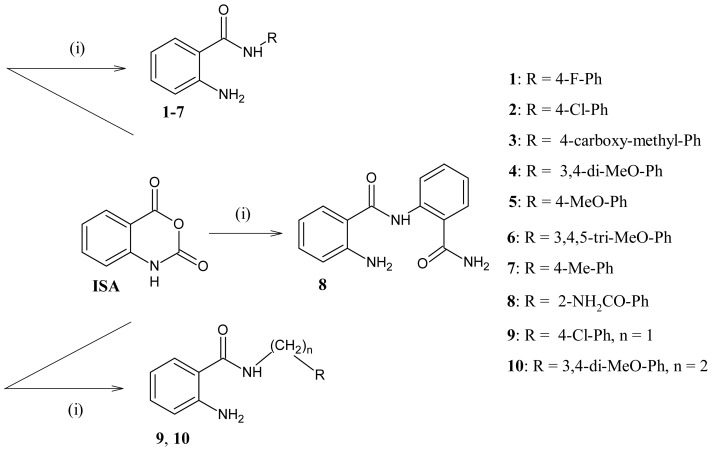
Scheme 1.
Synthesis of 2-aminobenzamide derivatives (1–10) from isatoic anhydride (i): R-NH2 in heating dimethylformamide (DMF).
2. Results and Discussion
2.1. Chemistry
The chemical advantages shown by ISA as precursor for a variety of medicinally important derivatives lie mainly in its capacity to react through two fundamental sites: (a) C8 position of the aromatic ring through electrophilic aromatic substitution or C10 if C8 is occupied by another atom or functional group; and (b) the more electrophilic carbonyl group though nucleophilic acyl substitution reaction generally by losing a CO2 group. In addition, it may also react through N atom due to its electronic properties to give some useful products [26].
In the present work, we utilized two different methods, the classical procedure (isatoic anhydride, 1.equiv. plus amine derivatives 1.equiv. in DMF solvent), and the simple, mild and time efficient solvent-free microwave mediated methodology for the synthesis of 2-aminobenzamide derivatives 1–10. Mechanistically, the products 1–10 were formed by initial nucleophilic attack of amine derivatives on electrophilic carbonyl group followed by ring opening and elimination of CO2 molecules. All the synthesized compounds were fully characterized on the basis of their physical, spectral (IR, NMR, MS) and microanalytical data.
In this context, the synthesis of 2-aminobenzamide derivatives has been proven either by classical method or simple, mild, time efficient, and environment friendly microwave irradiation procedure.
We tried our best to get all compounds of this new series by using the two methods but the microwave method led us to less important yields than conventional method. This is probably due to thermo-sensibility of compounds.
2.2. Biological Activity
Selected compounds 1–3, 5 and 7 were screened for their antimicrobial activities. Preliminary screening [27] of the synthesized compounds was performed against three Gram-positive bacteria (Bacillus subtilis (RCMB 000107) and Staphylococcus aureus (RCMB 000106), two Gram-negative bacteria (Pseudomonas aeruginosa (RCMB 000102) and Escherichia coli (RCMB 000103), and three fungi (Saccharomyces cerevisiae (RCMB 006002), Aspergillus fumigatus (RCMB 002003) and Candida albicans (RCMB 005002), using the bioassay technique of antibiotics specified in US pharmacopeia at (25 μg/mL). Most of the tested compounds showed moderate to good activity against one or more bacterial and/or fungal strains. However, the compound 5 was found to be the most active as compared to all other tested compounds with excellent antifungal activity (even more than the standard drug Clotrimazole) against Aspergillus fumigatus (RCMB 002003) (Table 1). It also showed excellent antifungal activity against Saccharomyces cerevislae (RCMB 006002) slightly less than the standard drug and good antibacterial activity against all bacterial strains . The results of the present investigation confirm the tremendous biological potential of compound 5 and suggest that it should be concentrated for further research. The results of antimicrobial studies are summarized in Table 1.
Table 1.
Antimicrobial activity (inhibition zones in mm) of some of compounds 1–10 [e].
| Compound | Bacteria | Fungi | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| (A) [a] | (B) [a] | (C) [b] | (D) [b] | (E) | (F) | (G) | |
| 1 | 9.9 | 9.7 | 9.1 | 9.4 | 11.8 | 11.2 | 11.9 |
| 2 | 7.2 | 7.8 | 7.4 | 6.9 | 9.4 | 8.2 | 8.4 |
| 3 | 11.9 | 11.1 | 10.4 | 9.5 | 11.8 | 11.5 | 11.2 |
| 5 | 17.9 | 19.2 | 10.9 | 12.4 | 19.7 | 20.1 | 15.8 |
| 7 | 9.1 | 8.8 | 7.9 | 7.6 | 12.2 | 9.4 | 7.2 |
| CLOZO [c] | - | - | - | - | 18.3 | 23.1 | 26.1 |
| STREPT [d] | 25.1 | 30.1 | 25.6 | 24.3 | - | - | - |
(A): Staphylococcus aureus (RCMB 000106); (B): Bacillis subtilis (RCMB 000107); (C): Pseudomonas aeruginosa (RCMB 000102); (D): Escherichia coli (RCMB 000103); (E): Aspergillus fumigatus (RCMB 002003); (F): Saccharomyces cerevislae (RCMB 006002); (G): Candida albicans (RCMB 005002);
Antibacterial Gram-positive;
Antibacterial Gram-negative;
CLOZO: Clotrimazole (St. 25 μL/mL);
STREPT; Streptomycin (St. 25 μL/mL);
Mean zone of inhibition in mm ± standard deviation beyond well diameter (6 mm) produced on a range of environmental and clinically pathogenic microorganism using (5 mg/mL) concentration of tested samples.
