Abstract
Fatty acids (FAs) are highly diverse in terms of carbon (C) chain-length and number of double bonds. FAs with C>20 are called very long-chain fatty acids (VLCFAs). VLCFAs are found not only as constituents of cellular lipids such as sphingolipids and glycerophospholipids but also as precursors of lipid mediators. Our understanding on the function of VLCFAs is growing in parallel with the identification of enzymes involved in VLCFA synthesis or degradation. A variety of inherited diseases, such as ichthyosis, macular degeneration, myopathy, mental retardation, and demyelination, are caused by mutations in the genes encoding VLCFA metabolizing enzymes. In this review, we describe mammalian VLCFAs by highlighting their tissue distribution and metabolic pathways, and we discuss responsible genes and enzymes with reference to their roles in pathophysiology.
Keywords: Sphingolipids, Glycerophospholipids, ELOVL, Ceramide, Ichthyosis, Leukodystrophy
CELLULAR FATTY ACIDS (FAS): CHAIN-LENGTH AND NUMBER OF DOUBLE BONDS
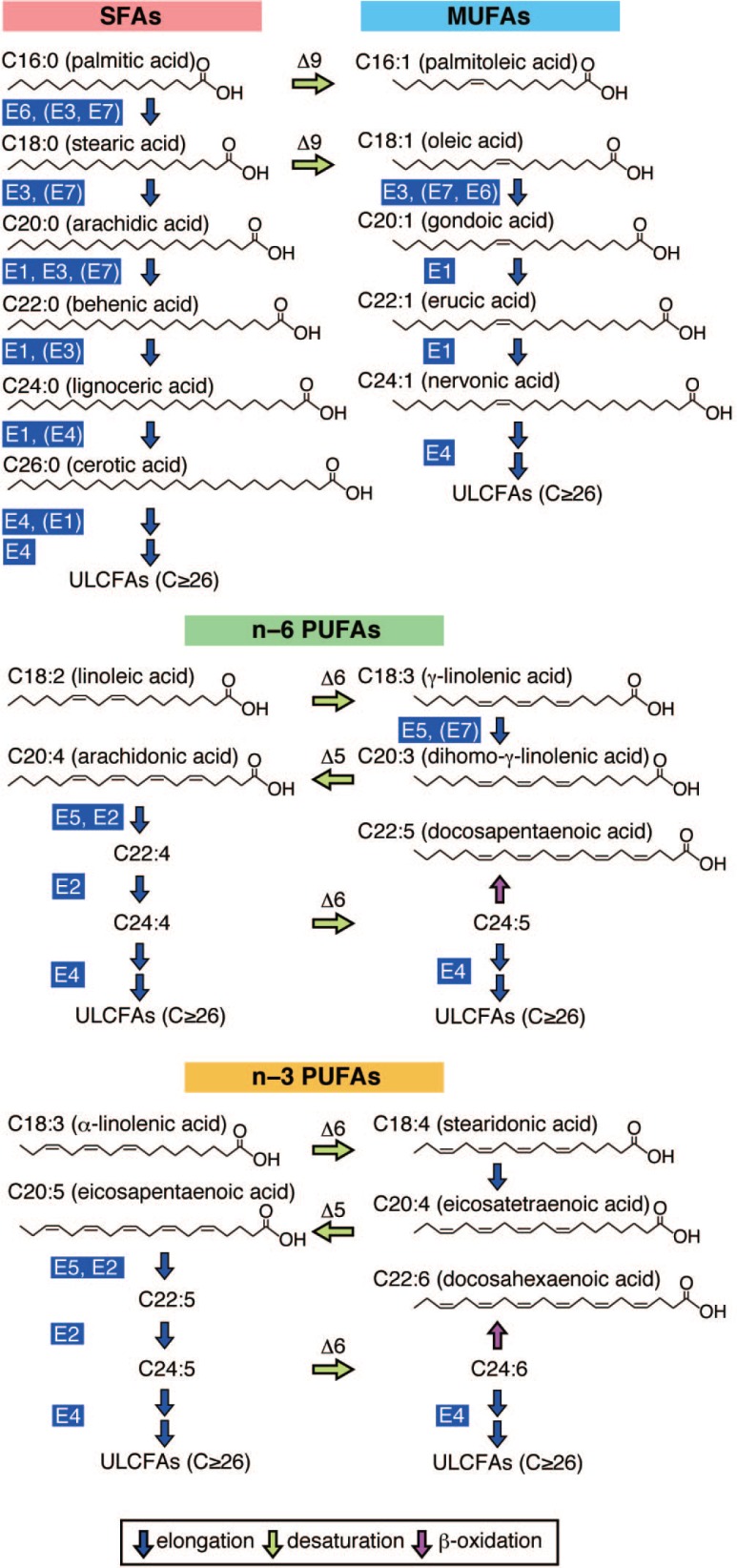
FAs are classified according to their carbon (C) chain-length and the number of double bonds (Fig. 1). Long-chain FAs (LCFAs) have chain-lengths of C11–20, of which C16 and C18 LCFAs are the most abundant FA species in mammalian cells. FAs longer than C20 (C>20) are called very long-chain FAs (VLCFAs) and are less abundant than LCFAs. VLCFAs with C22 and C24 are found ubiquitously throughout the body. VLCFAs with C≥26 are often sub-classified into ultra long-chain FAs (ULCFAs) and are found in specific tissues, including the skin, retina, meibomian gland, testis, and brain.
Fig. 1.
Human FA elongation pathways. The FA elongation pathways of SFAs, MUFAs and PUFAs are illustrated. ELOVL isozymes (E1-E7) responsible for each elongation step are indicated. Parentheses denote ELOVLs that exhibit weak activity toward the indicated substrates. Δ5, Δ6 and Δ9 represent Δ5-, Δ6- and Δ9-desaturase, respectively. FA: fatty acid; SFA: saturated FA; MUFA: monounsaturated FA; PUFA: polyunsaturated FA.
Another classification of FAs is based on the number of double bonds. FAs are classified into saturated FAs (SFAs; no double bond), monounsaturated FAs (MUFAs; one double bond) and polyunsaturated FAs (PUFAs; two or more double bonds) (Fig. 1). PUFAs are further sub-classified into n-3 (or ω3) and n-6 (or ω6) series depending on the position of the terminal double bond, i.e. the double bond most distant from the carboxyl group. In the n-x series, x indicates the ordinal number of carbon atom with a double bond from the end of the carbon chain.
By combining these two classifications, arachidonic acid for example, an n-6 FA with chain-length C20 and four double bonds is denoted by C20:4n-6. Linoleic acid (C18:2n-6) and a-linolenic acid (C18:3n-3) are essential FAs (EFAs) that must be consumed through food, since humans are unable to synthesize them.
