Abstract
In the present study, we investigated anti-cancer effect of snake venom activated NK cells (NK-92MI) in lung cancer cell lines. We used snake venom (4 μg/ml) treated NK-92MI cells to co-culture with lung cancer cells. There was a further decrease in cancer cell growth up to 65% and 70% in A549 and NCI-H460 cell lines respectively, whereas 30–40% was decreased in cancer cell growth by snake venom or NK-92MI alone treatment. We further found that the expression of various apoptotic proteins such as that Bax, and cleaved caspase-3 as well as the expression of various death receptor proteins like DR3, DR4 and Fas was also further increased. Moreover, consistent with cancer cell growth inhibition, the DNA binding activity of NF-κB was also further inhibited after treatment of snake venom activated NK-92MI cells. Thus, the present data showed that activated NK cells could further inhibit lung cancer cell growth.
Keywords: NK-92MI cells, Lung cancer, Snake venom, Death receptor, NF-κB
INTRODUCTION
Lung cancer does not show any symptoms in the initial stages, so it is the most difficult type of cancer to diagnose before it has spread to others organs (Latz et al., 2009; Magne et al., 2012). Thus, lung cancer leads to death more often than any other cancers (Mohammed et al., 2011). Although several anticancer agents have been used to treat lung cancer, chemo-resistance is a major obstacle hindering the successful treatment of lung cancer patients (Triller et al., 2006). Multimodal therapy approaches, such as combining chemotherapy agents with cellular immunotherapy may be effective to prevent toxic effects of chemotherapeutics. Hence we evaluated an anticancer strategy that focuses on overcoming such a barrier by combining snake venom and natural killer cells.
Snake venom is useful biological resource, containing several pharmacologically active components that could be of potential therapeutic value (Son et al., 2007). Particularly snake venom toxin from Vipera lebetina turanica was previously demonstrated as a possible chemotherapeutic against for growth of human prostate cancer cell and neuroblastoma cell through induction of apoptosis via modulating the expression of apoptosis regulatory proteins and ROS dependent mechanisms (Son et al., 2007; Park et al., 2009). Previously, we found that snake venom increases DRs expression in colon cancer cells (Park et al., 2012a). Thus, it is possible that the snake venom could enhance DRs expression in lung cancer cells, however, the combination effect of snake venom with immune cells like NK-92MI against NSCLC cell lines is not yet reported.
Natural killer (NK) cells are effecter lymphocytes of the innate immune system that control several types of tumors by limiting their spread and subsequent tissue damages (Cerwenka and Lanier, 2001; Cooper et al., 2001). NK cells play an important role in adoptive cellular immunotherapy against certain human cancers such as renal-cell carcinoma and malignant melanoma (Hercend et al., 1990; Burke et al., 2010), and are involved in the eradication of experimentally induced and spontaneously developing tumors in mice (Markovic and Murasko, 1991). The use of NK cells in human cancer immunotherapy has been proposed and such has recently entered clinical trials (Smyth et al., 2002). NK based cellular immunotherapy can be implemented by using any of the following approaches: administrating cytokines or immunomodulatory drugs to activate endogenous NK cells, or by increasing the susceptibility of cancer cells to NK cell mediated cytotoxicity. The susceptibility to natural killer cell-mediated lysis of colon cancer cells is enhanced by treatment with epidermal growth factor receptor inhibitors (Bae et al., 2012). Quercetin enhances susceptibility to NK cell-mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70 in K562, SNU1, and SNU-C4 cells (Bae et al., 2010). NK-92MI cells are highly cytotoxic established natural killer cell line. NK-92MI cells were used in immunotherapy of malignant melanoma in a SCID mouse model (Tam et al., 1999). A synergetic effect on tumor regression by genetic engineered IL18-IL2 fusion protein and natural killer cell cytotoxicity in enhancing IFN-γ production in mice models (Du et al., 2012). Though there are studies to increase cytotoxicity if NK cells by increasing the susceptibility of cancer cells, there is no study of using chemotherapeutics to activate NK cells and enhance the cytotoxicity to give an additive effect. Hence we used the combination of snake venom an established chemotherapeutic agent to activate NK-92MI cells against A549 and NCIH460.