2.3. Identification of Antimicrobial Pharmacophore Sites of 1–10
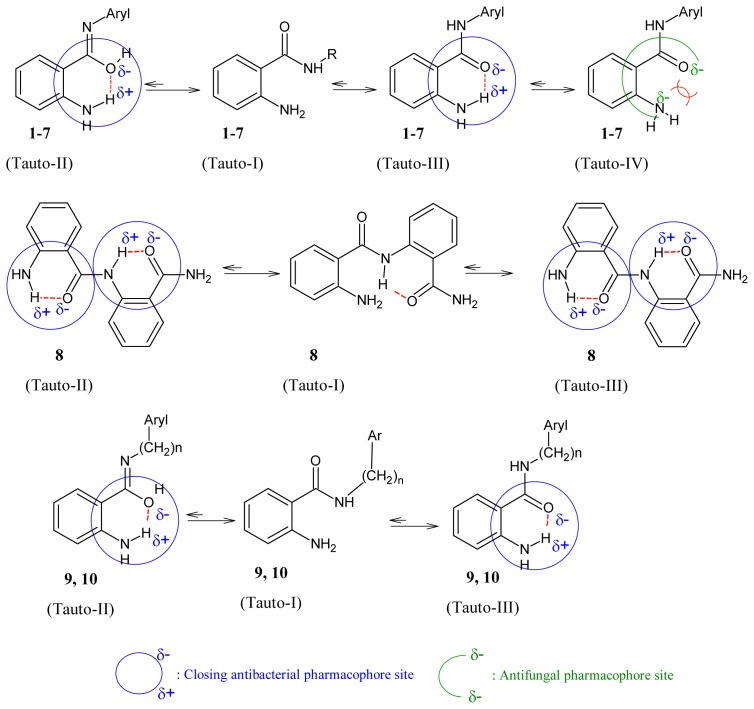
It was believed that amide can exist in two major tautomeric forms, keto-amine and hydroxy-enamine, although keto-amine form is predominant in the solid state [28] as shown in Figure 1. We investigated the potential pharmacophores of various bioactive compounds for their antibacterial [29–33], antifungal [34,35] and antiviral activity [36], and verified them further with Petra/Osiris/Molinspiration (POM) analyses. On the basis of our previously published findings in antibacterial, antiviral and antifungal fields, we can safely conclude that our present series, ABD 1–10, also contain a pharmacophore responsible for antifungal and antibacterial activities which is elaborated in Figure 2. It was also hypothesised that the difference in charges between two heteroatoms of the same dipolar pharmacophoric site may facilitate the inhibition of bacteria more than viruses and fungi. For antibacterial activity, the compounds possess (Xδ−–Yδ−) pharmacophore site (II, III) and for antiviral activity the compounds possess (Xδ−–Yδ−) pharmacophore site.
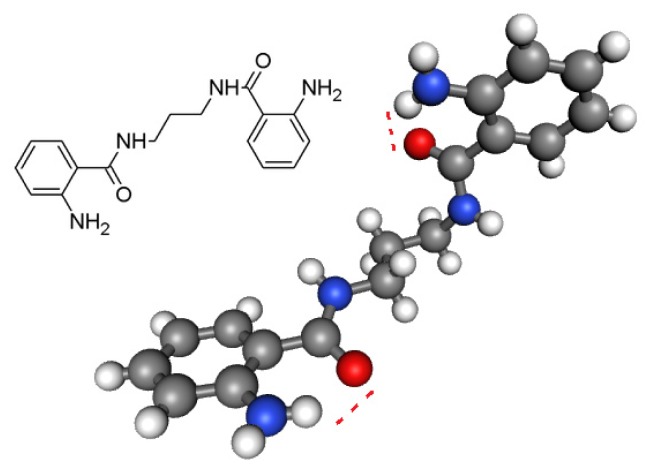
Figure 1.
The structural parameters do not indicate a tautomeric equilibrium but a single amino/amido form. The main differences between the two crystalline forms lie in the intramolecular hydrogen of NH2 bonding and its relative orientation to oxygen of amide [28]. Attractive intramolecular interactions occur and are responsible for the closure of pharmacophore site (C=Oδ−–NH2 δ+).
Figure 2.
Impact of tautmerism on opening/closing antimicrobial pharmacophore site of 1–10.