ENZYMES RESPONSIBLE FOR FA ELONGATION
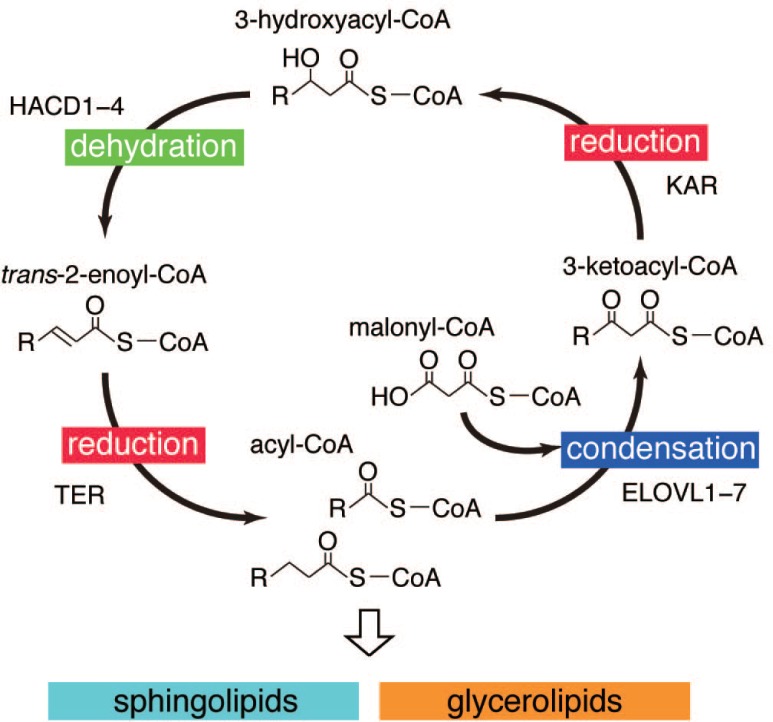
FAs are elongated in the form of acyl-CoA, in which FAs are covalently linked to coenzyme A via thioester bonds. Elongation of ≥C16 FAs that are either synthesized de novo by the FA synthase in the cytoplasm or absorbed from food, occurs in the endoplasmic reticulum (ER). FA elongation proceeds through repetition of the FA elongation cycle whereby two carbons are added to the carboxyl end in each cycle (Fig. 2) (Jakobsson et al., 2006; Guillou et al., 2010). The FA elongation cycle consists of four sequential reactions: condensation, reduction, dehydration, and reduction. The first condensation reaction is the rate-limiting step, in which 3-ketoacyl-CoA is produced by condensation of acyl-CoA with malonyl-CoA. This reaction is catalyzed by FA elongase. FA elongases constitute the ELOVL family of proteins, and there are seven isozymes (ELOVL1-7) in mammals (Jakobsson et al., 2006; Guillou et al., 2010; Ohno et al., 2010). In the second reduction step, 3-ketoacyl-CoA is converted to 3-hydroxyacyl-CoA using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor. The 3-ketoacyl-CoA reductase responsible for this reaction is KAR (Moon and Horton, 2003). In the third dehydration step, 3-hydroxyacyl-CoA is converted to trans-2-enoyl-CoA, and this reaction is catalyzed by 3-hydroxyacyl-CoA dehydratase (HACD) (Ikeda et al., 2008). This third step appears to be another rate-limiting step, since some 3-hydroxyacyl-CoAs accumulate at low but significant levels in in vitro FA elongation assays (Abe et al., 2013). There are four mammalian HACD isozymes (HACD1-4) (Ikeda et al., 2008). In the last reduction step, trans-2-enoyl-CoA is converted to acyl-CoA, which is longer than the original acyl-CoA by two carbons. The trans-2-enoyl-CoA reductase responsible for this reaction is TER, and the reaction requires NADPH as a cofactor (Moon and Horton, 2003).
Fig. 2.
Mammalian FA elongation cycle. The FA elongation cycle and enzymes involved in each step are illustrated. In each cycle, acyl-CoA incorporates two carbon units from malonyl-CoA.
In vitro FA elongation assays and knockdown or knockout (KO) of ELOVL1-7 genes uncovered that ELOVL1-7 exhibits substrate specificity; each isozyme prefers acyl-CoAs with specific chain-lengths and/or degree of saturation (Fig. 1) (Guillou et al., 2010; Ohno et al., 2010). ELOVL1 elongates saturated and monounsaturated C20–C26 acyl-CoAs (Ohno et al., 2010). ELOVL1 production of C24:0 and C24:1 acyl-CoAs is essential for the synthesis of C24 sphingolipids (discussed later); in Elovl1 KO mice, C24 sphingolipids are severely reduced in various tissues (Sassa et al., 2013). ELOVL2 and ELOVL5 elongate PUFAs. ELOVL2 elongates C20–C22 polyunsaturated acyl-CoAs, while ELOVL5 elongates C18–C20 polyunsaturated acyl-CoAs (Ohno et al., 2010). In Elovl2 KO mice, the levels of C22:4n-6 and C22:5n-3 FAs are increased, whereas the levels of derivatives thereof are severely reduced, indicating that Elovl2 is critical for the elongation of C22-CoAs of both the n-3 and n-6 series (Zadravec et al., 2011). Moreover, Elovl2 deficiency causes the near absence of ultra long-chain (ULC)-PUFAs (C28:5n-6 and C30:5n-6) in the testis and male infertility with a complete arrest of spermatogenesis (Zadravec et al., 2011). Analysis of Elovl5 KO mice demonstrated that Elovl5 is essential for the elongation of C18-CoAs of both n-3 and n-6 series in the liver (Moon et al., 2009). Elovl5 KO mice exhibit hepatic steatosis due to the activation of sterol regulatory element-binding protein (SREBP)-1c and its target genes involved in FA and triglyceride synthesis (Moon et al., 2009). ELOVL3 and ELOVL7 elongate both saturated and unsaturated C16–C22 acyl-CoAs, with the highest activity toward C18-CoAs (Ohno et al., 2010; Naganuma et al., 2011). Elovl3 is expressed in the skin sebaceous glands and hair follicles as well as brown adipose tissue. Elovl3 KO mice exhibit accumulation of C20:1 FAs in the skin that is associated with a sparse hair coat and defects in water repulsion (Westerberg et al., 2004). ELOVL4 also elongates both saturated and unsaturated acyl-CoAs, but it is specialized for the synthesis of ULCFAs (Ohno et al., 2010). In Elovl4 KO mice and homozygous Stargardt disease 3 (STGD3) model mice that carry a pathogenic mutation in the Elovl4 gene, ULCFAs in the epidermis were completely missing (Li et al., 2007; Vasireddy et al., 2007). ELOVL6 elongates shorter acyl-CoAs as compared to other ELOVLs, with activity toward C12:0-C16:0 acyl-CoAs (Moon et al., 2001). Consistent with this, the levels of C16:0 and C16:1 FAs are increased whereas the levels of C18:0 and C18:1 FAs are reduced in the liver of Elovl6 KO mice (Matsuzaka et al., 2007). Interestingly, Elovl6 KO mice are protected from obesity-induced insulin resistance despite becoming obese and developing hepatosteatosis (Matsuzaka et al., 2007).
C24 VLCFAS IN SPHINGOLIPIDS
Most saturated and monounsaturated VLCFAs are found as acyl moieties of sphingolipids. Sphingolipids contain ceramide (Cer) as a backbone, in which FAs are amide-linked to the sphingoid base. Cers are converted to sphingomyelin (SM) and glycosphingolipids by addition of a polar head group of phosphocholine and sugars, respectively.
Cer synthase (CERS) catalyzes an amide bond formation between acyl-CoA and the sphingoid base (Mizutani et al., 2009; Tidhar and Futerman, 2013). Six mammalian CERS isozymes (CERS1-6) have been identified, and each CERS isozyme exhibits characteristic substrate specificity toward acyl-CoAs with specific chain-lengths (Table 1) (Mizutani et al., 2009; Tidhar and Futerman, 2013). CERSs exhibit ubiquitous or tissue specific distribution patterns and may determine the tissue distribution patterns of sphingolipids with specific chain-lengths (Table 1) (Mizutani et al., 2009; Tidhar and Futerman, 2013).
Table 1.
Substrate specificity and tissue distribution of mammalian CERS isozymes
| Isozyme | Preferred substrates | mRNA expression |
|---|---|---|
| CERS1 | C18 | Brain, skeletal muscle |
| CERS2 | C22–C24 | Ubiquitous, high in liver, kidney, lung |
| CERS3 | ≥C26 | Skin, testis |
| CERS4 | C18–C20 | Lung, heart |
| CERS5 | C16 | Ubiquitous, high in brain, kidney, testis |
| CERS6 | C16 | Ubiquitous, high in brain, liver, thymus |
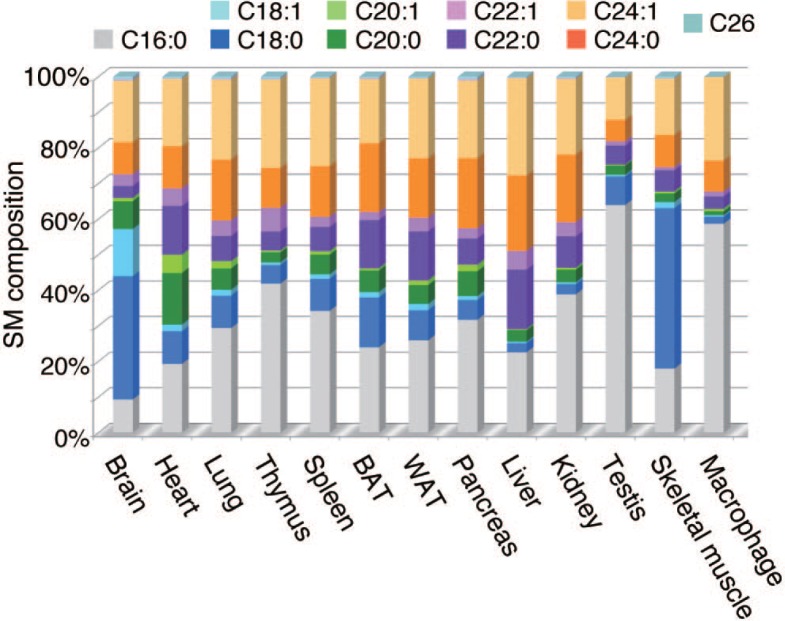
In most tissues, chain-lengths of sphingolipids range from C16 to C24 with the major FAs being C16:0 palmitic acid, C24:0 lignoceric acid, and C24:1 nervonic acid. However, the proportion of each FA varies considerably among tissues. FA compositions of SM in various mouse tissues are shown in Fig. 3. C24 VLCFAs constitute almost 50% of SMs in the liver, whereas they constitute less than 20% in the testis. As an exception, C18 LCFAs (C18:0 stearic acid and C18:1 oleic acid) rather than C16 LCFAs or C24 VLCFAs are predominant in the brain and skeletal muscle. FA composition of sphingolipids varies significantly depending on the cell type. In the nervous system, for example, neurons synthesize mainly C18 sphingolipids, while oligodendrocytes and Schwann cells, which wind tightly around axons to form myelin sheaths, predominantly synthesize C24 sphingolipids (Becker et al., 2008; Imgrund et al., 2009; Ginkel et al., 2012).