Apoptosis is the best characterized form of programmed cell death and is an intracellular suicide program possessing morphologic characteristics and biochemical features. Programmed cell death (PCD) has an important role in anti-cancer effects of chemotherapeutics (O’Donovan et al., 2011). Apoptosis can be induced by stimulation of death receptors (DRs) (Kang et al., 2011; Zhang et al., 2011). DRs belong to tumor necrosis factor (TNF) family cell surface receptors, which are activated by TNF family ligands like TNF alpha, Apo3L, TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL) (O’Donovan et al., 2011). NK cells can release several tumor necrosis factor (TNF) family ligands such as TNF, TNF-related apoptosis-inducing ligand (TRAIL), Apo3L and FAS ligand (FASL) to induce cancer cell apoptosis (Smyth et al., 2002; Park et al., 2004; Park et al., 2011; Bae et al., 2012; Jo et al., 2012) by activation of death receptors and subsequent downstream caspases, including caspases-9 and -3, as well as Bax (Elrod and Sun, 2008; Sun, 2011).
The nuclear factor kappa B (NF-κB) enhances proliferation, invasiveness, metastasis and inflammation in various human cancer cells (Nakshatri et al., 1997; Karin and Lin, 2002; Baud and Karin, 2009). NF-κB is involved in proliferative and anti-apoptotic activities that could contribute to the resistance of tumor cells to various therapeutic approaches. In lung cancer cells, the inactivation of NF-κB leads to enhanced therapeutic effect (Karin and Greten, 2005). In our previous study, we reported the anti-cancer effect of snake venom in ovarian cancer cells by inactivation of NF-κB (Song et al., 2012). Recent studies on the signaling mechanisms of the DR have revealed that members of the NF-κB and caspase families are key regulators of cell death (Sancho-Martinez and Martin-Villalba, 2009).
In the present study we tried to enhance the anti-cancer effect of both snake venom and NK-92MI cells by using both in a combination. The activation of NK-92MI cell lines with snake venom showed an additive effect in cell viability and also increased expression of apoptotic proteins (Bax, and cleaved caspase -3) and death receptor proteins Fas in A549 and DR3 in NCI-H460 cell lines. The combination of snake venom and NK-92MI cells also lead to increased inactivation of NF-κB compared to only snake venom or only NK-92MI cells. Thus in present study we show that a co-culture with snake venom treated NK-92MI cells have higher anti-cancer activity on lung cancer cells mediated by death receptors induced apoptotic pathway and inactivation of NF-κB.
MATERIALS AND METHODS
Chemicals
Snake Venom from Vipera lebetina turanica was purchased from Sigma (St. Louis, MO, USA).
Cell culture
NCI-H460 and A549 human lung cancer cells and NK-92MI human natural killer cells were obtained from the American Type Culture Collection (Cryosite, Lane Cove, NSW, Australia). NCI-H460 and A549 human lung cancer cells were grown in RPMI1640 and DMEM respectively, with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2 humidified air. NK-92MI cells were grown in MEM supplemented with 12.5% FBS, 12.5% horse serum, 100 U/ml penicillin, and 100 μg/ml streptomycin, 1% L-glu and 0.1% of 55 mM 2-mercaptoethanol at 37°C in 5% CO2 humidified air. The A549 and NCI-H460 lung cancer cells are grown as adherent cultures in monolayer, however NK-92MI cells were grown as suspension cultures forming aggregates of cells as a cluster.
Treatment and co-culture
The A549 and NCI-H460 cells are plated 24 well or 6 well plates (2.5×104 cells per well). The NK-92MI (5×104 cells per well) cells were cultured in semi-permeable inserts (trans-well, costar) suitable to 24 well or 6 well plates. After 24 h, the NK-92MI cells were pre-treated with 4 μg/ml snake venom for 1 h and transferred to 24 well or 6 well plates containing cancer cells (Park et al., 2012b), and cultured for different time period.
Cell viability assay
At different time points, the cultured cells were trypsinized with TrypLE Express (Invitrogen, Carlsbad, CA, USA) and then the cells were pelleted by centrifugation for 5 min at 1,500 rpm. Cells were resuspended in PBS and 0.2% trypan blue was added to the cancer cell suspension. Subsequently, a drop of suspension was placed into a Neubauer chamber and the living cancer cells were counted. Cells that showed signs of staining were considered to be dead, whereas those that excluded trypan blue were considered viable. Each assay was carried out in triplicate.