The following crystallographic results (Figure 1) support our hypothesis that 2-aminobenzamide derivatives (1–10) or any heterocyclic ring present in adjacent position to NH could generate at least four tautomeric forms and apparently two closed pharmacophore sites through the formation of six-membered intramolecular ring assisted by hydrogen bonding, which are responsible for decreasing both antibacterial (C=Oδ−–δ+HN) and antifungal activity (C-OHδ+–δ−N=C).
3. Experimental Section
3.1. General
Melting points (M.P.) were measured on a Gallenkamp melting point apparatus in open glass capillaries and are uncorrected. IR spectra were measured as KBr pellets on a Perking Elmer FT 1000 spectrophotometer (Madison, WI, USA). The NMR spectra were recorded on a Varian Mercury Jeol-400 NMR spectrometer (Tokyo, Japan). 1H-NMR (400 MHz) and 13C-NMR (100 MHz) were run in (DMSO-d6). Chemical shifts (δ) are referred in ppm and coupling constants J are given in Hz. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX mass spectrometer (Tokyo, Japan) at 70 eV. Elemental analysis was carried out on an Elementar Vario EL analyzer (Vernon Hills, IL, USA). Sample Availability: Samples of the compounds 1–10 are available from the authors.
3.2. General Procedure for the Synthesis of Compounds 1–10
3.2.1. Procedure A
To a solution of isatoic anhydride (5–10 mmol, 1.equiv.) in 5–10 mL DMF were added a solution of amine derivatives (5–10 mmol, 1.equiv.) in 5–10 mL DMF and the reaction mixture was refluxed for 6 h. The reaction mixture was monitored by TLC (EtOH:CHCl3). After completion of reaction, the reaction mixture was cooled to room temperature. The precipitated solid product formed was then filtered off and recrystallized to afford the respective product.
3.2.2. Procedure B
A mixture of isatoic anhydride (5–10 mmol, 1.equiv.) and amine derivatives (5–10 mmol, 1.equiv.) in the presence of few drops from DMF was exposed to microwave irradiation (140–420 W) for about 4–10 min. The reaction mixture was then cooled to room temperature and ice cooled water (5 mL) was added to the reaction mixture which resulted in the formation of solid precipitates. These precipitates were filtered off and recrystallized to get the pure products.
3.2.2.1. 2-Amino-N-(4-fluorophenyl)benzamide (1)
Compound 1 was prepared according to method A or method B (10 min,140 W), isolated as brown powder; yield (72a, 65b %); mp 122 °C; IR νmax (KBr) 3470.3, 3366.19, 3275.55, 1636.53 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 10.04 (1H, br s, NH), 7.71–7.74 (2H, dd), 7.62 (1H, d, J = 8.0 Hz), 7.14–7.20 (3H, m), 6.75 (1H, d, J = 8.0 Hz), 6.59 (1H, t, J = 8.0 Hz), 6.33 (2H, br s, NH2);13C-NMR: δ 115.3, 115.7, 116.9, 122.9, 129.1, 132.6, 136.1, 168.3; MS m/z (%): 230 (M+, 100); Anal. for C13H11N2OF (230.24) calcd; C, 67.82; H, 4.82; N, 12.17. Found: C, 67.81; H, 4.80; N, 12.25.
3.2.2.2. 2-Amino-N-(4-chlorophenyl)benzamide (2)
Compound 2 was prepared according to method A or method B (5 min, 140 W), isolated as brown powder; yield (80a, 70b %); mp 147 °C; IR νmax (KBr) 3463, 3364, 3285, 1638 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 10.12 (1H, br s, NH), 7.76 (2H, d, J = 8.8 Hz), 7.62 (1H, d, J = 7.7 Hz), 7.38 (2H, d, J = 8.8 Hz), 7.21 (1H, t, J = 7.7 Hz), 6.76 (1H, d, J = 7.7 Hz), 6.59 (1H, t, J = 7.7 Hz), 6.40 (2H, br s, NH2);13C-NMR: δ 115.2, 115.4, 116.9, 122.5, 127.5, 128.9, 129.2, 132.8, 138.8, 150.3, 168.4; MS m/z (%): 246 (M+, 100) [M+] (C13H11N2OCl) (73.85), 216 (6.25), 165 (10.94), 109 (25.00), 74 (43.08), 30 (100); Anal. for C13H11ClN2O (246.69) calcd; C, 63.29; H, 4.49; N, 11.36; Found: C, 63.30; H, 4.51; N, 11.33.