Fig. 3.
FA compositions of SM. FA compositions of SM in indicated mouse tissues determined by liquid chromatography-mass spectrometry analysis are illustrated. SM: sphingomyelin; BAT: brown adipose tissue; WAT: white adipose tissue.
CERS2 is primarily responsible for the synthesis of C24 sphingolipids. In CerS2 KO mice, the levels of C24 sphingolipids are severely reduced in tissues that normally contain high levels of C24 sphingolipids, such as the liver, kidney and brain (Imgrund et al., 2009; Pewzner-Jung et al., 2010b). ELOVL1 provides C24-CoAs for C24 sphingolipid synthesis via CERS2. Elovl1 KO mice also exhibit a severe reduction in C24 sphingolipid levels in various tissues (Sassa et al., 2013). CerS2 KO mice exhibit defects in myelin sheath stability associated with the near absence of myelin C22–C24 galactosyl-Cer (GalCer) and sulfatide, a sulfated form of GalCer (Imgrund et al., 2009). CerS2 KO mice also develop hepatocellular carcinomas (Imgrund et al., 2009; Pewzner-Jung et al., 2010a).
At the cellular and molecular levels, C24 sphingolipids have unique biophysical properties not possessed by C16 sphingolipids such as effects on membrane fluidity, lipid microdomain formation, and signaling across the membrane (Simons and Ikonen, 1997; Kasahara and Sanai, 2000; Sonnino et al., 2009; Silva et al., 2012). Studies using artificial lipid bilayers have suggested that the C24 FA moiety in sphingolipids may interdigitate with the opposing leaflet and facilitate the formation of lipid microdomains (Morrow et al., 1995). In neutrophils, C24 lactosylCers (LacCers) but not C16 LacCers are associated with the Src family kinase LYN in the plasma membrane microdomain; this association is necessary for neutrophil functions such as superoxide generation and migration (Iwabuchi et al., 2008). In HeLa cells, knockdown of ELOVL1 or CERS2 causes a shift in sphingolipid composition from C24 to C16 and increases susceptibility to apoptosis induced by diverse stimuli such as anticancer drugs (i.e. cisplatin), ultraviolet radiation, or C6 Cers (Sassa et al., 2012).
Yeast sphingolipids consist exclusively of saturated C26 VLCFAs (Ejsing et al., 2009). VLCFA synthesis is essential for yeast viability, and viable mutant strains with limited VLCFA synthesis exhibit defects in vesicular transport mainly in the late endosome/multivesicular body (Obara et al., 2013). VLCFAs may have a conserved role in vesicular trafficking systems, where a highly curved membrane is constantly appearing and disappearing to support vesicle budding and fusion.
ULCFAS IN EPIDERMAL CERS
Saturated and monounsaturated ULCFAs are found in the skin. The stratum corneum (SC), the outermost layer of the skin epidermis, is essential for the epidermal permeability barrier, which protects terrestrial animals against desiccation by transepidermal water loss and invasion of pathogens and toxic chemicals (Proksch et al., 2008). In the SC, the intercellular spaces are filled with multilamellar lipid layers called extracellular lipid lamella (ELL) (Breiden and Sandhoff, 2014; Rabionet et al., 2014). Cers account for ∼50% of lipids in the ELL, although Cers are less abundant in most tissues since they are utilized as precursors of complex sphingolipids such as SMs and glycosphingolipids. C26–C36 ULCFAs are found in Cers in the ELL (Masukawa et al., 2008; t’Kindt et al., 2012). Most C≥30 ULCFAs in the epidermal Cers are ω-hydroxylated and esterified with linoleic acid (C18:2n-6) to form ω-O-acyl-Cers or covalently bound to proteins (mainly involucrin) of corneocytes, the latter form corneocyte lipid envelopes (CLEs) (Breiden and Sandhoff, 2014; Rabionet et al., 2014). EFA deficiency in human and other mammals causes ichthyosis-like cutaneous abnormalities including scaly skin and impaired permeability barriers (Prottey, 1977; Yamanaka et al., 1980). In EFA deficiency, linoleic acid (C18:2n-6) esterified with ω-OH ULCFAs in ω-O-acylCers is replaced by oleic acid (C18:1n-9) with a concomitant decrease in the epidermal permeability barrier, suggesting an essential function of linoleate-esterified ω-O-acylCers in constructing and maintaining epidermal permeability barrier integrity (Prottey, 1977; Yamanaka et al., 1980).
Our understanding on the pathway(s) and the enzymes involved in the synthesis of epidermal Cers is still incomplete. However, recent studies using KO mice identified the enzymes responsible for the synthesis of ULC-Cers. ULC-Cers are essential for the epidermal permeability barrier, thus a deficiency in ULC-Cers synthesis can be easily identified in KO mice by examining transepidermal water loss and dye penetration, and epidermal histology. Among the six CERS isozymes, CERS3 is exclusively required for ULC-Cer synthesis (Jennemann et al., 2012). Epidermal ULC-Cers are completely lost in CerS3 KO mice, precluding the formation of the ELL and CLE, which leads to severe transepidermal water loss and early postnatal lethality (Jennemann et al., 2012). Consistent with the involvement of ELOVL4 in ULCFA synthesis (Fig. 1), Elovl4 KO mice exhibit essentially identical skin phenotypes to that of CerS3 KO mice (Li et al., 2007; Vasireddy et al., 2007). CERS3 and ELOVL4 mRNA increases during keratinocyte differentiation (Mizutani et al., 2013). Elovl1 KO mice also exhibit lethal epidermal permeability barrier deficiency (Sassa et al., 2013). ELOVL1 activity is regulated differently by CERS2 or CERS3 (Ohno et al., 2010; Mizutani et al., 2013; Sassa et al., 2013). In most tissues, ELOVL1 cooperates with CERS2 and elongates acyl-CoAs up to C24-CoAs, which are utilized for C24 sphingolipid synthesis by CERS2. However, in the upper epidermis, where ULC-Cers present in the ELL and CLE are synthesized, CERS3 is highly expressed and instructs ELOVL1 to elongate up to C26-CoAs, which are either utilized for the synthesis of C26-Cers by CERS3 or subject to further elongation up to C36-CoAs by ELOVL4 (Sassa et al., 2013).
Recently, recessive mutations in the CERS3 gene on chromosome 15 and the ELOVL4 gene on chromosome 6 have been identified in ichthyosis patients (Table 2) (Aldahmesh et al., 2011; Eckl et al., 2013; Radner et al., 2013). Lipid analysis of the CERS3 mutant keratinocytes derived from the patients revealed severe reductions in the levels of ULC-Cers including ω-O-acylCers and protein bound ω-OH Cers (Eckl et al., 2013; Radner et al., 2013).
Table 2.