Reverse transcription (RT)-PCR
Total RNAs were isolated from cultured cells using RNeasy plus Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s manual. The RNA pellet obtained in the final step was dissolved in 30 μl of sterile diethylpyro-carbonate (DEPC)-treated water, and its concentration was determined using a UV spectrophotometer at 260 nm. RNA was kept in DEPC-treated water at −70°C until use. Reverse transcription was performed using High Capacity RNA-to-cDNA Kit (AP, Foster, CA, USA). PCR amplifications were then carried out with the primers. The PCR primers used were 5′-ACAAGCCTGTAGCCCATGTT-3′ and 5′-AAAGATGACCTGCCCAGACT-3′ for TNF alpha, 5′-ACCAATGCCACAAAGGAAC-3′ and 5′-CTG CAATTGAAGCACTGGAA-3′ for the human TNF receptor 1, 5′-CTCAGGAGCATG GG G-ATAAA-3′ and 5′-AGCCAGCCAGTCTGACATCT-3′ for the human TNF receptor-2, 5′-ATGGCGATGGCTGCGTGTCCTG-3′ and 5′-AGCGCCTCCTGGGTCTCGGGGTAG-3′ for the human DR3, 5′-ACTTTGGTTGTTCCGTTGCTG TTG-3′ and 5′-GGCTTTCCATTTGCTGCTCA-3′ for the human DR 4, and 5′-CAAAGCCCATTTTTCTTCCA-3′ and 5′-GACAAAGCCACCCCAAGTTA-3′ for human FAS, 5′-TCG CAGAAGTGC A CCTAA AG-3′ and 5′-AGCCTTCCCCTCATCAAAGT-3′ for human ApoL, 5′-CAGCTCTTCCACCTACAG AAG G-3′ and 5′-AAGATTGAACACTGCCCCCAGG-3′ for FasL, 5′-AGACCTGCGTGCTGATCGTG-3′ and 5′-TTATTTTGCGGCCCAGAGCC-3′ for human TRAIL, 5′-GAAGGTGAAGGTCGGAGT-3′ and 5′-CTTCTACCACTACCCTAAAG-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively. The reaction mixture containing 10 μl premix, 1 μl of each forward and reverse primers and 1 μl of cDNA are made upto 20 μl. The reverse transcription PCR is carried out in three steps. Step1: 95°C-3 min, Step2: 95°C-30 sec, 55–65°C (ambient temp)-30 sec and 70°C-1 min and Step3: 72°C-10 min. The Step 2 is repeated for 30 cycles and lid temp-105°C. The samples are loaded into horizontal gel electrophoresis and detected under UV light using MyImage software.
Western blot analysis
Harvested cells were homogenized with lysis buffer [50 mM Tris pH 8.0, 150 mM NaCl, 0.02% sodium azide, 0.2% SDS, 1 mM PMFS, 10 μl/ml aprotinin, 1% igapel 630 (Sigma Aldrich), 10 mM NaF, 0.5 mM EDTA, 0.1 mM EGTA, and 0.5% sodium deoxycholate] and centrifuged at 23,000×g for 15 min. The protein concentration was measured by the Bradford method (Bio-Rad Protein Assay; Bio-Rad Laboratories Inc., Hercules, CA, USA), and equal amount of proteins (50 μg) were separated on a SDS/10%-polyacrylamide gel and then transferred to a Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). Blots were blocked for 2 h at room temperature with 5% (w/v) non-fat dried milk in Tris buffered saline [10mM Tris (pH 8.0) and 150 mM NaCl] solution containing 0.05% tween-20. The membrane was incubated for 5 h at room temperature with specific antibodies: mouse polyclonal antibodies against Bax, IκB, p- IκB, p65, histone-H1, TNF-R1 and Fas (1:500 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA); rabbit polyclonal for p50, TNFR-2, DR3 and DR6 (1:500 dilution, Santa Cruz Biotechnology Inc.); and for caspase-3, cleaved caspase3, cleaved caspase-9, inhibitor of apoptosis protein (cIAP) 1 and 2 (1:1,000 dilution; Cell Signaling Technology, Inc., Beverly, MA, USA). The blot was then incubated with the corresponding conjugated anti-rabbit and anti-mouse immunoglobulin G-horseradish peroxidase (1:4,000 dilution, Santa Cruz Biotechnology Inc.). Immunoreactive proteins were detected with the ECL Western blotting detection system. The relative density of the protein bands was scanned by densitometry using My Image (SLB) and quantified by Lab works 4.0 software (UVP Inc., Upland, CA, USA).
