Abstract
Soil tillage practices have a profound influence on the physical properties of soil and the greenhouse gas (GHG) balance. However there have been very few integrated studies on the emission of carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) and soil biophysical and chemical characteristics under different soil management systems. We recorded a significantly higher net global warming potential under conventional tillage systems (26–31% higher than zero tillage systems). Crucially the 3-D soil pore network, imaged using X-ray Computed Tomography, modified by tillage played a significant role in the flux of CO2 and CH4. In contrast, N2O flux was determined mainly by microbial biomass carbon and soil moisture content. Our work indicates that zero tillage could play a significant role in minimising emissions of GHGs from soils and contribute to efforts to mitigate against climate change.
Globally, agriculture accounts for 10–12% of total anthropogenic emissions of greenhouse gases (GHGs), estimated to be 5.1–6.1 Gt CO2-eq yr−1 in 20051. Conservation tillage is one among many different mitigation options suggested to reduce GHG emissions from agriculture. Conservation tillage practices such as reduced/minimum/zero tillage, direct drilling and strip cropping are also widely recommended to protect soil against erosion and degradation of structure2, create greater aggregate stability3,4, increase soil organic matter content, enhance sequestration of carbon5,6, mitigate GHG emissions7 and improve biological activity8. Derpsch9 estimated that approximately 45 million hectares was managed by conservation tillage worldwide in 2001 and this figure had more than doubled by 2007.
Minimum tillage practices have been reported to reduce GHG emissions through decreased use of fossil fuels in field preparation and by increasing carbon sequestration in soil10. For example, Hermle et al.13 observed net carbon sequestration to a depth of 50 cm after 20 years of no tillage. However, reduced tillage can lead to a stratification of soil organic carbon at the surface11 in contrast to the more uniform distribution of carbon in conventionally tilled soils12. The crop residues accumulated on the soil surface under reduced tilled conditions may result in carbon being lost to the atmosphere upon decomposition10. Furthermore, climate change mitigation benefits such as reduced CO2 emissions, by virtue of increased sequestration of carbon and increased CH4 uptake under reduced tillage, could be offset by increased emissions of N2O, a greenhouse gas with higher warming potential than both CO2 and CH413,14,15. Increased N2O emissions have been linked to increased denitrification under reduced tillage due to the formation of micro-aggregates within macro-aggregates that create anaerobic micro sites13 with increased microbial activity leading to greater competition for oxygen16.
Reduction of tillage can also create increased soil densification and a subsequent decrease in the volume of macropores17 leading to reduction in gaseous exchange. Soil aggregation and the resultant geometry of the pore structure are vitally important characteristics affected by tillage practices which impact on the physico-chemical and hydro-thermal regime in soil, and ultimately crop yield. Additionally, the effect of tillage on the environment varies across farms geographically since the impacts of cultivation on soil organic matter and net greenhouse balance depends on soil type, climatic variables and management15.
No previous studies have considered the effect of the soil porous architecture created by tillage on net balance of greenhouse gas emissions. Traditional methods for inferring soil structure such as soil moisture retention curves are limited as they are destructive and do not provide the soil pore size distribution in three dimensions18. However, imaging technologies such as X-ray Computed Tomography (CT) can be used to reveal the undisturbed structure, aggregation and pore characteristics at high resolutions (e.g. microscale <100 μm). In this study we sought to evaluate the impact of zero tillage and conventional tillage on soil pore characteristics, carbon sequestration and GHG emissions. We hypothesised that zero tillage improves C sequestration and reduces GHG emissions compared with conventional tillage through the enhanced development of the soil porous network associated with less anthropogenic disturbance.
Results
Soil physical properties
Soil texture varied between the different experimental sites ranging from heavy clay soils to lighter sandy soils (see supplementary Table 1). Crucially there was no significant variation in soil texture between paired fields of conventional and zero tilled soils (P > 0.05). Zero tilled soils had a higher bulk density (1.16 Mg m−3) than tilled soils (1.09 Mg m−3) (Table 1, P < 0.001) which was not influenced by length of zero tillage management which ranged from 5–10 years (P > 0.05). Zero tilled soils had an increased shear strength (26 MPa) compared to tilled fields (12 MPa) (Table 1, P < 0.001), which was also independent of the duration of zero tillage (P > 0.05). Soil moisture content (volumetric) was significantly higher under zero tilled soils (29.6%) compared to tilled soils (26.0%) (P < 0.01), regardless of the duration of zero tillage (P > 0.05).
Table 1. Selected physico-chemical properties of soils under zero tillage and conventional tillage*.
| Tillage | Depth (cm) | Bulk density (Mg m−3) | Shear strength (MPa) | Soil moisture (%) | pH | SOM (%) | NH4-N (mg kg−1soil) | NO3-N (mg kg−1soil) | Microbial C (mg kg−1soil) | Microbial N (mg kg−1soil) |
|---|---|---|---|---|---|---|---|---|---|---|
| Zero tilled | 0–10 | 1.16 ± 0.04 | 25.7 ± 1.47 | 31.29 ± 1.40 | 6.98 ± 0.13 | 7.81 ± 0.44 | 2.59 ± 0.10 | 0.66 ± 0.05 | 591.8 ± 55.0 | 104.9 ± 7.92 |
| 10–20 | ND** | ND | 27.90 ± 1.36 | 7.32 ± 0.10 | 7.41 ± 0.42 | 2.42 ± 0.08 | 0.45 ± 0.04 | 442.2 ± 26.6 | 77.3 ± 5.11 | |
| Tilled | 0–10 | 1.09 ± 0.04 | 12.0 ± 1.12 | 26.98 ± 1.06 | 7.22 ± 0.14 | 6.59 ± 0.42 | 2.51 ± 0.16 | 0.62 ± 0.06 | 434.9 ± 44.3 | 73.4 ± 5.11 |
| 10–20 | ND | ND | 24.96 ± 1.11 | 7.29 ± 0.13 | 6.15 ± 0.40 | 2.30 ± 0.14 | 0.54 ± 0.06 | 402.5 ± 39.7 | 66.6 ± 3.79 |
*Mean ± Standard Error of mean (n = 33).