VLCFA-related genes mutated in inherited diseases
| Gene | Chromosome | Function | Disease |
|---|---|---|---|
| ELOVL4 | 6 | FA elongase (condensation) | Stargardt-like macular dystrophy (STGD3) (dominant) Ichthyosis, nervous system abnormalities (recessive) |
| HACD1 (PTPLA) | 10 | 3-Hydroxyacyl-CoA dehydratase | Myopathy |
| TER (TECR) | 19 | Trans-2-enoyl-CoA reductase | Non-syndromic mental retardation |
| CERS3 | 15 | Cer synthase | Ichthyosis |
| ABCA12 | 2 | Glucosylceramide transport into LB | Ichthyosis |
| FA2H | 16 | 2-Hydroxylation of FA | Leukodystrophy with spastic paraparesis and dystonia |
| ABCD1 | X | VLCFA-CoA transport into peroxisome | X-linked adrenoleukodystrophy (X-ALD) |
| ACOX1 | 17 | VLCFA β-oxidation in peroxisome | Leukodystrophy, other nervous system abnormalities |
| HSD17β4 | 5 | VLCFA β-oxidation in peroxisome | Leukodystrophy, other nervous system abnormalities |
Inherited ichthyoses form part of a large clinically and etiologically heterogeneous group of disorders of cornification, and most ichthyoses are associated with the impaired epidermal permeability barrier (Oji et al., 2010; Elias et al., 2012). Notably, along with CERS3 and ELOVL4, some nonsyndromic and syndromic forms of inherited ichthyosis include genes involved in lipid metabolism. Given the crucial role of ULC-Cers in epidermal permeability barrier, it is plausible that at least some of these genes may be involved in the synthesis or transport of ULC-Cers necessary for the formation of the ELL and CLE. Indeed, the ABCA12 gene, which is mutated in harlequin ichthyosis, the most severe form of autosomal recessive congenital ichthyosis, encodes a member of the ATP-binding cassette (ABC) transporters likely involved in the transport of glucosylCers (GlcCers) into lamellar bodies (LB) (Table 2) (Akiyama et al., 2005; Akiyama, 2014). GlcCers in LBs are precursors of Cers including ω-O-acylCers and are converted to Cers upon secretion in the SC (Akiyama, 2014; Breiden and Sandhoff, 2014; Rabionet et al., 2014).
VLCFAS IN THE MEIBUM
The ocular surface is covered by a structure called the tear film (TF), which consists of aqueous tears and a mixture of diverse lipids (meibum) that are produced by lachrymal glands and meibomian glands, respectively (Butovich, 2013). The physiological functions of the TF are the maintenance of a smooth surface for light refraction, and, in analogy with the epidermal permeability barrier, the protection of underlying ocular structures including the cornea and conjunctiva from desiccation and infection. In humans, there are 30–40 and 20–30 meibomian glands in the upper and lower eyelids, respectively. In dry eye patients, the number of functional (yielding liquid secretion) meibomian glands is significantly decreased compared with asymptomatic controls (Korb and Blackie, 2008).
The major components of meibum are cholesteryl esters and wax esters (WEs), each accounting for ∼30% of the total meibum lipids (Ohashi et al., 2006). The FA residues of human meibum cholesteryl esters are ∼80% saturated, and their chain-lengths range from C18 up to C32, with saturated C24–C27 VLCFAs being the major species (Butovich, 2010). WEs are esters of FAs with fatty alcohols. The major WE species in human meibum have saturated C24–C26 VLC fatty alcohols that are esterified to oleic acid (C18:1) (Butovich et al., 2009). Meibum also contains ω-O-acylVLCFAs, which consist of ω-OH monounsaturated ULCFAs (C30–C34) esterified with monounsaturated C16 or C18 FA (Butovich et al., 2009). However, neither ω-O-acylCers nor regular Cers were found in the human meibum, and FAs (palmitoleic acid, C16:1n-7 and oleic acid, C18:1n-9) esterified to ω-OH ULCFAs are different from those (linoleic acid, C18:2n-6) esterified to epidermal ω-OH Cers (Butovich et al., 2009).
DOCOSAHEXAENOIC ACID (DHA) IN THE TESTIS, BRAIN, AND RETINA
VLC-PUFAs include DHA (C22:6n-3) (Fig. 1). DHA is abundant in glycerophospholipids in the testis, brain, and retina. In the spermatozoa, DHA is almost exclusively found in phosphatidylcholine (PC) or phosphatidylethanolamine (PE), whereas n-6 PUFAs, including docosapentaenoic acid (DPA; C22: 5n-6), are uniformly distributed in various glycerophospholipid species (Lin et al., 1993). In the brain gray matter, DHA is predominantly found in phosphatidylserine (PS) and PE (Sastry, 1985). About 50–60% of the PE in the brain is present in the form of plasmalogen, which contains a vinyl ether bond at the sn-1 position instead of an ester bond. DHA is enriched in both PE and PE plasmalogen (Sastry, 1985). In the retina, DHA is the major FA (∼30%) in the disc membrane of retinal photoreceptor outer segment, where photopigment rhodopsin processes phototransduction (Sangiovanni and Chew, 2005). DHA accounts for 20–30% of the FAs in PC, PE, and PS of outer segment disc membranes. Newborns with n-3 FA deficiency exhibit reduced light sensitivity of retinal rod photoreceptors, and DHA supplementation enhances visual resolution acuity (Uauy et al., 2001).
One of the functions of DHA is to generate lipid mediators, which actively turn off the inflammatory responses in tissues. The lipid mediators formed from DHA include D-series resolvin (resolvin D1–D4), protectin D1 (neuroprotectin D1), and maresin 1 (Bannenberg and Serhan, 2010). These molecules constitute novel families of lipid mediators that are structurally unrelated to authentic eicosanoids, such as prostaglandin or leuztriene, and that display potent anti-inflammatory and tissue-protective actions such as reduced neutrophil migration and activation of phagocytosis by macrophages (Bannenberg and Serhan, 2010).
In the retina, continuous light absorption by photoreceptors induces oxidative stress in the photoreceptor outer segments. To replace the damaged outer segments, photoreceptors shed the distal tips of the outer segments, which are phagocytosed by retinal pigment epithelial (RPE) cells. RPE cells respond to oxidative stress by synthesizing protectin D1 from DHA in the phagocytosed outer segment membranes (Bazan et al., 2010). Protectin D1 promotes the survival of RPE cells, and, as a consequence, photoreceptor cell integrity (Mukherjee et al., 2007).
ULC-PUFAS IN THE TESTIS, BRAIN, AND RETINA
ULC-PUFAs are found in the testis, brain, and retina (Agbaga et al., 2010). In the mammalian testis and spermatozoa, n-6 and n-3 ULC-PUFAs with chain-lengths of C26–C32 and 3–6 double bonds are present uniquely in sphingolipids including SMs, Cers, and fucosylated glycosphingolipids (FGSLs) (Sandhoff et al., 2005; Furland et al., 2007b; Rabionet et al., 2008). The level of ULC-PUFAs in these sphingolipids increase with the onset of spermatogenesis, and ULC-PUFAs account for up to 15% and 40% of rat testicular SMs and Cers, respectively (Furland et al., 2007b). Galgt1 gene KO mice (encoding GM2 synthase) lack a subset of ULC-PUFAs-containing FGSLs and are infertile with an arrest of spermatogenesis at the stage of round spermatids (Sandhoff et al., 2005). Interestingly, ULC-PUFAs-containing SMs and Cers are enriched in the head of the spermatozoa, and ULC-PUFAs-containing SMs are converted to the corresponding Cers during sperm capacitation in vitro, suggesting a role for ULC-PUFAs-containing SMs and Cers in acquiring competency for fertilization (Furland et al., 2007a).
In the brain, n-6 and n-3 series of ULC-PUFAs with chain-lengths of C26–C38 and 4–6 double bonds are found in PCs at the sn-1 position, with n-6 series ULC-PUFAs being predominant (Poulos et al., 1988). In rats, the levels of PCs containing ULC-PUFAs in the brain are higher in the neonatal and early postnatal periods than in adults, suggesting a role in postnatal brain development (Robinson et al., 1990).
ELOVL4 is involved in the synthesis of ULC-PUFAs as well as saturated and monounsaturated ULCFAs (Fig. 1). The recessive mutations in the ELOVL4 gene mentioned above are associated with not only ichthyosis but also seizures, intellectual abnormality, and spastic quadriplegia, suggesting the importance of ULC-PUFAs in brain development and physiology (Aldahmesh et al., 2011).
In the retina, similar to the brain, ULC-PUFAs are found at the sn-1 position of PCs. These ULC-PUFAs are predominantly n-3 series with chain-lengths of C26–C36 and 3–6 double bonds (Aveldano and Sprecher, 1987). These ULC-PUFA-containing PCs also have PUFAs (predominantly DHA) at the sn-2 position, thus as many as 12 double bonds are present in a single PC molecule (Aveldano and Sprecher, 1987). In retina-specific Elovl4 KO mice, ULC-PUFA-containing PCs in the adult retina were severely decreased (Harkewicz et al., 2012).