Gel electro mobility shift assay
Gel shift assay was performed according to the manufacturer’s recommendations (Promega, Madison, WI, USA). Briefly, the sample of 1×106 cells/ml was washed twice with 1×PBS, followed by the addition of 1 ml of PBS, and the cells were scraped into a cold Eppendorf tube. Cells were pelleted by centrifugation at 151×g for 5 min, and the resulting supernatant was removed. Solution A (50 mM HEPES, pH 7.4, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 0.1 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 10 μg/ml soybean trypsin inhibitor, 10 μg/ml aprotinin, and 0.5% Nonidet P-40) was added to the pellet and allowed to incubate on ice for 10 min and centrifuged at 3220×g for 6 min and cytoplasmic extract was separated. Solution C (solution A+10% glycerol and 400 mM KCl) was added to the pellet and vortexed on ice for 20 min. The cells were centrifuged at 13,000×g for 12 min, and the resulting nuclear extract supernatant was collected in a chilled Eppendorf tube. Consensus oligonucleotides were end-labeled using T4 polynucleotide kinase and [γ-P32] ATP for 10 min at 37°C. Gel shift reactions were assembled and allowed to incubate at room temperature for 10 min followed by the addition of 1 μl (50,000–200,000 cpm) of labeled oligonucleotide and another 20 min of incubation at room temperature. Subsequently 1 μl of gel loading buffer was added to each reaction and loaded onto a 4% non-denaturing gel and electrophoresis was performed until the dye was three-fourths of the way down the gel. The gel was dried at 80°C for 50 min and exposed to film overnight at −70°C. The relative density of the DNA-protein binding bands was scanned by densitometry using My Image (SLB, Seoul, Korea), and quantified by Lab works 4.0 software (UVP Inc).
RESULTS
Effect of snake venom on the cells viability of lung cancer cells (A549 and NCI-H460) and natural killer cells (NK-92MI)
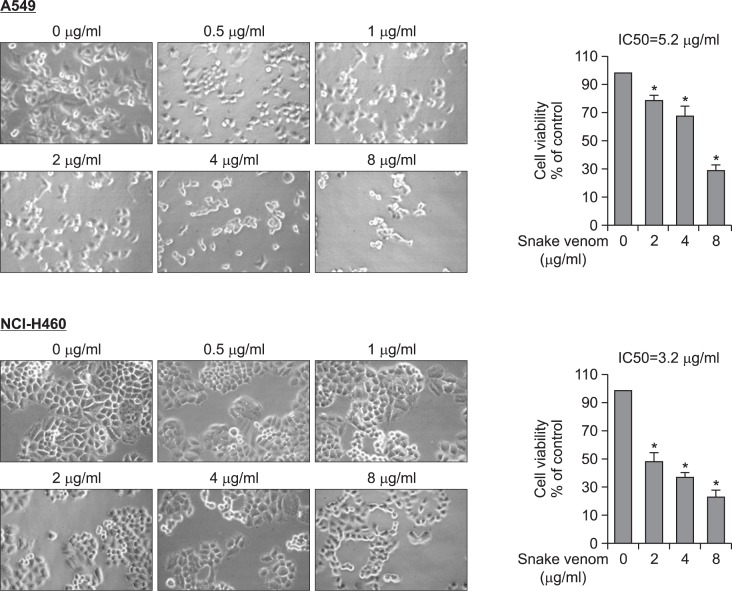
Initially to examine the effect of snake venom on A549 and NCI-H460 human lung cancer cell growth, we analyzed cell growth pattern of the cells after treatment with various concentrations. The IC50 value is found to be 5.2 μg/ml and 3.2 μg/ml respectively in A549 and NCI-H460 cells (Fig. 1). However, the treatment of snake venom (4 μg/ml) does not show any significant effect in NK-92MI cell viability (Supplementary Data, A) as well as expression of granzyme B (Supplementary Data, B) and NO generation which are representative indicators of activation NK cells. Moreover, the occulted cancer cells with activated NK-93MI cell did not further increased NO generation (Supplementary Data, C). These data indicated that granzyme and NO generation did not contribute to anti-cancer effect of snake venom activated NK cells.
Fig. 1.
Effect of snake venom on the cells viability of lung cancer cells (A549 and NCI-H460). Concentration-dependent effect of snake venom on cell viability of A549 and NCI-H460 at 48 h, the morphological changes were observed and numbers of viable cells were counted under a microscope (magnification, ×200). Values are mean ± SD of three experiments with replicates. Values are mean ± SD of three experiments with replicates. *p≤0.05 indicates statistically significant differences from the control group.