**ND- not determined.
Soil pore characteristics
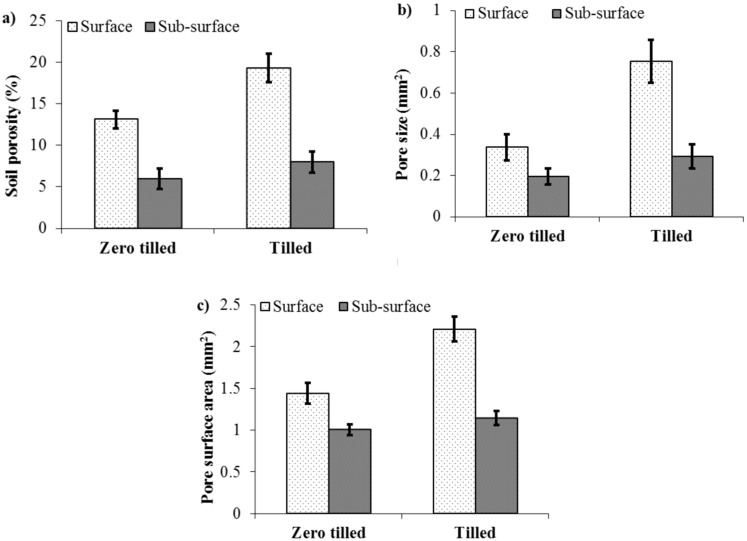
X-ray CT measured soil porosity was significantly higher under tilled soil (13.6%) than zero tilled soil (9.6%) (P < 0.001, Figure 1a). The porosity in the surface layer (0–10 cm) of tilled soils were 46.9% higher than under zero tilled soils and 33.2% higher in tilled compared to zero tilled soils in the 10–20 cm layer (P < 0.001). Soil pore size followed similar pattern to soil porosity (Figure 1b). Pore size significantly varied with tillage type and soil depth with increased pore size at the surface layers of tilled soil (Table 2, P < 0.05). Pores in tilled soils were twice as large (0.52 mm2) as those in zero tilled soils (0.27 mm2) (P < 0.01). The largest pore sizes were recorded in the 0–10 cm layer (0.55 mm2) as opposed to the 10–20 cm layer (0.24 mm2) (P < 0.001). The surface area of the total soil pore system was higher in tilled soils (Figure 1c, P < 0.001). The surface area of pores was also greater in the 0–10 cm depth (1.83 mm2) than the 10–20 cm depth (1.07 mm2) across both tilled and zero tilled soil treatments (P < 0.01).
Figure 1. Soil pore characteristics under zero tilled and tilled managed soil derived from X-ray CT.
(a) soil porosity (b), mean soil pore size (c) and surface area of soil pores at the surface (0–10 cm) and sub-surface layers (10–20 cm) in zero tilled and tilled soils (average values for different sites and standard error of the mean are shown, n = 33).
Table 2. Statistical output from linear mixed modelling (texture, tillage, duration, depth) for the physico-chemical characteristics of soils under zero tillage and conventional tillage (F(df1,df2) statistic).
| Parameter | Clay (%) | Tillage | Duration of non-tillage | Depth | Tillage × depth | Duration of non-tillage × depth |
|---|---|---|---|---|---|---|
| Moisture content | 6.97(1,58)* | 17.86(1,10)** | ns | 52.29(1,63)*** | ns | ns |
| Porosity | 6.70(1,32)* | 16.49(1,9)*** | ns | 59.3(1,63)*** | 15.86(1,63)*** | ns |
| Pore size | 11.31(1,21)** | 14.21(1,9)** | ns | 17.2663*** | 4.89(1,63)** | ns |
| Pore area | 14.71(1,36)*** | 17.01(1,9)*** | ns | 47.71(1,63)*** | 8.36(1,63)** | ns |
| pH | 6.72(1,46)* | ns | ns | 38.49(1,63)*** | 15.78(1,63)*** | ns |
| SOM | ns | 33.24(1,9)*** | ns | 84.13(1,63)*** | ns | ns |
| NH4-N | 3.86(1,44)* | ns | ns | 7.52(1,63)** | ns | ns |
| NO3-N | ns | ns | ns | 29.8(1,63)*** | 5.03(1,63)* | ns |
| MBC | ns | 33.96(1,9)*** | ns | 37.14(1,63)*** | 35.67(1,63)*** | 4.82(1,63)* |
| MBN | ns | 25.85(1,8)*** | ns | 20.42(1,63)*** | 7.44(1,63)** | ns |
| CO2a | ns | 8.91(1,13)* | ns | |||
| CO2b | ns | 11.12(1,11)** | ns | |||
| CH4a | ns | 5.79(1,19)* | ns | |||
| CH4b | ns | 4.99(1,18)* | ns | |||
| N2Oa | ns | 10.04(1,14)** | ns | |||
| N2Ob | ns | 6.38(1,14)* | ns |
Subscripted numbers indicate degrees of freedom for F value; df1 = numerator df, df2 = denominator df, ns: non-significant, SOM: soil organic matter, MBC: microbial biomass carbon, MBN: microbial biomass nitrogen, superscripts a and b following CO2, CH4 and N2O represents potentials expressed in mg m−2 h−1 and ng g−1 h−1, respectively).
***p < 0.001.
**p < 0.01.
*p < 0.05.
Soil chemical and biological properties
Zero tilled soils contained significantly more soil organic matter (SOM) than tilled soils (P < 0.001). Soil from the 0–10 cm layer contained more SOM than soils from the 10–20 cm layers in both zero tilled (7.8 and 7.4% at 0–10 cm and 10–20 cm respectively) and tilled soils (6.6% at 0–10 cm and 6.2% at 10–20 cm) (Table 1, P < 0.001). There were no significant effects for duration of zero tillage on soil organic matter (Table 2).
Neither ammonium (NH4-N) nor nitrate (NO3-N) content in soil was affected by tillage. Soil from the upper 10 cm contained significantly higher NH4-N than the 10–20 cm layer (Table 1, P < 0.01). Nitrate (NO3-N) followed a similar trend to NH4-N. Tillage type and duration did not influence the NO3-N content (P > 0.05). Soil depth significantly influenced NO3-N content (P < 0.001) with highest amount in the surface layer (0–10 cm) under both zero tillage and conventional tillage.