STDG3 is an early onset, autosomal dominant form of macular degeneration that is characterized by decreased visual acuity, flecks in fundus flavimaculatus, and macular dystrophy (Donoso et al., 2001). STDG3 is caused by mutations in the ELOVL4 gene on chromosome 6 (Table 2) (Zhang et al., 2001; Vasireddy et al., 2010). All three STDG3 causative mutations are located in the last exon (exon 6) of ELOVL4 and result in the production of C-terminally truncated proteins lacking the ER retention signal. These dominant mutations are located closer to the C-terminus of the ELOVL4 protein than the recessive mutations that result in ichthyosis and other nervous system abnormalities, and it may explain the differences in the symptoms and the mode of inheritance. A knock-in mouse model carrying one of the STGD3 mutations was generated and displayed characteristic features associated with the STGD3, such as the accumulation of lipofuscin in RPE and photoreceptor degeneration (Vasireddy et al., 2006). STGD3 mutant ELOVL4 proteins exhibited no activity in vitro and were misrouted to perinuclear aggresomes (Karan et al., 2005; Okuda et al., 2010). Moreover, STGD3 mutant ELOVL4 proteins are able to form hetero-oligomeric complexes with other components of the elongation machinery as well as homo-oligomeric complex with wild type ELOVL4 (Okuda et al., 2010).
2-HYDROXYLATED (2-OH) VLCFAS IN THE MYELIN
A subset of sphingolipids contains 2-OH VLCFAs. 2-OH VLCFAs are found almost exclusively in sphingolipids and are abundant in Cers in the epidermis and GalCers in the brain and kidney (Hama, 2010). In the brain, myelin contains abundant amounts of C24 GalCers and C24 sulfatides. The levels of 2-OH GalCers and 2-OH sulfatides increase during the course of myelination (Alderson et al., 2006).
2-Hydroxylation of FAs is catalyzed by the fatty acid 2-hydroxylase (FA2H) (Mizutani et al., 2008; Hama, 2010). Mutations in the FA2H gene on chromosome 16 are associated with leukodystrophy with spastic paraparesis and dystonia (Table 2) (Edvardson et al., 2008), indicating the importance of 2-OH GalCer/sulfatide for myelin formation and maintenance (Hama, 2010).
OTHER DISEASE-ASSOCIATED MUTATIONS IN THE VLCFA SYNTHESIS PATHWAY
A mutation in the HACD1 gene (PTPLA) on chromosome 10 was identified in congenital myopathy characterized by the severe hypotonicity and the absence of deep tendon reflexes (Table 2) (Muhammad et al., 2013). HACD1 is one of the four mammalian HACD isozymes (HACD1-4) and catalyzes the third step of the FA elongation cycle: dehydration of 3-hydroxyacyl-CoA to trans-2-enoyl-CoA (Fig. 2) (Ikeda et al., 2008). The expression of HACD1 gene is highly specific to the heart and skeletal muscle (Li et al., 2000). The mutant HACD1 protein, which is C-terminally truncated by the introduction of nonsense codon at the Tyr residue 248 (Tyr248Stop), exhibits no activity toward 3-hydoroxy C16:0-CoA in vitro (Muhammad et al., 2013). A mutation of the dog HACD1 gene caused by the insertion of a short interspersed element (SINE) in exon 2 also causes myopathy (Pelé et al., 2005). SINE insertion leads to multiple splicing defects and severely reduces the amount of wild type transcripts.
A mutation in the TER (TECR) gene on chromosome 19 encoding trans-2-enoyl-CoA reductase has been identified in an autosomal recessive form of non-syndromic mental retardation (Table 2) (Çalişkan et al., 2011). TER catalyzes the fourth step and last of the FA elongation cycle: NADPH-dependent reduction of trans-2-enoyl-CoA to acyl-CoA (Fig. 2) (Moon and Horton, 2003). The mutation substitutes the Pro residue at 182 to Leu (P182L) in the TER protein (Çalişkan et al., 2011). TER is an ER resident membrane protein with six predicted membrane-spanning domains, and the Pro-182 residue is likely to be located in the second luminal loop. The P182L mutation reduces both the activity and stability of the TER protein, thereby impairing the FA elongation cycle (Abe et al., 2013). Lipid analysis of B-lymphoblastoid cell lines derived from patients revealed a change in the sphingolipid profile and decreased levels of C24 Cers and C24 SMs (Abe et al., 2013).
MUTATIONS IN THE VLCFA DEGRADATION PATHWAY
Impairment in VLCFA degradation pathway leads to several diseases (Table 2). VLCFAs are transported, as VLCFA-CoAs, into peroxisomes, where they are subjected to β-oxidization into long-chain or shorter acyl-CoAs, followed by transport to the mitochondria and further cycles of β-oxidization (Wanders, 2014). Peroxisomal membrane protein ABCD1, a member of ABC transporters encoded by the ABCD1 gene on X chromosome, transports VLCFA-CoAs into peroxisomes (van Roermund et al., 2008; Morita and Imanaka, 2012). Mutations in the ABCD1 gene cause X-linked adrenoleukodystrophy (X-ALD), the most common peroxisomal disorder with more than 600 different mutations characterized by progressive demyelination and adrenal insufficiency (Table 2) (Mosser et al., 1993; Berger et al., 2010; Engelen et al., 2012). A defect in ABCD1 impairs the VLCFA degradation process, resulting in elevated levels of saturated C24–C26 VLCFAs in the plasma, brain, adrenal grand, and other tissues (Berger et al., 2010; Kemp et al., 2012). The accumulation of saturated VLCFAs is believed to play a crucial role in the pathogenesis of X-ALD such as demyelination with inflammation (Paintlia et al., 2003). As mentioned earlier, ELOVL1 is responsible for the synthesis of saturated and monounsaturated C24–C26 VLCFAs such as those accumulated in X-ALD. In fibroblasts derived from X-ALD patients, suppression of VLCFA synthesis by knockdown of ELOVL1 partially restores C26:0 levels, suggesting ELOVL1 is a potential target for the treatment of X-ALD (Ofman et al., 2010). Lorenzo’s oil, a 4:1 mixture of glyceryl trioleate and glyceryl trierucate, has been used to reduce the saturated VLCFA level in the plasma of X-ALD patients (Rizzo et al., 1989; Berger et al., 2010). Biochemical analysis demonstrated that oleic and erucic acids inhibit ELOVL1, suggesting that inhibition of ELOVL1 may be an underlying mechanism by which Lorenzo’s oil exerts its action (Sassa et al., 2014)
Peroxisomal β-oxidation of VLCFAs consists of four sequential reactions: dehydrogenation, hydration, dehydrogenation, and thiolytic cleavage (Wanders, 2014). The first dehydrogenation step, which converts acyl-CoA to trans-2-enoyl-CoA, is catalyzed by acyl-CoA oxidase 1 (ACOX1). Mutations in the ACOX1 gene on chromosome 17 result in autosomal recessive peroxisomal acyl-CoA deficiency (or pseudoneonatal adrenoleukodystrophy) with similar clinical features to those of X-ALD including the accumulation of VLCFAs and leukodystrophy (Table 2) (Poll-The et al., 1988). Both the second and third steps of VLCFA β-oxidation, hydration of trans-2-enoyl-CoA, and dehydrogenation of 3-hydroxyacyl-CoA, respectively, are catalyzed by single enzyme 17β-hydroxysteroid dehydrogenase 4 (HSD17β4; D-bifunctional protein). Homozygous mutations in the HSD17β4 gene on chromosome 5 have been identified in the disorder called D-bifunctional protein deficiency with clinical features similar to those of peroxisomal acyl-CoA deficiency and X-ALD (Table 2) (Watkins et al., 1995). The fourth step, which converts 3-ketoacyl-CoA to acyl-CoA, is catalyzed by peroxisomal 3-oxoacyl-CoA thiolase encoded by the acetyl-CoA acyltransferase 1 (ACAA1) gene on chromosome 3 (Wanders, 2014). No mutations in the ACAA1 gene associated with disorders have been identified.