3.2.2.3. 2-[4-(2-Aminobenzamido)phenyl]acetic acid (3)
Compound 3 was prepared according to method A or method B (4 min, 280 W) isolated as yellowish green powder; yield (99a, 60b %); mp 188.5 °C; IR νmax (KBr) 3483, 2600, 1698.08, 1639.97 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 12.38 (1H, br s, OH), 9.98 (1H, br s, NH), 7.62–7.67 (3H, m), 7.18–7.23 (3H, m), 6.76 (1H, d, J = 7.7 Hz), 6.59 (1H, t, J = 7.7 Hz), 6.36 (2H, br s, NH2), 3.54 (2H, s, CH2); 13C-NMR: δ 40.2, 115.2, 115.8, 116.9, 121.0, 129.2, 129.9, 130.5, 132.6, 138.3, 150.2, 168.3, 173.4; MS m/z (%): 270 [M+] (C15H14N2O3) (100), 224 (22.12), 196 (25.74), 150 (56.55), 138 (13.34), 74 (10.12); Anal. for C15H14N2O3 (270.28) calcd; C, 66.66; H, 5.22; N, 10.36; Found: 66.67; H, 5.23; N, 10.29.
3.2.2.4. 2-Amino-N-(3,4-dimethoxyphenyl)benzamide (4)
Compound 4 was prepared according to method A or method B (4 min, 400 W), recrystallized from ethanol and isolated as black powder; yield (65a, 68b %); mp 166 °C; IR νmax (KBr) 3439, 3332, 3376, 1643 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 9.85 (1H, br s, NH), 7.60 (1H, d, J = 8.0 Hz), 7.42 (1H, s), 7.27 (1H, d, J = 8.8 Hz), 7.18 (1H, t, J = 8.0 Hz), 6.91 (1H, d, J = 8.8 Hz), 6.74 (1H, d, J = 8.0 Hz), 6.58 (1H, t, J = 8.0 Hz), 6.34 (2H, br s, NH2), 3.73, 3.74 (3H, s, 2(OCH3); 13C-NMR: δ 55.9, 56.2, 106.2, 112.3, 113.0, 115.1, 115.9, 116.8, 129.0, 132.4, 133.3, 145.5, 148.9, 150.2, 168.0; MS m/z (%): 272 [M+] (C15H16N2O3) (100), 273 [M+ + 1] (98.97), 257 (15.62), 241 (12.34), 230 (38.46), 201 (46.92); Anal. for C15H16N2O3 (272.30) calcd; C, 66.16; H, 5.92; N, 10.29; Found: C, 66.22; H, 5.88; N, 10.26.
3.2.2.5. 2-Amino-N-(4-methoxyphenyl)benzamide (5)
Compound 5 was prepared according to method A or method B (4 min, 140 W) and isolated as beige powder; yield (99a, 84b %); m.p. 121 °C; IR νmax (KBr) 3454, 3366, 3274, 1643 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 7.75 (1H, br s, NH), 7.42–7.44 (3H, m), 7.22 (1H, t, J = 7.7 Hz), 6.86–6.88 (2H, m), 6.65–6.69 (2H, m), 5.12 (2H, br s, NH2), 3.78 (3H, s, OCH3); 13C-NMR: δ 55.5, 114.2, 116.3, 116.8, 117.5, 122.7, 127.2, 130.8, 132.6, 148.9, 156.7, 167.6; MS m/z (%): 242 [M+] (C14H14N2O2) (80.00), 225 (17.22), 212 (11.34), 194 (100), 77 (37.43), 76 (25.12); Anal. for C14H14N2O2 (242.27) calcd; C, 69.41; H, 5.82; N, 11.56; Found: C, 69.43; H, 5.81; N, 11.55.
3.2.2.6. 2-Amino-N-(3,4,5-trimethoxyphenyl)benzamide (6)
Compound 6 was prepared according to method A and isolated as brown cubes; yield (75a %); mp 217 °C; IR νmax (KBr) 3461, 3345, 3146, 1654 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 9.89 (1H, br s, NH), 7.60 (1H, d, J = 7.8 Hz), 7.18–7.22 (3H, m), 6.75 (1H, d, J = 7.8 Hz), 6.59 (1H, t, J = 7.8 Hz), 6.35 (2H, br s, NH2), 3.76 (6H, s, 2(OCH3*)), 3.64 (3H, s, OCH3); 13C-NMR: δ 56.2, 60.6, 98.6, 115.1, 115.7, 116.9, 129.0, 132.6, 134.0, 135.9, 150.2, 153.0, 168.2; MS m/z (%): 302 [M+] (C16H18N2O4) Anal. for C16H18N2O4 (302.33) calcd; C, 63.56; H, 6.00; N, 9.27; Found: C, 63.59; H, 6.03; N, 9.29.
3.2.2.7. 2-Amino-N-(p-tolyl)benzamide (7)
Compound 7 was prepared according to method A, recrystallized from benzene and isolated as beige powder; yield (97a %); mp 149 °C; IR νmax (KBr) 3464.73, 3361.59, 3273.20, 1636.80 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 7.76 (1H, br s, NH), 7.41–7.45 (3H, m), 7.23 (1H, t, J = 7.7 Hz), 7.15 (2H, d, J = 8.0 Hz), 6.68–6.70 (2H, m), 5.43 (2H, br s, NH2), 2.33 (3H, s, CH3); 13C-NMR: δ 21.0, 116.4, 116.8, 117.5, 120.8, 127.3, 129.6, 132.7, 134.2, 135.3, 148.9, 167.6; MS m/z (%): 226 [M+] (C14H14N2O) (31.25), 209 (92.31), 191 (15.62), 133 (29.69), 118 (100), 105 (31.25); Anal. for C14H14N2O (226.27) calcd; C, 74.31; H, 6.24; N, 12.38; Found: 74.29; H, 6.25; N, 12.37.