Effect of snake venom activated NK-92MI cells on the cells viability of lung cancer cells (A549 and NCI-H460)
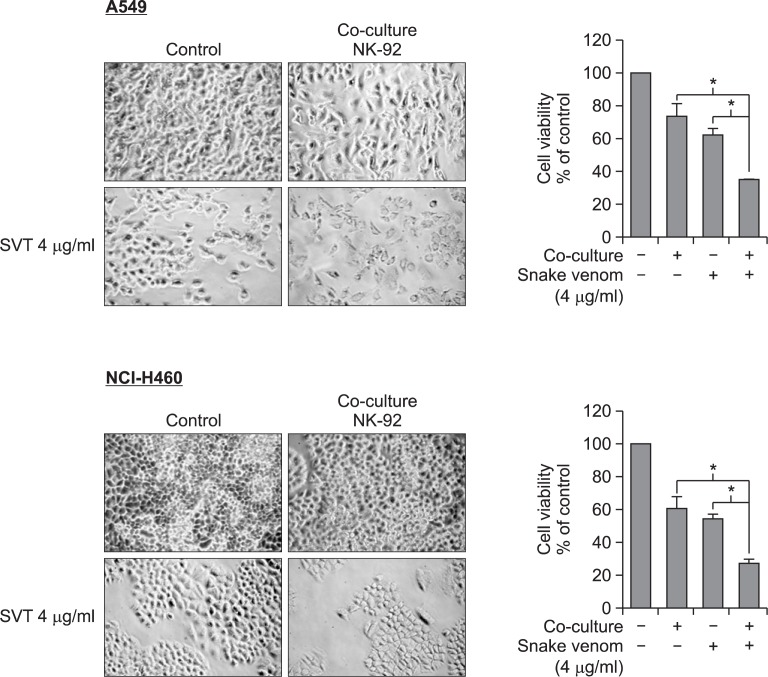
We co-cultured snake venom (4 μg/ml) activated NK-92MI cells with NSCLC cells at a ratio of 1:2 (NSCLC cells: NK-92MI). Morphological features of the A549 and NCI-H460 cells show a reduction in cell size and rounding up of cells in the presence of snake venom (4 μg/ml) activated NK-92MI cells when compared to either snake venom (4 μg/ml) or NK-92MI cells alone. In addition, there is a significant decrease in cell viability (65% and 70% respectively) of A549 and NCI-H460 cells in the presence of snake venom (4 μg/ml) activated NK-92MI cells (Fig. 2).
Fig. 2.
Effect of snake venom activated NK-92MI cells on the cells viability of lung cancer cells (A549 and NCI-H460). The cells were co-culture with NK-92MI cells treated with snake venom (4 μg/ml) and the morphological changes were observed and numbers of viable cells were counted under a microscope (magnification, ×200). Values are mean ± SD of three experiments with replicates. *p≤0.05 indicates statistically significant differences from the control group.
Effect of snake venom activated NK-92MI on the expression of granules and TNF family cytokines in NK-92MI
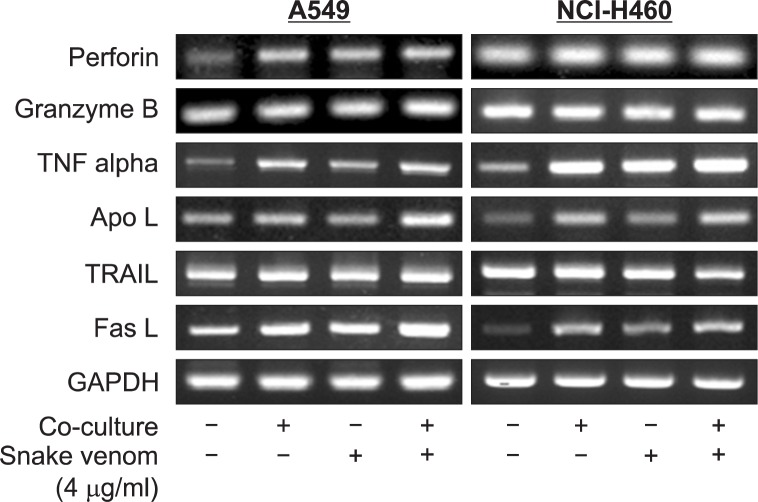
NK-92MI cells can activate TNF family receptors on cancer cells by the release of various ligands. Hence we performed RT-PCR to find the expression levels of various ligands including TNF alpha and death receptors ligands. The results showed a significant increase in the expression of TNF alpha, Apo3L and FasL (Fig. 3). NK-92MI cells can also release granzyme B and perforin to induce apoptosis in cancer cells. Hence we also performed RT-PCR for the granzyme B and perforin. There is an increase in perforin expression in A549, but not NCI-H460 cells (Fig. 3).