CONCLUSIONS
VLCFAs are FAs with a chain-length of C>20. VLCFAs with C≥26 are sub-classified as ULCFAs and found in limited tissues including the skin, retina, brain, testis, and meibomian gland. VLCFAs are variously present in sphingolipids, glycerophospholipids, and other forms of lipids including ω-O-acyl-ULCFAs. VLCFAs are synthesized by the FA elongation cycle in the ER. Identification of the enzymes that participate in FA elongation, modification, transport, and degradation enabled the study of specific VLCFA species. VLCFAs play multiple roles not substituted by LCFAs. KO mice for VLCFA-related genes exhibit various phenotypes, and mutations in VLCFA-related genes cause inherited disorders including ichthyosis, macular dystrophy, myopathy, mental retardation, and demyelination. VLCFAs may regulate cellular functions by affecting membrane properties including membrane fluidity, permeability, curvature, and lipid microdomain formation. Nevertheless, our present knowledge of VLCFAs is still the tip of the iceberg. Further identification of novel VLCFA species, VLCFA-related metabolic pathways, genes, and disorders will reveal the diverse and unique functions of VLCFAs, and may lead to intervention for lipid-associated diseases.
REFERENCES
- Abe K, Ohno Y, Sassa T, Taguchi R, Çalişkan M, Ober C, Kihara A. Mutation for nonsyndromic mental retardation in the trans-2-enoyl-CoA reductase TER gene involved in fatty acid elongation impairs the enzyme activity and stability, leading to change in sphingolipid profile. J Biol Chem. 2013;288:36741–36749. doi: 10.1074/jbc.M113.493221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbaga MP, Mandal MN, Anderson RE. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J Lipid Res. 2010;51:1624–1642. doi: 10.1194/jlr.R005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M. The roles of ABCA12 in epidermal lipid barrier formation and keratinocyte differentiation. Biochim Biophys Acta. 2014;1841:435–440. doi: 10.1016/j.bbalip.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Akiyama M, Sugiyama-Nakagiri Y, Sakai K, McMillan JR, Goto M, Arita K, Tsuji-Abe Y, Tabata N, Matsuoka K, Sasaki R, Sawamura D, Shimizu H. Mutations in lipid transporter ABCA12 in harlequin ichthyosis and functional recovery by corrective gene transfer. J Clin Invest. 2005;115:1777–1784. doi: 10.1172/JCI24834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldahmesh MA, Mohamed JY, Alkuraya HS, Verma IC, Puri RD, Alaiya AA, Rizzo WB, Alkuraya FS. Recessive mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. Am J Hum Genet. 2011;89:745–750. doi: 10.1016/j.ajhg.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson NL, Maldonado EN, Kern MJ, Bhat NR, Hama H. FA2H-dependent fatty acid 2-hydroxylation in postnatal mouse brain. J Lipid Res. 2006;47:2772–2780. doi: 10.1194/jlr.M600362-JLR200. [DOI] [PubMed] [Google Scholar]
- Aveldaño MI, Sprecher H. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J Biol Chem. 1987;262:1180–1186. [PubMed] [Google Scholar]
- Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker I, Wang-Eckhardt L, Yaghootfam A, Gieselmann V, Eckhardt M. Differential expression of (dihydro)ceramide synthases in mouse brain: oligodendrocyte-specific expression of CerS2/Lass2. Histochem Cell Biol. 2008;129:233–241. doi: 10.1007/s00418-007-0344-0. [DOI] [PubMed] [Google Scholar]
- Berger J, Pujol A, Aubourg P, Forss-Petter S. Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20:845–856. doi: 10.1111/j.1750-3639.2010.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiden B, Sandhoff K. The role of sphingolipid metabolism in cutaneous permeability barrier formation. Biochim Biophys Acta. 2014;1841:441–452. doi: 10.1016/j.bbalip.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Butovich IA. Fatty acid composition of cholesteryl esters of human meibomian gland secretions. Steroids. 2010;75:726–733. doi: 10.1016/j.steroids.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA. Tear film lipids. Exp Eye Res. 2013;117:4–27. doi: 10.1016/j.exer.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–2485. doi: 10.1194/jlr.M900252-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çalişkan M, Chong JX, Uricchio L, Anderson R, Chen P, Sougnez C, Garimella K, Gabriel SB, dePristo MA, Shakir K, Matern D, Das S, Waggoner D, Nicolae DL, Ober C. Exome sequencing reveals a novel mutation for autosomal recessive non-syndromic mental retardation in the TECR gene on chromosome 19p13. Hum Mol Genet. 2011;20:1285–1289. doi: 10.1093/hmg/ddq569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso LA, Edwards AO, Frost A, Vrabec T, Stone EM, Hageman GS, Perski T. Autosomal dominant Stargardt-like macular dystrophy. Surv Ophthalmol. 2001;46:149–163. doi: 10.1016/s0039-6257(01)00251-x. [DOI] [PubMed] [Google Scholar]
- Eckl KM, Tidhar R, Thiele H, Oji V, Hausser I, Brodesser S, Preil ML, Önal-Akan A, Stock F, Müller D, Becker K, Casper R, Nürnberg G, Altmüller J, Nürnberg P, Traupe H, Futerman AH, Hennies HC. Impaired epidermal ceramide synthesis causes autosomal recessive congenital ichthyosis and reveals the importance of ceramide acyl chain length. J Invest Dermatol. 2013;133:2202–2211. doi: 10.1038/jid.2013.153. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Hama H, Shaag A, Gomori JM, Berger I, Soffer D, Korman SH, Taustein I, Saada A, Elpeleg O. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am J Hum Genet. 2008;83:643–648. doi: 10.1016/j.ajhg.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, Ekroos K, Klemm RW, Simons K, Shevchenko A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc Natl Acad Sci USA. 2009;106:2136–2141. doi: 10.1073/pnas.0811700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Williams ML, Feingold KR. Abnormal barrier function in the pathogenesis of ichthyosis: therapeutic implications for lipid metabolic disorders. Clin Dermatol. 2012;30:311–322. doi: 10.1016/j.clindermatol.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen M, Tran L, Ofman R, Brennecke J, Moser AB, Dijkstra IM, Wanders RJ, Poll-The BT, Kemp S. Bezafibrate for X-linked adrenoleukodystrophy. PLoS One. 2012;7:e41013. doi: 10.1371/journal.pone.0041013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furland NE, Oresti GM, Antollini SS, Venturino A, Maldonado EN, Aveldaño MI. Very long-chain polyunsaturated fatty acids are the major acyl groups of sphingomyelins and ceramides in the head of mammalian spermatozoa. J Biol Chem. 2007a;282:18151–18161. doi: 10.1074/jbc.M700709200. [DOI] [PubMed] [Google Scholar]
- Furland NE, Zanetti SR, Oresti GM, Maldonado EN, Aveldaño MI. Ceramides and sphingomyelins with high proportions of very long-chain polyunsaturated fatty acids in mammalian germ cells. J Biol Chem. 2007b;282:18141–18150. doi: 10.1074/jbc.M700708200. [DOI] [PubMed] [Google Scholar]
- Ginkel C, Hartmann D, vom Dorp K, Zlomuzica A, Farwanah H, Eckhardt M, Sandhoff R, Degen J, Rabionet M, Dere E, Dörmann P, Sandhoff K, Willecke K. Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes. J Biol Chem. 2012;287:41888–41902. doi: 10.1074/jbc.M112.413500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010;49:186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Hama H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim Biophys Acta. 2010;1801:405–414. doi: 10.1016/j.bbalip.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkewicz R, Du H, Tong Z, Alkuraya H, Bedell M, Sun W, Wang X, Hsu YH, Esteve-Rudd J, Hughes G, Su Z, Zhang M, Lopes VS, Molday RS, Williams DS, Dennis EA, Zhang K. Essential role of ELOVL4 protein in very long chain fatty acid synthesis and retinal function. J Biol Chem. 2012;287:11469–11480. doi: 10.1074/jbc.M111.256073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Kanao Y, Yamanaka M, Sakuraba H, Mizutani Y, Igarashi Y, Kihara A. Characterization of four mammalian 3-hydroxyacyl-CoA dehydratases involved in very long-chain fatty acid synthesis. FEBS Lett. 2008;582:2435–2440. doi: 10.1016/j.febslet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Imgrund S, Hartmann D, Farwanah H, Eckhardt M, Sandhoff R, Degen J, Gieselmann V, Sandhoff K, Willecke K. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J Biol Chem. 2009;284:33549–33560. doi: 10.1074/jbc.M109.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi K, Prinetti A, Sonnino S, Mauri L, Kobayashi T, Ishii K, Kaga N, Murayama K, Kurihara H, Nakayama H, Yoshizaki F, Takamori K, Ogawa H, Nagaoka I. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj J. 2008;25:357–374. doi: 10.1007/s10719-007-9084-6. [DOI] [PubMed] [Google Scholar]
- Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Jennemann R, Rabionet M, Gorgas K, Epstein S, Dalpke A, Rothermel U, Bayerle A, van der Hoeven F, Imgrund S, Kirsch J, Nickel W, Willecke K, Riezman H, Gröne HJ, Sandhoff R. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum Mol Genet. 2012;21:586–608. doi: 10.1093/hmg/ddr494. [DOI] [PubMed] [Google Scholar]
- Karan G, Yang Z, Howes K, Zhao Y, Chen Y, Cameron DJ, Lin Y, Pearson E, Zhang K. Loss of ER retention and sequestration of the wild-type ELOVL4 by Stargardt disease dominant negative mutants. Mol Vis. 2005;11:657–664. [PubMed] [Google Scholar]
- Kasahara K, Sanai Y. Functional roles of glycosphingolipids in signal transduction via lipid rafts. Glycoconj J. 2000;17:153–162. doi: 10.1023/a:1026576804247. [DOI] [PubMed] [Google Scholar]
- Kemp S, Berger J, Aubourg P. X-linked adrenoleukodystrophy: Clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta. 2012;1822:1465–1474. doi: 10.1016/j.bbadis.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27:1142–1147. doi: 10.1097/ICO.0b013e3181814cff. [DOI] [PubMed] [Google Scholar]
- Li D, Gonzalez O, Bachinski LL, Roberts R. Human protein tyrosine phosphatase-like gene: expression profile, genomic structure, and mutation analysis in families with ARVD. Gene. 2000;256:237–243. doi: 10.1016/s0378-1119(00)00347-4. [DOI] [PubMed] [Google Scholar]
- Li W, Sandhoff R, Kono M, Zerfas P, Hoffmann V, Ding BC, Proia RL, Deng CX. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int J Biol Sci. 2007;3:120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DS, Connor WE, Wolf DP, Neuringer M, Hachey DL. Unique lipids of primate spermatozoa: desmosterol and docosahexaenoic acid. J Lipid Res. 1993;34:491–499. [PubMed] [Google Scholar]
- Masukawa Y, Narita H, Shimizu E, Kondo N, Sugai Y, Oba T, Homma R, Ishikawa J, Takagi Y, Kitahara T, Takema Y, Kita K. Characterization of overall ceramide species in human stratum corneum. J Lipid Res. 2008;49:1466–1476. doi: 10.1194/jlr.M800014-JLR200. [DOI] [PubMed] [Google Scholar]
- Matsuzaka T, Shimano H, Yahagi N, Kato T, Atsumi A, Yamamoto T, Inoue N, Ishikawa M, Okada S, Ishigaki N, Iwasaki H, Iwasaki Y, Karasawa T, Kumadaki S, Matsui T, Sekiya M, Ohashi K, Hasty AH, Nakagawa Y, Takahashi A, Suzuki H, Yatoh S, Sone H, Toyoshima H, Osuga J, Yamada N. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Chiba H, Tojo H, Igarashi Y. 2-Hydroxy-ceramide synthesis by ceramide synthase family: enzymatic basis for the preference of FA chain length. J Lipid Res. 2008;49:2356–2364. doi: 10.1194/jlr.M800158-JLR200. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Mitsutake S, Tsuji K, Kihara A, Igarashi Y. Ceramide biosynthesis in keratinocyte and its role in skin function. Biochimie. 2009;91:784–790. doi: 10.1016/j.biochi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Mizutani Y, Sun H, Ohno Y, Sassa T, Wakashima T, Obara M, Yuyama K, Kihara A, Igarashi Y. Cooperative synthesis of ultra long-chain fatty acid and ceramide during keratinocyte differentiation. PLoS One. 2013;8:e67317. doi: 10.1371/journal.pone.0067317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YA, Hammer RE, Horton JD. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. J Lipid Res. 2009;50:412–423. doi: 10.1194/jlr.M800383-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon YA, Horton JD. Identification of two mammalian reductases involved in the two-carbon fatty acyl elongation cascade. J Biol Chem. 2003;278:7335–7343. doi: 10.1074/jbc.M211684200. [DOI] [PubMed] [Google Scholar]
- Moon YA, Shah NA, Mohapatra S, Warrington JA, Horton JD. Identification of a mammalian long chain fatty acyl elongase regulated by sterol regulatory element-binding proteins. J Biol Chem. 2001;276:45358–45366. doi: 10.1074/jbc.M108413200. [DOI] [PubMed] [Google Scholar]
- Morita M, Imanaka T. Peroxisomal ABC transporters: structure, function and role in disease. Biochim Biophys Acta. 2012;1822:1387–1396. doi: 10.1016/j.bbadis.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Morrow MR, Singh D, Lu D, Grant CW. Glycosphingolipid fatty acid arrangement in phospholipid bilayers: cholesterol effects. Biophys J. 1995;68:179–186. doi: 10.1016/S0006-3495(95)80173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- Muhammad E, Reish O, Ohno Y, Scheetz T, DeLuca A, Searby C, Regev M, Benyamini L, Fellig Y, Kihara A, Sheffield VC, Parvari R. Congenital myopathy is caused by mutation of. HACD1 Hum Mol Genet. 2013;22:5229–5236. doi: 10.1093/hmg/ddt380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, de Rivero Vaccari JC, Gordon WC, Jackson FE, Bazan NG. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced apoptosis with concomitant neuroprotectin D1 synthesis. Proc Natl Acad Sci USA. 2007;104:13158–13163. doi: 10.1073/pnas.0705963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma T, Sato Y, Sassa T, Ohno Y, Kihara A. Biochemical characterization of the very long-chain fatty acid elongase ELOVL7. FEBS Lett. 2011;585:3337–3341. doi: 10.1016/j.febslet.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Obara K, Kojima R, Kihara A. Effects on vesicular transport pathways at the late endosome in cells with limited very long-chain fatty acids. J Lipid Res. 2013;54:831–842. doi: 10.1194/jlr.M034678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofman R, Dijkstra IM, van Roermund CW, Burger N, Turkenburg M, van Cruchten A, van Engen CE, Wanders RJ, Kemp S. The role of ELOVL1 in very long-chain fatty acid homeostasis and X-linked adrenoleukodystrophy. EMBO Mol Med. 2010;2:90–97. doi: 10.1002/emmm.201000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Dogru M, Tsubota K. Laboratory findings in tear fluid analysis. Clin Chim Acta. 2006;369:17–28. doi: 10.1016/j.cca.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Suto S, Yamanaka M, Mizutani Y, Mitsutake S, Igarashi Y, Sassa T, Kihara A. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci USA. 2010;107:18439–18444. doi: 10.1073/pnas.1005572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oji V, Tadini G, Akiyama M, Blanchet Bardon C, Bodemer C, Bourrat E, Coudiere P, DiGiovanna JJ, Elias P, Fischer J, Fleckman P, Gina M, Harper J, Hashimoto T, Hausser I, Hennies HC, Hohl D, Hovnanian A, Ishida-Yamamoto A, Jacyk WK, Leachman S, Leigh I, Mazereeuw-Hautier J, Milstone L, Morice-Picard F, Paller AS, Richard G, Schmuth M, Shimizu H, Sprecher E, Van Steensel M, Taïeb A, Toro JR, Vabres P, Vahlquist A, Williams M, Traupe H. Revised nomenclature and classification of inherited ichthyoses: results of the first ichthyosis consensus conference in sorèze 2009. J Am Acad Dermatol. 2010;63:607–641. doi: 10.1016/j.jaad.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Okuda A, Naganuma T, Ohno Y, Abe K, Yamagata M, Igarashi Y, Kihara A. Hetero-oligomeric interactions of an ELOVL4 mutant protein: implications in the molecular mechanism of Stargardt-3 macular dystrophy. Mol Vis. 2010;16:2438–2445. [PMC free article] [PubMed] [Google Scholar]