Zero tilled soils contained significantly more microbial biomass carbon than tilled soils (P < 0.001). The mean microbial biomass carbon under zero tilled soil was 517.0 mg kg−1 soil compared with 418.7 mg kg−1 soil in tilled soils. Microbial biomass carbon was significantly higher in the 0–10 cm layer (517 mg kg−1 soil) than the 10–20 cm layer (419 mg kg−1 soil) under zero tillage and conventional tillage (P < 0.001, Table 1). Significantly higher microbial biomass carbon was recorded at the 0–10 cm layer in zero tilled soil (591.8 mg kg−1 soil) with a significant tillage and depth interaction (P < 0.001). However there was no significant effect of duration of zero tillage (Table 2).
Tillage and soil depth significantly influenced soil microbial biomass nitrogen (Table 1 and 2). Zero tilled soils contained a higher microbial biomass nitrogen (91.1 mg kg−1 soil) than tilled soil (70.0 mg kg−1 soil) (P < 0.001). Surface layers (0–10 cm) maintained more microbial biomass nitrogen than sub surface layers (10–20 cm) under both zero tilled soils and tilled soils.
Fluxes of greenhouse gases
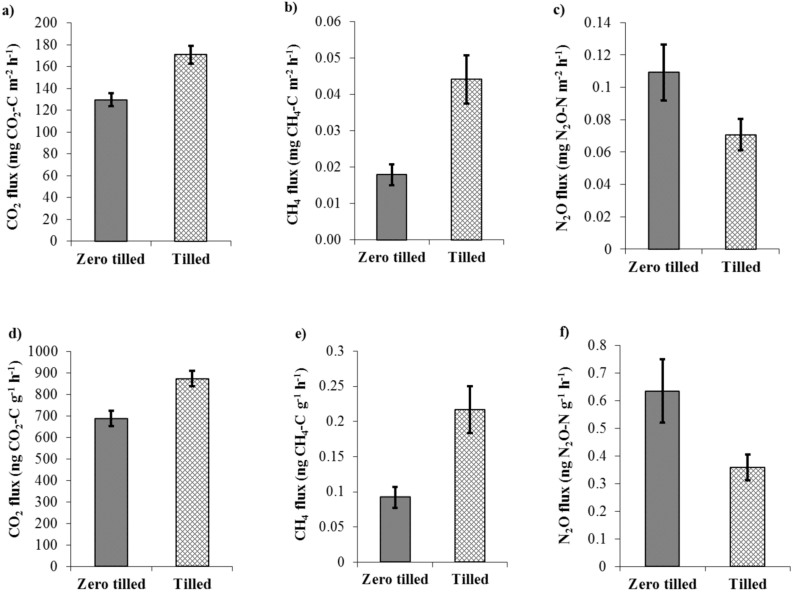
Potential CO2 flux was higher from tilled soil than zero tilled soil (P < 0.05, Figure 2a). Potential CO2 fluxes under zero tilled soil ranged from 47 to 216 mg m−2 h−1 with a mean value of 141 mg m−2 h−1 whilst under tilled soil it ranged from 119 to 236 mg m−2 h−1 with a mean value of 171 mg m−2 h−1. The potential CO2 flux on a per soil weight basis was also higher under tilled soil (873 ng g−1 h−1 soil) compared to zero tilled soil (688 ng g−1 h−1 soil) (P < 0.01, Figure 2d).
Figure 2. Fluxes of greenhouse gas from zero tilled and tilled soil.
(a) CO2 expressed in mg CO2-C m−2 h−1, (b) CH4 expressed in mg CH4-C m−2 h−1, (c) N2O expressed in mg N2O-N m−2 h−1, (d) CO2 expressed in ng CO2-C g−1 h−1, (e) CH4 expressed in ng CH4-C g−1 h−1 and (f) N2O expressed in ng N2O-N g−1 h− (average values for different sites and standard error of the mean are shown, n = 33).
Potential CH4 fluxes were generally positive and higher from tilled soils (0.044 mg m−2 h−1 or 0.22 ng g−1 soil) compared to zero tilled soil (0.018 mg m−2 h−1 or 0.09 ng g−1 h−1 soil) (P < 0.05, Figure 2b and 2e). In contrast, potential N2O emissions were higher under zero tilled soil (0.63 ng g−1 h−1) than tilled soil (0.36 ng g−1 h−1) (54% higher under zero tilled soil when measured on a soil area basis and 77% on a soil dry weight basis compared to tilled soil) (P < 0.01, Figure 2c and 2f).
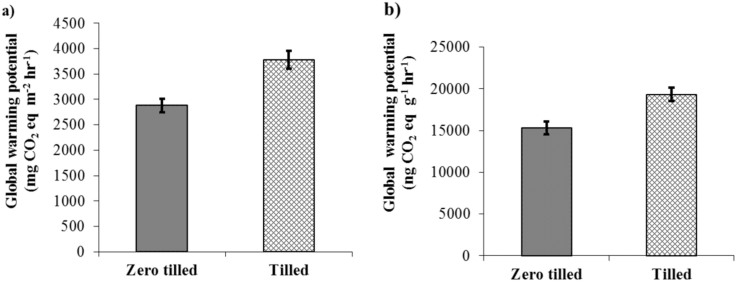
The net global warming potential calculated as per IPCC19 was significantly higher from tilled soil than zero tilled soil. Tilled soil produced 31% on an area basis or 26% on a weight basis greater global warming potential (GWP) than zero tilled soil (P < 0.05, Figure 3). There was no evidence to suggest that the different duration of zero tillage considered in this study, (5–10 years) affected net emissions of greenhouse gases.
Figure 3. Global warming potential under zero tilled and tilled soils.
(Average values for different sites and standard error of the mean are shown, n = 33). (a) GWP expressed in terms of mg m−2 h−1and (b) GWP expressed in terms of ng g−1 h−1.