3.2.2.8. 2-Amino-N-(2-carbamoylphenyl)benzamide (8)
Compound 8 was prepared according to method A or method B (6 min, 280 W) and isolated as beige powder; yield (60a, 64b %); m.p. 110 °C; IR νmax (KBr) 3411, 3325, 3187, 1660, 1618 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): 1.73 (1H, br s, NH), 7.91 (1H, d, J = 7.5 Hz, H-2′), 7.73 (1H, t, J = 7.5 Hz, H-4′), 7.52 (1H, d, J = 7.5 Hz, H-5′), 7.24 (1H, t, J = 7.5 Hz, H-3′), 7.10–7.16 (2H, m, H-4, 6), 6.67 (1H, d, J = 7.7 Hz, H-3), 7.59, 7.05 (each 2H, s, 2(NH2)), 6.47 (1H, t, J = 7.7 Hz, H-5); 13C-NMR: δ 110.79, 114.22, 114.91, 115.88, 116.94, 124.05, 129.29, 129.48, 132.43, 137.47, 141.93, 147.65, 160.43, 171.85; MS m/z (%): 255.27 [M+] (C14H13N3O2]; Anal. for C14H13N3O2 (255.27) calcd; C, 65.87; H, 5.13; N, 16.46; Found: C, 65.90; H, 5.11; N, 16.45.
3.2.2.9. 2-Amino-N-(4-chlorobenzyl)benzamide (9)
Compound 9 was prepared according to method A or method B (10 min, 280 W), recrystallized from methanol and isolated as white powder; yield (95a, 62b %); mp 137 °C; IR νmax (KBr) 3472, 3358, 3308, 1638 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 7.55 (1H, d, J = 8.0 Hz), 7.38 (2H, d, J = 8.0 Hz), 7.33 (2H, d, J = 8.0 Hz), 7.14 (1H, t, J = 8.0 Hz), 6.70 (1H, d, J = 8.0 Hz), 6.52 (1H, t, J = 8.0 Hz), 6.45 (2H, br s, NH2), 4.40 (2H, s, CH2); 13C-NMR: δ 40.2, 114.7, 115.1, 116.9, 128.6, 128.7, 129.5, 131.7, 132.4, 139.5, 150.3, 169.4; MS m/z (%): 260 [M+] (C14H13N2OCl) (86.22), 261 [M+ + 1] (100), 229 (21.33), 225 (32.40), 214 (28.86), 192 (65.90); Anal. for C14H13ClN2O (260.72) calcd; C, 64.49; H, 5.03; N, 10.74; Found: C, 64.53; H, 5.08; N, 10.68.
3.2.2.10. 2-Amino-N-(3,4-dimethoxyphenethyl)benzamide (10)
Compound 10 was prepared according to method A and isolated as beige powder; yield (100a %); mp 105 °C; IR νmax (KBr) 3326, 3415, 1637 cm−1; 1H-NMR (400 MHz, DMSO-d6) (ppm): δ 8.26 (1H, br s, NH), 7.44 (1H, d, 7.5 Hz), 7.12 (1H, t, J = 7.5 Hz), 6.82–6.87 (2H, m), 6.73–6.76 (1H, dd, J = 7.5 Hz), 6.69 (1H, d, J = 8.0 Hz), 6.50 (1H, t, J = 7.5 Hz), 6.40 (2H, br s, NH2), 3.72–3.71 (each 3H, s, 2(OCH3)), 3.41 (2H, t, J = 6.9 Hz, CH2), 2.76 (2H, t, J = 7.3 Hz,CH2); 13C- NMR: δ 39.8, 40.4, 55.8, 56.0, 112.3, 113.0, 115.0, 115.5, 116.8, 121.0, 28.5, 132.1, 132.6, 147.7, 149.1, 150.1, 169.3; MS m/z (%): 300 [M+] (C17H20N2O3) 301 [M+ + 1] (100), 302 [M+ + 2] (21.33), 287 (4.12), 184 (10.62), 179 (12.10); Anal. for C17H20N2O3 (300.35) calcd; C, 67.98; H, 6.71; N, 9.33; Found: C, 68.00; H, 6.72; N, 9.38.
4. Antifungal Activity
Tested samples were screened separately in vitro for their antifungal activity various fungi viz. Aspergillus fumigatus (RCMB 002003), Saccharomyces cerevislae (RCMB 006002), and Candida albicans (RCMB 005002) . The culture of fungi was purified by a single spore isolation technique. The antifungal activity was by an agar well diffusion method by the following procedure.