- Paintlia AS, Gilg AG, Khan M, Singh AK, Barbosa E, Singh I. Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X-ALD: implications for potential therapies. Neurobiol Dis. 2003;14:425–439. doi: 10.1016/j.nbd.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Pelé M, Tiret L, Kessler JL, Blot S, Panthier JJ. SINE exonic insertion in the PTPLA gene leads to multiple splicing defects and segregates with the autosomal recessive centronuclear myopathy in dogs. Hum Mol Genet. 2005;14:1417–1427. doi: 10.1093/hmg/ddi151. [DOI] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, Horn-Saban S, Amann-Zalcenstein D, Raanan C, Berkutzki T, Erez-Roman R, Ben-David O, Levy M, Holzman D, Park H, Nyska A, Merrill AH, Jr, Futerman AH. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J Biol Chem. 2010a;285:10911–10923. doi: 10.1074/jbc.M109.077610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, Erez-Roman R, Brügger B, Sachsenheimer T, Wieland F, Prieto M, Merrill AH, Jr, Futerman AH. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J Biol Chem. 2010b;285:10902–10910. doi: 10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poll-The BT, Roels F, Ogier H, Scotto J, Vamecq J, Schutgens RB, Wanders RJ, van Roermund CW, van Wijland MJ, Schram AW, Tagar JM, Saudubray JM. A new peroxisomal disorder with enlarged peroxisomes and a specific deficiency of acyl-CoA oxidase (pseudo-neonatal adrenoleukodystrophy) Am J Hum Genet. 1988;42:422–434. [PMC free article] [PubMed] [Google Scholar]
- Poulos A, Sharp P, Johnson D, Easton C. The occurrence of polyenoic very long chain fatty acids with greater than 32 carbon atoms in molecular species of phosphatidylcholine in normal and peroxisome-deficient (Zellweger’s syndrome) brain. Biochem J. 1988;253:645–650. doi: 10.1042/bj2530645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Prottey C. Investigation of functions of essential fatty acids in the skin. Br J Dermatol. 1977;97:29–38. doi: 10.1111/j.1365-2133.1977.tb15424.x. [DOI] [PubMed] [Google Scholar]
- Rabionet M, Gorgas K, Sandhoff R. Ceramide synthesis in the epidermis. Biochim Biophys Acta. 2014;1841:422–434. doi: 10.1016/j.bbalip.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Rabionet M, van der Spoel AC, Chuang CC, von Tümpling-Radosta B, Litjens M, Bouwmeester D, Hellbusch CC, Körner C, Wiegandt H, Gorgas K, Platt FM, Gröne HJ, Sandhoff R. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: a link to ceramide synthase-3. J Biol Chem. 2008;283:13357–13369. doi: 10.1074/jbc.M800870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radner FP, Marrakchi S, Kirchmeier P, Kim GJ, Ribierre F, Kamoun B, Abid L, Leipoldt M, Turki H, Schempp W, Heilig R, Lathrop M, Fischer J. Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans. PLoS Genet. 2013;9:e1003536. doi: 10.1371/journal.pgen.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo WB, Leshner RT, Odone A, Dammann AL, Craft DA, Jensen ME, Jennings SS, Davis S, Jaitly R, Sgro JA. Dietary erucic acid therapy for X-linked adrenoleukodystrophy. Neurology. 1989;39:1415–1422. doi: 10.1212/wnl.39.11.1415. [DOI] [PubMed] [Google Scholar]
- Robinson BS, Johnson DW, Poulos A. Unique molecular species of phosphatidylcholine containing very-long-chain (C24–C38) polyenoic fatty acids in rat brain. Biochem J. 1990;265:763–767. doi: 10.1042/bj2650763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhoff R, Geyer R, Jennemann R, Paret C, Kiss E, Yamashita T, Gorgas K, Sijmonsma TP, Iwamori M, Finaz C, Proia RL, Wiegandt H, Gröne HJ. Novel class of glycosphingolipids involved in male fertility. J Biol Chem. 2005;280:27310–27318. doi: 10.1074/jbc.M502775200. [DOI] [PubMed] [Google Scholar]
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Sassa T, Ohno Y, Suzuki S, Nomura T, Nishioka C, Kashiwagi T, Hirayama T, Akiyama M, Taguchi R, Shimizu H, Itohara S, Kihara A. Impaired epidermal permeability barrier in mice lacking Elovl1, the gene responsible for very-long-chain fatty acid production. Mol Cell Biol. 2013;33:2787–2796. doi: 10.1128/MCB.00192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa T, Suto S, Okayasu Y, Kihara A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim Biophys Acta. 2012;1821:1031–1037. doi: 10.1016/j.bbalip.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Sassa T, Wakashima T, Ohno Y, Kihara A. Lorenzo’s oil inhibits ELOVL1 and lowers the level of sphingomyelin with a saturated very long-chain fatty acid. J Lipid Res. 2014;55:524–530. doi: 10.1194/jlr.M044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry PS. Lipids of nervous tissue: composition and metabolism. Prog Lipid Res. 1985;24:69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- Silva LC, Ben David O, Pewzner-Jung Y, Laviad EL, Stiban J, Bandyopadhyay S, Merrill AH, Jr, Prieto M, Futerman AH. Ablation of ceramide synthase 2 strongly affects biophysical properties of membranes. J Lipid Res. 2012;53:430–436. doi: 10.1194/jlr.M022715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Prinetti A, Nakayama H, Yangida M, Ogawa H, Iwabuchi K. Role of very long fatty acid-containing glycosphingolipids in membrane organization and cell signaling: the model of lactosylceramide in neutrophils. Glycoconj J. 2009;26:615–621. doi: 10.1007/s10719-008-9215-8. [DOI] [PubMed] [Google Scholar]
- t’Kindt R, Jorge L, Dumont E, Couturon P, David F, Sandra P, Sandra K. Profiling and characterizing skin ceramides using reversed-phase liquid chromatography-quadrupole time-of-flight mass spectrometry. Anal Chem. 2012;84:403–411. doi: 10.1021/ac202646v. [DOI] [PubMed] [Google Scholar]
- Tidhar R, Futerman AH. The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochim Biophys Acta. 2013;1833:2511–2518. doi: 10.1016/j.bbamcr.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001;36:885–895. doi: 10.1007/s11745-001-0798-1. [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Visser WF, Ijlst L, van Cruchten A, Boek M, Kulik W, Waterham HR, Wanders RJ. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- Vasireddy V, Jablonski MM, Mandal MN, Raz-Prag D, Wang XF, Nizol L, Iannaccone A, Musch DC, Bush RA, Salem N, Jr, Sieving PA, Ayyagari R. Elovl4 5-bp-deletion knock-in mice develop progressive photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2006;47:4558–4568. doi: 10.1167/iovs.06-0353. [DOI] [PubMed] [Google Scholar]
- Vasireddy V, Uchida Y, Salem N, Jr, Kim SY, Mandal MN, Reddy GB, Bodepudi R, Alderson NL, Brown JC, Hama H, Dlugosz A, Elias PM, Holleran WM, Ayyagari R. Loss of functional ELOVL4 depletes very long-chain fatty acids (≥C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasireddy V, Wong P, Ayyagari R. Genetics and molecular pathology of Stargardt-like macular degeneration. Prog Retin Eye Res. 2010;29:191–207. doi: 10.1016/j.preteyeres.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanders RJ. Metabolic functions of peroxisomes in health and disease. Biochimie. 2014;98:36–44. doi: 10.1016/j.biochi.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Watkins PA, McGuinness MC, Raymond GV, Hicks BA, Sisk JM, Moser AB, Moser HW. Distinction between peroxisomal bifunctional enzyme and acyl-CoA oxidase deficiencies. Ann Neurol. 1995;38:472–477. doi: 10.1002/ana.410380322. [DOI] [PubMed] [Google Scholar]
- Westerberg R, Tvrdik P, Undén AB, Månsson JE, Norlén L, Jakobsson A, Holleran WH, Elias PM, Asadi A, Flodby P, Toftgård R, Capecchi MR, Jacobsson A. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J Biol Chem. 2004;279:5621–5629. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
- Yamanaka WK, Clemans GW, Hutchinson ML. Essential fatty acids deficiency in humans. Prog Lipid Res. 1980;19:187–215. doi: 10.1016/0163-7827(80)90004-1. [DOI] [PubMed] [Google Scholar]
- Zadravec D, Tvrdik P, Guillou H, Haslam R, Kobayashi T, Napier JA, Capecchi MR, Jacobsson A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J Lipid Res. 2011;52:245–255. doi: 10.1194/jlr.M011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kniazeva M, Han M, Li W, Yu Z, Yang Z, Li Y, Metzker ML, Allikmets R, Zack DJ, Kakuk LE, Lagali PS, Wong PW, MacDonald IM, Sieving PA, Figueroa DJ, Austin CP, Gould RJ, Ayyagari R, Petrukhin K. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat Genet. 2001;27:89–93. doi: 10.1038/83817. [DOI] [PubMed] [Google Scholar]