Relationship between greenhouse gas fluxes and soil properties
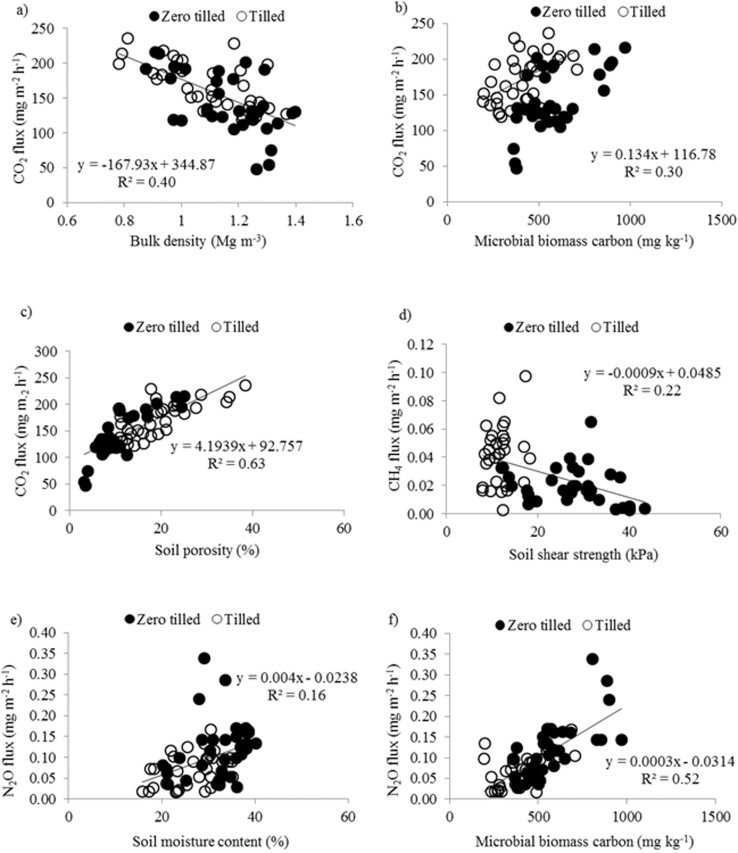
Potenital CO2 fluxes were predicted by a multiple regression model (P < 0.001) including bulk density (BD), microbial biomass carbon (MBC) and soil porosity (P) which accounted for 69.9% of the variation. The optimal model for the potential CO2 flux is provided in the equation (1).
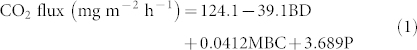 |
In this model the soil porosity contributed to c. 40% of variation, much higher than the individual contribution by any other parameter, as illustrated by retaining the parameter when fitting last to the model. Together microbial biomass carbon and bulk density contributed to 30% of the total variation (Figures 4a, 4b and 4c).
Figure 4. Illustration of important relationships between soil biophysical properties and GHG release.
(a) soil bulk density and CO2 flux from soil; F1,64 = 42.08, P < 0.001 (b) microbial biomass carbon and CO2 flux; F1,64 = 5.89, P < 0.05 (c) soil porosity and CO2 flux; F1,64 = 110.14, P < 0.001 (d) soil shear strength and CH4 flux; F1,64 = 14.08, P < 0.001 (e) soil moisture content and N2O flux; F1,64 = 12.62, P < 0.001 and (f) microbial biomass carbon and N2O flux; F1,64 = 69.5, P < 0.001.
Only soil shear strength (SS) explained variation (18%) in the potential CH4 flux (Equation 2, Figure 4d, P < 0.01).
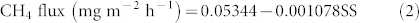 |
The optimal model in equation (3) for potential N2O flux accounted for 62.0% of the variation and included soil moisture (SM), microbial biomass nitrogen (MBN) and microbial biomass carbon (MBC) (Figures 4e and 4f, P < 0.001).
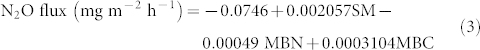 |
Individually microbial biomass carbon explained the greatest proportion (20.8%) of the total variation when fitted last in the model. Removing soil moisture and microbial biomass nitrogen separately from the model did not substantially decrease the amount of variation explained suggesting that these factors were confounded.
Discussion
We have demonstrated tillage practice has the potential to strongly influence release of CO2, CH4 and N2O, through its impact on soil biophysical properties across a wide range of soil textures. However, the main driving factors and the direction of change varied among the three GHGs measured. The higher CO2 release found in response to tillage highlights the role of ploughing in the breakdown of soil aggregates and exposure of organic materials for microbial decomposition20. Soil pore characteristics (previously ignored in similar studies), such as total porosity and pore size, were a stronger predictor of CO2 flux than soil organic matter and microbial biomass carbon, which has not been previously reported. The effects of zero tillage was to reduce soil porosity by 33%, which lead to 21% reduction in potential CO2 efflux. These results demonstrate the increased soil porosity under conventional tillage favours the respiration of aerobic organisms by improving movement of water and air through the soils21 with important implications for CO2 emissions. In parallel, strong effects of soil bulk density on CO2 production from soil cores have been shown by Beare et al.22 who found 2.3 times more CO2 production under uncompacted soil than in compacted soil. The potential CO2 flux data presented here (47 to 235 mg m−2 h−1) is in the range of that reported from laboratory incubations of soils from 13 European sites including, arable land (47 mg m−2 h−1) and grassland (186 mg m−2 h−1)23. Similar effects of tillage on CO2 fluxes were found by Ball et al.24 investigating in situ CO2 fluxes, they attributed the greater CO2 efflux to the larger pores created by tillage. Potential CH4 flux ranged from 0.0025 to 0.16 mg m−2 h−1, which is high compared to values reported by Schaufler et al.23: e.g. average CH4 flux in arable land was 0.0014 mg m−2 h−1 and in grassland it was 0.0005 mg m−2 h−1. Despite the less porous and wetter status of zero tilled soils, which normally promote CH4 production31, the opposite was the case here which may be due to increased activity of methanotrophic bacteria32. The reduced potential CH4 flux under zero tillage was best predicted by soil shear strength which reflects the reduced porosity and high bulk density in zero tilled soils17,25,26.Increased bulk density in soil can prevent flow of CH4 in soil and the resulting enhanced retention of CH4 in soil may improve oxidation by methanotrophs27 resulting in lower CH4 emissions. Furthermore, the development of methanotrophic populations is negatively affected by tillage28 which are slow to recover29,30. The potential N2O fluxes measured were comparable to field measurement by Regina et al.33 in Finnish soils after 5–7 years of zero till management (0.003 to 0.23 mg m−2 h−1) with significantly higher N2O fluxes under zero tillage. They reported 21 to 86% higher N2O flux in zero till soils when compared to tilled soils. The average increased emission of in situ N2O flux under zero tilled soils obtained by Oorts et al.34 was 39% for a 30 year experiment. As with CH4, N2O is produced under reducing conditions in waterlogged and poorly aerated soils35,36, so we attribute the increased potential N2O emissions from zero tilled soils in part to the wetter and denser soils found under this management regime. In contrast to the potential CO2 and CH4 fluxes, the potential production of N2O was most strongly related to the soil microbial biomass. The greater total soil microbial biomass found under zero tillage may hence play a very important role in N2O release. One important aspect of zero tillage is enhanced crop residue retention resulting in greater SOM content. Given the importance of an adequate supply of labile substrates for the denitrifying bacteria35, it may also be that the crop retention under zero tillage drives greater N2O release.