Sabourad dextrose agar plates: A homogeneous mixture of glucose-peptone-agar (40:10:15) was sterilized by autoclaving at 121 °C for 20 min. The sterilized solution (25 mL) was poured into each sterilized petri dish in laminar flow and left for 20 min to form the solidified sabourad dextrose agar plate. These plates were inverted and kept at 30 °C in incubator to remove the moisture and to check for the contamination.
Fungal strain was grown in 5 mL sabourad dextrose broth (glucose: peptone; 40:10) for 3–4 days to achieve 105 CFU/mL cells. The fungal culture (0.1 mL) was spread out uniformly on the sabourad dextrose agar plates by sterilized triangular folded glass rod. Plates were left for 5–10 min so that culture is properly adsorbed on the surface of sabourad dextrose agar plates. Now, small wells of size 4 mm × 2 mm were cut into the plates with the help of a well cutter, and the bottom of the wells were sealed with 0.8% soft agar to prevent the flow of test sample at the bottom of the well. One hundred μL of the tested samples (10 mg/mL) were loaded into the wells of the plates. All compounds were prepared in dimethyl sulfoxide, DMSO was loaded as control. The plates were kept for incubation at 30 °C for 3–4 days and then the plates were examined for the formation of a zone of inhibition. Each inhibition zone was measured three times by caliper to get an average value. The test was performed three times for each fungus. Clotrimazole was used as antifungal standard drug.
5. Antibacterial Activity
Antibacterial activities were investigated using an agar well diffusion method. The activity of tested samples was studied against the Staphylococcus aureus (RCMB 000106) and Bacillis subtilis (RCMB 000107), as Gram positive bacteria and Pseudomonas aeruginosa (RCMB 000102) and Escherichia coli (RCMB 000103), as Gram negative bacteria. The solution of 5 mg/mL of each compound in DMSO was prepared for testing against bacteria. Centrifuged pellets of bacteria from 24 h old culture containing approximately 104–106 CFU (colony forming unit) per ml were spread on the surface of nutrient agar (typetone 1%, yeast extract 0.5%, NaCl 0.5%, agar 1000 mL of distilled water, PH 7.0) which was autoclaved under 121 °C for at least 20 min. Wells were created in medium with the help of sterile metallic bores and then cooled down to 45 °C. The activity was determined by measuring the diameter of the inhibition zone (in mm). One hundred μL of the tested samples (10 mg/mL) were loaded into the wells of the plates. All compounds was prepared in DMSO, DMSO was loaded as control. The plates were kept for incubation at 37 °C for 24 h and then the plates were examined for the formation of a zone of inhibition. Each inhibition zone was measured three times by caliper to get an average value. The test was performed three times for each bacterium. Streptomycin was used as antibacterial standard drug.
6. Conclusions
In conclusion, we have successfully prepared 2-Aminobenzamide derivatives 1–10 starting from isatoic anhydride ISA and reacting it with appropriate N-nucleophile. A simple, mild, time efficient, high yielding and environmentally friendly microwave irradiation procedure has been introduced in addition to a classical method for the synthesis of benzamide derivatives. Furthermore, the antimicrobial potential of the selected synthesized compounds was also evaluated. Compound 5 showed excellent antimicrobial potential against all tested bacterial and fungal strains. However, it was found more potent than the standard drug against Aspergillus fumigatus species. All other compounds exhibited moderate to good activity against one or more tested strains of bacteria and fungi.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the research group project Number RGP-VPP-007.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Author Contributions
Yahia N. Mabkhot designed the study, carried out the synthesis; Abdullah M. Al-Majid designed the study, carried out the synthesis. Assem Barakat wrote some research and audit. Salim S. Al-Showiman wrote some research and audit. Munirah S. Al-Har student did the experiments. Smaail Radi carried out Petra analyses. Muhammad Moazzam Naseer edited the English language. Taibi B. Hadda carried out and compiled Petra/Osiris/Molinspiration (POM) analyses with comparison to experimental data.