Fig. 3.
Effect of snake venom activated NK-92MI cells on the expression of granules and TNF family ligands in NK-92MI. The total RNA were extracted and examined for expressions of Granzyme B, Perforin, TNF alpha, ApoL, TRAIL, FasL and GAPDH by RT-PCR. GA PDH was used as an internal control to show equal RNA loading
Effect of snake venom activated NK-92MI cells on the expression of TNF family receptors in lung cancer cells (A549 and NCI-H460)
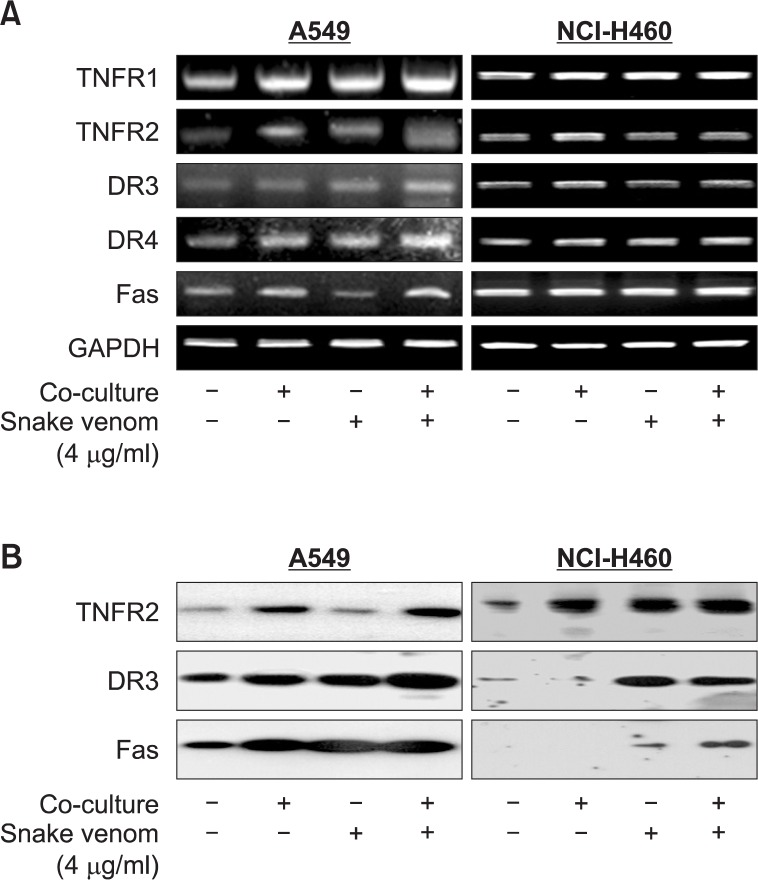
NK-92MI cells can stimulate expression of many death receptors in cancer cells. Hence we performed RT-PCR and western blotting to find the expression levels of various receptors including TNF receptors and death receptors. The results showed a significant increase in the expression of TNFR1, TNFR2, DR3, DR4 and Fas expressions in A549 cell lines and TNFR1, TNFR2, and DR3 in NCI-H460 cells (Fig. 4A). To further investigate the expression of TNF family receptors, we also analyzed the expression levels of various TNF family receptors proteins. The results showed a significant increase in the expression of TNFR2, DR3 and Fas in both lung cancer cells by co-cultured with snake venom activated NK-92MI (Fig. 4B).
Fig. 4.
Effect of snake venom activated NK-92MI cells on the expression of TNF family receptors in lung cancer cells (A549 and NCI-H460). (A) The total RNA were extracted and examined for expressions of TNF-R1, TNF-R2, FAS, DR-3, and GAPDH by RTPCR. GAPDH was used as an internal control to show equal RNA loading. (B) Equal amounts of total proteins (50 μg/lane) were subjected to 10% SDSPAGE. Expressions of TNFR2, DR3, and Fas were detected by Western blotting using specific antibodies. Each band is representative for three experiments.