Considering the GHGs together, tilled soil produced 20% greater net global warming than zero tilled soil indicating a potential for zero tillage system to mitigate climate change after only 5 to 10 years since conversion (earlier than this was not measured here). In parallel with this Del Grosso et al.37 also reported a 33% reduction in global warming potential under zero tillage (0.29 Mg C ha−1 y−1) compared with tilled soil (0.43 Mg C ha−1 y−1) for major non-rice cropping systems in US based on simulation using DAYCENT ecosystem model. Also in subtropical conditions, zero tillage has been found to reduce GWP by c. 20%38.
Zero tilled soils had enhanced SOM, microbial biomass carbon and nitrogen. Importantly, the time during which the soils had been under conservation tillage did not influence the SOM content in the soil (although only changes between 5 and 10 years were measured), suggesting that increases in SOM occurred within five years following conversion to zero tillage. However West and Post5 in similar work recorded a large increase in soil between 5–10 years. The time required to reach a steady state for carbon sequestration will vary with respect to climate, soil types and the management practices followed39.
A very important question that remains to be addressed is how the impact of the change in the soil porous architecture brought by tillage/zero tillage on net GHG release and the GWP varies spatio-temporaly across a greater range of soils types, crops and climate than those explored in our study. With reduced tillage practices becoming more prevelant globally, it is important to further understand the impacts of this on the biophysical evolution of the soil environment at both micro and macroscales. It is clear from this study that the modification of soil structure by tillage plays a crucial role for GHG release. Our study was based on analysis of intact cores removed from the field. To fully account for the impact of zero tillage on GHG release it is important to extend this work to in situ field measurement through the year to account for variation in weather and crop development. In conclusion, we have shown soils under zero tillage increased potential N2O emissions, but this is counterbalanced by a significant reduction in potential CO2 and CH4 emissions which is closely linked to the geometry of the soil porous architecture. To evaluate the potential of zero tillage as a tool for mitigation of climate change, there is a need to further assess its impact on yield to ensure a balance between climate change mitigation and food security is achieved.
Methods
Soils
A selection of 22 farms from Leicestershire, Nottinghamshire and Lincolnshire in the East Midlands of the U.K. were chosen for this study. All sampling sites comprised pairs of intensely tilled farms and farms where zero tillage practices were followed, located directly adjacent to each other. The zero tilled soils had been managed in this way for a minimum of 5 years to a maximum of 10 years. In fields under zero tillage, stubble was left at the surface after harvest of the previous crop. Seed drilling was carried out between the root stocks of previous crop using a range of min-till seed drills. The crops cultivated under zero tilled and tilled sites were wheat, oil seed rape and oats. The tilled soil sites were annually ploughed to depths of 20–25 cm and contained the same crops as the zero tilled fields at the time of sampling. Sampling was undertaken shortly after seedbed preparation and sowing so as to minimize any effect of the emerging root system on soil structure.
Intact soil cores were collected using a manual core sampler that used transparent sample liner tubes (Van Walt Ltd, Haslemere, UK). The core sampling was performed to a depth of 20 cm with a diameter of 5 cm and in triplicate. The samples were labelled and sealed in plastic bags before transporting to the laboratory. Samples were stored at 4°C until measurements were taken (<2 weeks). Bulk soil samples of about 1 kilogram were also collected from two depth ranges (0 to 10 cm and 10 to 20 cm) and stored at 4°C until measurement. Smaller soil cores were collected in the field using stainless steel cylinders (radius 3.4 cm, height 4 cm) for measurement of bulk density40.
Soil physical properties
Soil shear strength was recorded in the field using a Pilcon 120 kPa hand vane from the upper 50 mm of soil. Similarly the volumetric water content of the surface layer of soil (0–10 cm depth) was recorded using a Delta-T Theta probe connected to a Theta meter. All observations were recorded in triplicate for each field. Particle size analysis was performed using the hydrometer method41. Soil textural classification was made according to European classification using 60 μm as the upper limit for silt42.
X-ray Computed Tomography (CT)
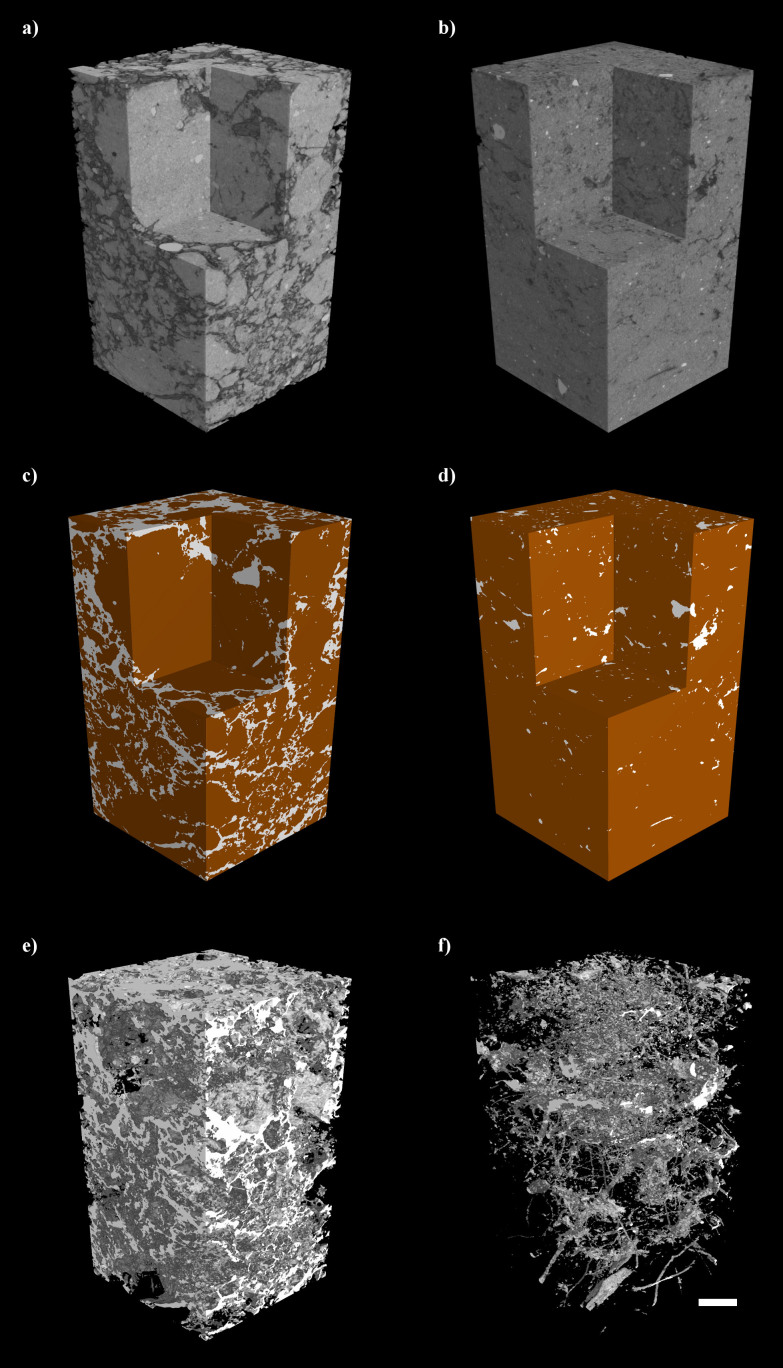