References
- 1.Friedlander P., Wleugel S. Zur Constitution des Anthranils. Ber. Disch. Chem. Ges. 1883;16:2227–2229. [Google Scholar]
- 2.Coppola G.M. The chemistry of isatoic anhydride. Synthesis. 1980;7:505–536. [Google Scholar]
- 3.Shvekhgeimer M.G.A. Synthesis of heterocyclic compounds based on isatoic anhydrides (2H-31-Benzoxazine-24-diones) Chem. Heterocycl. Compd. 2001;37:385–443. [Google Scholar]
- 4.Weissleder R., Kelly K., Sun E.Y., Shtatland T., Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 5.Kozminykh E.N., Goncharov V.I., Aitken R.A., Kozminykh V.O., Lomidze K.S. Synthesis of 3-(24-dinitrophenyl)hydrazones of 5-arylfuran-23-diones. Chem. Heterocycl. Comp. 2006;42:1107–1108. [Google Scholar]
- 6.Kappe T., Stadlbauer W. Isatoic Anhydrides and Their Uses in Heterocyclic Synthesis. Vol. 28. Academic Press; New York, NY, USA; London, UK; Paris, France: 1981. p. 127. [Google Scholar]
- 7.Ren J., Goss D.J. Synthesis of a fluorescent 7-methylguanosine analog and a fluorescence spectroscopic study of its reaction with wheatgerm cap binding proteins. Nucleic Acids Res. 1996;24:3629–3634. doi: 10.1093/nar/24.18.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nawrot B., Milius W., Ejchart A., Limmer S., Sprinz M. The structure of 3′-O-anthraniloyladenosine an analogue of the 3′-end of aminoacyl-tRNA. Nucleic Acids Res. 1997;25:948–954. doi: 10.1093/nar/25.5.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Honkanen E., Pippuri A., Kairisalo P., Nore P., Karppanen H., Paakkari I. Synthesis and antihypertensive activity of some new quinazoline derivatives. J. Med. Chem. 1983;26:1433–1438. doi: 10.1021/jm00364a014. [DOI] [PubMed] [Google Scholar]
- 10.Zhou Y., Murphy D.E., Sun Z., Gregor V.E. Novel parallel synthesis of N-(4-oxo-2-substituted -4-H-quinazolin-3-yl)-substituted sulfonamides. Tetrahedron Lett. 2004;45:8049–8051. [Google Scholar]
- 11.Aziza M.A., Nassar M.W., Abdel-Hamide S.G., El-Hakim A.E., El-Azab A.S. Synthesis and antimicrobial activities of some new 3-heteroaryl-quinazolin-4-ones. Indian J. Heterocycl. Chem. 1996;6:25–30. [Google Scholar]
- 12.El-Azab A.S. Synthesis of some new substituted 2-mercaptoquinazoline analogs as potential antimicrobial agents. Phosphorus Sulfur Silicon Relat. Elem. 2007;183:333–348. [Google Scholar]
- 13.Refaie F.M., Esmat A.Y., Gawad S.M.A., Ibrahim A.M., Mohamed M.A. The antihyperlipidemic activities of 4 (3H) quinazolinone and two halogenated derivatives in rats. Lipids Health Dis. 2005;4:22–32. doi: 10.1186/1476-511X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habib N.S., Ismail K.A., El-Tombary A.A., Abdel-Aziem T. Antilipidemic agents Part IV: Synthesis and antilipidemic testing of some heterocyclic derivatives of hexadecyl and cyclohexyl hemisuccinate esters. Pharmazie. 2000;55:495–499. [PubMed] [Google Scholar]
- 15.Al-Omar M.A., El-Azab A.S., El-Obeid H.A., Abdel Hamide S.G. Synthesis of some new 4-(3H)-quinazoline analogs as potential antioxidant agents. J. Saudi Chem. Soc. 2006;10:113–127. [Google Scholar]
- 16.Alafeefy A.M., Kadi A.A., El-Azab A.S., Abdel-Hamide S.G., Daba M.H. Synthesis analgesic and anti-inflammatory evaluation of some new 3H-quinazolin-4-one derivatives. Arch. Pharm. (Weinheim.) 2008;341:377–385. doi: 10.1002/ardp.200700271. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A., Sharma S., Archana A., Bajaj K., Sharma S., Panwar H., Singh T., Srivastava V.K. Some new 236-trisubstituted quinazolinones as potent anti-inflammatory analgesic and COX-II inhibitors. Bioorg. Med. Chem. 2003;11:5293–5299. doi: 10.1016/s0968-0896(03)00501-7. [DOI] [PubMed] [Google Scholar]
- 18.Alagarsamy V., Solomon V.R., Dhanabal K. Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazolin-4-one as analgesic anti-inflammatory agents. Bioorg. Med. Chem. 2007;15:235–241. doi: 10.1016/j.bmc.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 19.El-Azab A.S., Kamal E.H., Attia S.M. Synthesis and anticonvulsant evaluation of some novel 4(3H)-quinazolinones. Monatsh. Chem. 2011;142:837–848. [Google Scholar]
- 20.Kashaw S.K., Kashaw V., Mishra P., Jain N.K., Stables J.P. Synthesis anticonvulsant and CNS depressant activity of some new bioactive 1-(4-substituted-phenyl)-3-(4-oxo-2-phenyl/ethyl- 4H-quinazolin-3-yl)-urea. Eur. J. Med. Chem. 2009;44:4335–4343. doi: 10.1016/j.ejmech.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Archana V., Srivastava K., Kumar A. Synthesis of some newer derivatives of substituted quinazolinonyl-2-oxo/thiobarbituric acid as potent anticonvulsant agents. Bioorg. Med. Chem. 2004;12:1257–1264. doi: 10.1016/j.bmc.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 22.El-Azab A.S., ElTahir K.E. Synthesis and anticonvulsant evaluation of some new 238-trisubstituted-4(3H)-quinazoline derivatives. Bioorg. Med. Chem. Lett. 2012;22:327–333. doi: 10.1016/j.bmcl.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Al-Omary F.A., Abou-Zeid L.A., Nagi M.N., Habib E.S.E., Abdel-Aziz A.A., El-Azab A.S., Abdel-Hamide S.G., Al-Omar M.A., Al-Obaid A.M., El-Subbagh H.I. Non-classical antifolates Part 2: Synthesis biological evaluation and molecular modeling study of some new 26-substituted-quinazolin-4-ones. Bioorg. Med. Chem. 2009;18:2849–2863. doi: 10.1016/j.bmc.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Al-Obaid A.M., Abdel-Hamide S.G., El-Kashef H.A., Abdel-Aziz A.A., El-Azab A.S., Al-Khamees H.A., El-Subbagh H.I. Substituted quinazolines part 3 Synthesis in vitro antitumor activity and molecular modeling study of certain 2-thieno-4(3H)-quinazolinone analogs. Eur. J. Med. Chem. 2009;44:2379–2391. doi: 10.1016/j.ejmech.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 25.El-Azab A.S., Al-Omar M.A., Abdel-Aziz A.A., Abdel-Aziz N.I., El-Sayed M.A., Aleisa A.M., Sayed-Ahmed M.M., Abdel-Hamid S.G. Design synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: molecular docking study. Eur. J. Med. Chem. 2010;45:4188–4198. doi: 10.1016/j.ejmech.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Ramos-Morales F.R., Durand-Niconoff S., Correa-Basurto J., Meléndez-Bustamante F.J., Cruz-Sánchez S.J. Theoretical study of reactivity based on the Hard-Soft/Acid-Base (HSAB) in isatoic anhydride and some derivatives. J. Mex. Chem. Soc. 2008;52:241–248. [Google Scholar]
- 27.Smania A.J., Delle Monache F., Smania E.F.A., Cuneo R.S. Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Medl. Mush. 1999:325–330. [Google Scholar]
- 28.Sreedasyam J.S., Sunkari J., Kundha S., Gundapaneni R.R. N,N′-(Propane-1,3-diyl)bis(2- aminobenzamide) Acta Cryst. 2013;E69:o673. doi: 10.1107/S160053681300888X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarrahpour A., Fathi J., Mimouni M., Hadda T.B., Sheikh J., Chohan Z.H., Parvez A. Petra Osiris and Molinspiration (POM) together as a successful support in drug design: Antibacterial activity and biopharmaceutical characterization of some azo schiff bases. Med. Chem. Res. 2012;21:1984–1990. [Google Scholar]
- 30.Jarrahpour A., Motamedifar M., Zarei M., Youssoufi M.H., Mimouni M., Chohan Z.H., Hadda T.B. Petra osiris and molinspiration together as a guide in drug design: predictions and correlation structure/antibacterial activity relationships of new n-sulfonyl monocyclic β-lactams (Part II) Phosphorus Sulfur Silicon Relat. Elem. 2010;185:491–497. [Google Scholar]
- 31.Parvez A., Jyotsna M., Tiwari V., Sheikh J., Dongre R., Youssoufi M.H., Hadda T.B. Pharmacophores modeling in terms of prediction of theoretical physicochemical properties and verification by experimental correlations of novel coumarin derivatives produced via Betti’s protocol. Eur. J. Med. Chem. 2010;45:4370–4378. doi: 10.1016/j.ejmech.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Parvez A., Jyotsna M., Youssoufi M.H., Hadda T.B. Bioinformatic prediction and experimental verification of antibacterial potential of some monocyclic β-lactams containng two synergetic buried antibacterial pharmacophore sites (Part II) Phosphorus Sulfur Silicon Relat. Elem. 2010;185:1500–1510. [Google Scholar]
- 33.Anaflous A., Benchat N., Mimouni M., Abouricha S., Hadda T.B., Bali B.E., Hakkou A., Hacht B. Armed imidazo[12-a]pyrimidines (-pyridines): Evaluation of antibacterial activity. Lett. Drug Des. Discov. 2004;1:224–229. [Google Scholar]
- 34.Chohan Z.H., Youssoufi M.H., Jarrahpour A., Hadda T.B. Identification of antibacterial and antifungal pharmacophore sites for potent bacteria and fungi inhibition: Indolenyl sulfonamide derivatives. Eur. J. Med. Chem. 2010;45:1189–1199. doi: 10.1016/j.ejmech.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh J., Parvez A., Ingle V., Juneja H., Dongre R., Chohan Z.H., Youssoufi M.H., Hadda T.B. Synthesis biopharmaceutical characterization antimicrobial and antioxidant activities of 1-(4′-O-β-d-Glucopyranosyloxy-2′-hydroxyphenyl)-3-aryl-propane-13-diones. Eur. J. Med. Chem. 2011;46:1390–1399. doi: 10.1016/j.ejmech.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 36.Bennani B., Kerbal A., Daoudi M., Baba B.F., Houari G.A., Jalbout A.F., Mimouni M., Benazza M., Demailly G., Akkurt M., et al. Combined drug design of potential Mycobacterium tuberculosis and HIV-1 inhibitors: 3′4′-di-substituted-4′H-spiro[isothiochromene-35′-isoxazol]-4(1H)-one. Arkivoc. 2007;16:19–40. [Google Scholar]