Prior to the study of GHGs, the soil core samples were subjected to morphological analysis using an X-ray CT scanner (Nanotom, Phoenix X-ray, GE Sensing and Inspection Technologies GmbH, Germany) to visualise and measure the internal soil structure. The cores were scanned at a voltage of 140 kV and a current of 100 mA. A copper filter of thickness 0.25 mm was used to minimise artefacts such as beam hardening. The image resolution was 64 μm per voxel. The soil core was positioned vertically onto the scanner platform. Each scan lasted 100 minutes per core, scanning both top and bottom 10 cm portions in a split scan. Whilst it is possible to achieve much faster scan times than this, a larger scan time was used to achieve the highest possible image quality. For each scan 1000 images were collected. The obtained images were visualised using the software, VG StudioMax (Volume Graphics). The images were converted to the tiff format and analysed using ImageJ43 to study the soil pore characteristics. A rectangular region of interest (27.94 × 27.94 mm2) was selected to avoid the edges of the soil cores. In addition the first 100 images each from the beginning and end of the scan were discarded due to cone beam artefacts. The images were sharpened to highlight the image features and then smoothed by a median filter before being converted to the binary scale using the minimum threshold algorithm in ImageJ. Both dark and bright outliers were removed and the ‘fill holes' function was used to minimise noise. Measurements on soil physical features were obtained on the binary images which included porosity, number of pores, pore size and surface area of pores (Figure 5).
Figure 5. Non-destructive 3-D imaging of soil by X-ray CT.
Examples of Tilled (A–C) and Zero Tilled (D–F) soils. (A&D): 3D rendered grayscale density map of soil cores showing a virtual ‘cut-out' to the revealing clear differences soil structure between the two soils. (B&E): Thresholded 3D image highlighting ‘solid' soil in brown and ‘pore' space in white. (C&F): Visualisation of pore space only highlighting high connectivity of pores in the tilled soils and the presence of numerous biopores in the zero tilled soil. Scale bar = 10 mm.
Soil chemical and biological properties
Soil pH was determined on air dried 2 mm sieved soils using 1:2 soil to water ratio using a combined glass electrode. Total soil organic matter (SOM) content in soil was determined by loss on ignition following igniting oven dried soil at 550°C in a muffle furnace. For the measurement of ammonium and nitrate (NH4-N and NO3-N) concentration, 6 g of field moist soil was used. An extraction was carried out using 40 ml of 2 M KCl by shaking and filtration. Ammonium in the extracts was determined colourimetrically44. A suitable aliquot of the filtrate (1 ml) was made to react with phenol and hypochlorite to form a blue indophenol complex in solution. The concentration of ammonium in solution was measured by comparing the absorbance with known standards prepared using NH4Cl at a wavelength of 635 nm. For the determination of NO3-N, nitrate in a suitable aliquot of KCl extract was reduced to nitrite using spongy cadmium, which was further complexed to form a red azo-species in solution. The concentration of NO3-N was measured by comparing the absorbance with known standards of KNO3 at a wavelength of 543 nm45. Field moist soil samples (both surface 0–10 and subsurface 10–20 cm depths) were used for the estimation of microbial biomass carbon and nitrogen by the chloroform fumigation-extraction technique46. Samples were incubated in the chloroform environment in presence of soda lime. The extraction was carried out using 0.5 M K2SO4 at the start of fumigation in un-fumigated samples and 24 hour after fumigation in fumigated samples. Microbial biomass carbon and nitrogen in the extracts were analysed using a Shimadzu CN analyser (TOC-V CPH Shimadzu). The results were corrected using the value of 0.45 for both carbon and nitrogen47.
Potential fluxes of greenhouse gases
Cores were removed from the 4°C environment and kept at a constant temperature of 16°C for 48 hours to activate and stabilise the biological activity. Gas sampling was performed by placing cores in 1.5 litre plastic jars (20 cm height and 10 cm diameter) with a septum on the top to aid gas sampling using a 20 ml syringe. The air in the headspaces was mixed, before sampling at time intervals 0, 15, 30 and 60 minutes using 20 ml syringes. The collected gas samples were stored in airtight pre-evacuated glass vials and analysed for concentration of CO2, CH4 and N2O using gas chromatography equipped with a Thermal Conductivity Detector (TCD), Flame Ionization Detector (FID) and an electron capture detector (ECD) (GC-2014, Shimadzu). The fluxes of these samples were calculated using linear regression of the gas concentration against time. The GHG data was converted to mass per volume and mass per weight basis by the use of ideal gas equation and the molecular mass of each gas48.
 |
Where n is the number of moles of CO2, N2O or CH4, P is atmospheric pressure (≈1 atm), V is the volume of head space (dm−3), R is the ideal gas constant (0.08205746 L atm K−1 mol−1) and T is the temperature of sampling (273.15 + room temperature in °C). From this the flux of gas was measured.
 |
Where E = flux of each gas in mg m−2 h−1, n = number of moles of CO2, N2O or CH4, m = molar weight of CO2 (44.01), N2O (44.01) or CH4 (16.04), a = area of the soil core used and t is the time in hour. Finally total greenhouse balance or net global warming potential (GWP) was calculated in CO2-equivalents19 using the following equation.
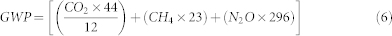 |
Statistical analysis
Each site consisted of a pair of fields; one of which was ploughed and the other had been tilled for a number of years. The sites were in areas consisting of a range of soil types in different geographical regions although at each site the tilled and zero tilled fields were located adjacent to each other. Samples were taken at a number of random locations in each field and at two soil depths (0–10 and 10–20 cm). The variation in soil properties in response to tillage and soil depth was analysed as split-split plot design in a linear mixed model with site, field and location within fields as random effects. Tilled vs non-tilled, soil depth and their interaction were considered as fixed effects. The variation among just the zero-tilled fields was further partioned to test for a trend in response to the number of years since adoption of zero tillage. This test was thus orthogonal to the tilled vs zero-tilled contrast as was its interactions with soil depth. To account for potential differences with respect to soil texture, the clay content of the soil was considered as a covariate by including it as a fixed effect in the model. The covariate could account for significant amounts of the random variation among fields and locations. In such cases, by reducing the unexplained residual variation, the model including the covariate is likely to be more sensitive for detecting tillage effects than a model without the covariate. Multiple linear regressions were used to predict the best model describing the fluxes of GHGs from soil. The maximal model consisted of all the physical, chemical and biological properties studied in this experiment. By using a stepwise backwards elimination process, only the variables that contributed significantly to the model and reduced the residual sum of squares were retained in the model. For illustrative purposes we also carried out the single linear regression between the parameters that contributed to the multiple regression models. All tests were performed using Genstat (14th Edition, VSN International Ltd, UK).
Author Contributions
Original ideas for the research came from S.S., D.S. and S.J.M; S.M., S.S. and S.J.M. undertook all sampling; S.M. and C.J.S. conducted the X-ray CT scanning and analysis; J.C. provided statistical advice; Construction of paper by S.M., S.S., C.J.S., J.C., D.S. and S.J.M.
Supplementary Material
Dataset 1
Acknowledgments
We acknowledge the research funding by the Indian Council of Agricultural Research, New Delhi through International Fellowship programme and the University of Nottingham through Research Excellence Scholarship. Thanks are also due to the farmers who permitted access to their land and to Boris Lazarevic for field assistance.
References
- Smith P. et al. [Agriculture] Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Metz, B. et al. (ed.)] (Cambridge University Press, Cambridge, United Kingdom and New York, USA, 2007). [Google Scholar]
- Petersen S. O., Mutegi J. K., Hansen E. M. & Munkholm L. J. Tillage effects on N2O emissions as influenced by a winter cover crop. Soil Biol. Biochem. 43, 1509–1517 (2011). [Google Scholar]
- Fernández R., Quiroga A., Zorati C. & Noellemeyer E. Carbon contents and respiration rates of aggregate size fractions under no-till and conventional tillage. Soil Tillage Res. 109, 103–109 (2010). [Google Scholar]
- Zotarelli L., Alves B. J. R., Urquiaga S., Boddey R. M. & Six J. Impact of tillage and crop rotation on light fraction and intra-aggregate soil organic matter in two Oxisols. Soil Tillage Res. 95, 196–206 (2007). [Google Scholar]
- West T. O. & Post W. M. Soil organic carbon sequestration rates by tillage andcrop rotation. Soil Sci. Soc. of Am. J. 66, 1930–1946 (2002). [Google Scholar]
- Six J., Elliott E. T. & Paustian K. Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 32, 2099–2103 (2000). [Google Scholar]
- Kong A. Y. Y., Fonte S. J., van Kessel C. & Six J. Transitioning from standard to minimum tillage: Trade-offs between soil organic matter stabilization, nitrous oxide emissions, and N availability in irrigated cropping systems. Soil Tillage Res. 104, 256–262 (2009). [Google Scholar]
- Helgason B. L., Walley F. L. & Germida J. J. No-till soil management increases microbial biomass and alters community profiles in soil aggregates. Appl. Soil Ecol. 46, 390–397 (2010). [Google Scholar]
- Derpsch R. & Friedrich T. Proceedings, Lead Papers, 4th World Congress on Conservation Agriculture 429–438 (New Delhi, India, 2009). [Google Scholar]
- Petersen S. O., Schjønning P., Thomsen I. K. & Christensen B. T. Nitrous oxide evolution from structurally intact soil as influenced by tillage and soil water content. Soil Biol. Biochem. 40, 967–977 (2008). [Google Scholar]
- Baker J. M., Ochsner T. E., Venterea R. T. & Griffis T. J. Tillage and soil carbon sequestration--What do we really know? Agric. Ecosyst. Environ. 118, 1–5 (2007). [Google Scholar]
- Campbell C. et al. Quantifying short-term effects of crop rotations on soil organic carbon in southwestern Saskatchewan. Can. J. Soil Sci. 80, 193–202 (2000). [Google Scholar]
- Hermle S., Anken T., Leifeld J. & Weisskopf P. The effect of the tillage system on soil organic carbon content under moist, cold-temperate conditions. Soil Tillage Res. 98, 94–105 (2008). [Google Scholar]
- Six J. et al. The potential to mitigate global warming with no-tillage management is only realized when practised in the long term. Global Change Biol. 10, 155–160 (2004). [Google Scholar]
- Chatskikh D. & Olesen J. E. Soil tillage enhanced CO2 and N2O emissions from loamy sand soil under spring barley. Soil Tillage Res. 97, 5–18 (2007). [Google Scholar]
- West T. O. & Marland G. Net carbon flux from agricultural ecosystems: methodology for full carbon cycle analyses. Environ. Pollut. 116, 439–444 (2002). [DOI] [PubMed] [Google Scholar]
- Schjønning P. & Rasmussen K. J. Soil strength and soil pore characteristics for direct drilled and ploughed soils. Soil Tillage Res. 57, 69–82 (2000). [Google Scholar]
- Gantzer C. J. & Anderson S. H. Computed tomographic measurement of macroporosity in chisel-disk and no-tillage seedbeds. Soil Tillage Res. 64, 101–111 (2002). [Google Scholar]
- IPCC. Climate Change 2001: The Scientific Basis, Contribution of Working Group I to The Third assessment Report Of The Intergovernmental Panel On Climate Change [Houghton, J. T. et al. (ed.)] (Cambridge University Press, 2001). [Google Scholar]
- Ussiri D. A. N. & Lal R. Long-term tillage effects on soil carbon storage and carbon dioxide emissions in continuous corn cropping system from an alfisol in Ohio. Soil Tillage Res. 104, 39–47 (2009). [Google Scholar]
- Udawatta R. P. & Anderson S. H. CT-measured pore characteristics of surface and subsurface soils influenced by agroforestry and grass buffers. Geoderma 145, 381–389 (2008). [Google Scholar]
- Beare M. H., Gregorich E. G. & St-Georges P. Compaction effects on CO2 and N2O production during drying and rewetting of soil. Soil Biol. Biochem. 41, 611–621 (2009). [Google Scholar]
- Schaufler G. et al. Greenhouse gas emissions from European soils under different land use: effects of soil moisture and temperature. Eur. J. Soil Sci. 61, 683–696 (2010). [Google Scholar]
- Ball B. C., Scott A. & Parker J. P. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res. 53, 29–39 (1999). [Google Scholar]
- Bhattacharyya R., Prakash V., Kundu S. & Gupta H. S. Effect of tillage and crop rotations on pore size distribution and soil hydraulic conductivity in sandy clay loam soil of the Indian Himalayas. Soil Tillage Res. 86, 129–140 (2006). [Google Scholar]
- Wu L., Swan J. B., Paulson W. H. & Randall G. W. Tillage effects on measured soil hydraulic properties. Soil Tillage Res. 25, 17–33 (1992). [Google Scholar]
- Smith P. et al. Enhancing the carbon sink in European agricultural soils: including trace gas fluxes in estimates of carbon mitigation potential. Nutr. Cycl. Agroecosys. 60 (2001). [Google Scholar]
- Mosier A. et al. Impact of agriculture on soil consumption of atmospheric CH4 and a comparison of CH4 and N2O flux in subarctic, temperate and tropical grasslands. Nutr. Cycl. Agroecosys. 49, 71–83 (1997). [Google Scholar]
- Hütsch B. W. Tillage and land use effects on methane oxidation rates and their vertical profiles in soil. Biol. Fert. Soils 27, 284–292 (1998). [Google Scholar]
- Nazaries L. et al. Response of methanotrophic communities to afforestation and reforestation in New Zealand. ISME J. 5, 1832–1836 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Bohme F., Rinlebe J., Neue H. & DeLaune R. D. Major biogeochemical processes in soils-A microcosm incubation from reducing to oxidizing conditions. Soil Sci. Soc. Am. J. 71, 1406–1417 (2007). [Google Scholar]
- Ussiri D., Lal R. & Jarecki M. K. Nitrous oxide and methane emissions from long-term tillage under a continuous corn cropping system in Ohio. Soil Tillage Res. 104, 247–255 (2009). [Google Scholar]
- Regina K. & Alakukku L. Greenhouse gas fluxes in varying soils types under conventional and no-tillage practices. Soil Tillage Res. 109, 144–152 (2010). [Google Scholar]
- Oorts K., Merckx R., Grehan E., Labreuche J. & Nicolardot B. Determinants of annual fluxes of CO2 and N2O in long-term no-tillage and conventional tillage systems in northern France. Soil Tillage Res. 95, 133–148 (2007). [Google Scholar]
- Choudhary M. A., Akramkhanov A. & Saggar S. Nitrous oxide emissions from a New Zealand cropped soil: tillage effects, spatial and seasonal variability. Agric. Ecosyst. Environ. 93, 33–43 (2002). [Google Scholar]
- Gregorich E. G., Rochette P., St-Georges U. F. & Chan C. Tillage effects on N2O emission from soils under corn and soybeans in Eastern Canada. Can. J. Soil Sci. 88, 153–161 (2008). [Google Scholar]
- Del Grosso S. J., Mosier A. R., Parton W. J. & Ojima D. S. DAYCENT model analysis of past and contemporary soil N2O and net greenhouse gas flux for major crops in the USA. Soil Tillage Res. 83, 9–24 (2005). [Google Scholar]
- Piva J. T. et al. No-till reduces global warming potential in a subtropical Ferralsol. Plant Soil 361, 359–373 (2012). [Google Scholar]
- Post W. M. et al. Enhancement of carbon sequestration in US soils. Bioscience 54, 895–908 (2004). [Google Scholar]
- Page A. L., Miller R. H. & Keeney D. R. Methods Of Soil Analysis, Part 2- Chemical And Microbiological Properties-Second Edition. (American Society of Agronomy and Soil Science Society of America, Madison, WI, USA, 1982). [Google Scholar]
- Bouyoucos G. J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 54, 464–465 (1961). [Google Scholar]
- Rowell D. L. Soil Science: Methods And Applications (Longman Scientific and Technical, UK, 1994). [Google Scholar]
- Rasband W. NIH ImageJ, Research Service Branch, National Institute of Mental Health, National Institute of Health, Bethesda, MD (2002) Available online at http://rsb.info.nih.gov/ij/docs/intro.html (Date of access: October 21, 2013) (2002).
- Kempers A. J. Determination of sub-microquantities of ammonium and nitrates in soils with phenol, sodiumnitroprusside and hypochlorite. Geoderma 12, 201–206 (1974). [Google Scholar]
- Jones M. N. Nitrate reduction by shaking with cadmium: Alternative to cadmium columns. Water Res. 18 (1984). [Google Scholar]
- Vance E. D., Brookes P. C. & Jenkinson D. S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19, 703–707 (1987). [Google Scholar]
- Jenkinson D. S., Brookes P. C. & Powlson D. S. Measuring soil microbial biomass. Soil Biol. Biochem. 36, 5–7 (2004). [Google Scholar]
- Denef K., Zotarelli L., Boddey R. M. & Six J. Microaggregate-associated carbon as a diagnostic fraction for management-induced changes in soil organic carbon in two Oxisols. Soil Biol. Biochem. 39, 1165–1172 (2007). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset 1