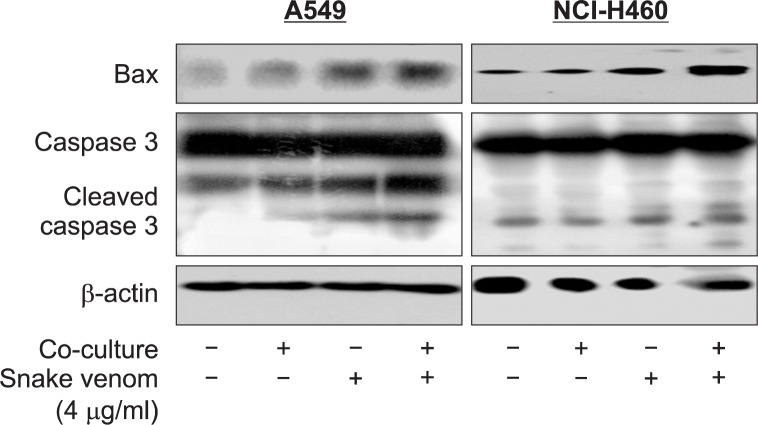
Effect of snake venom activated NK-92MI cells on the expression of apoptosis regulatory protein in lung cancer cells (A549 and NCI-H460)
To further investigate the apoptotic cell death, we also analyzed the expression levels of various apoptotic signaling proteins. The results showed an increase in apoptotic regulatory protein like cleaved caspase-3 and BAX in both lung cancer cells by co-cultured with snake venom activated NK-92MI (Fig. 5).
Fig. 5.
Effect of snake venom activated NK-92MI cells on the expression of apoptosis protein in lung cancer cells (A549 and NCI-H460). Equal amounts of total proteins (50 μg/lane) were subjected to 10% SDSPAGE. Expressions of Bax, caspase3 and cleaved caspase3 were detected by Western blotting using specific antibodies. Each band is representative for three experiments.
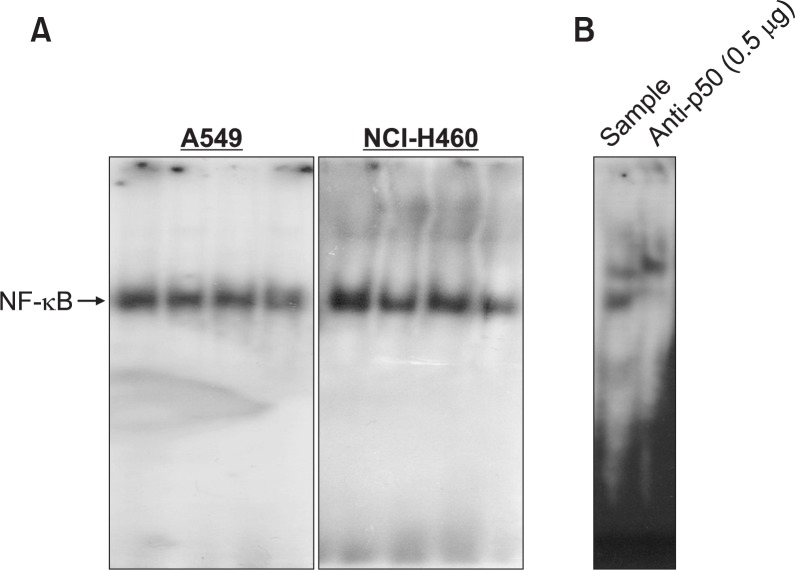
Effect of snake venom activated NK-92MI on the activation of NF-κB in lung cancer cells (A549 and NCI-H460)
Decrease in the activation of NF-κB has been involved in expression of apoptotic cell death of many cancer cells. Hence we examined the DNA binding activity of NF-κB with EMSA. The results showed that there is a significant inactivation of NF-κB in the lung cancer cells by co-cultured with snake venom activated NK-92MI (Fig. 6A). This band was confirmed as a NF-κB by supershift assay with anti-p50 antibody (Fig. 6B).
Fig. 6.
Effect of snake venom activated NK-92MI cells on the activation of NF-κB in lung cancer cells (A549 and NCI-H460). Nuclear extract from A549 and NCI-H460 lung cancer cells co-administered with snake venom and NK-92MI cells for 1h was incubated in binding reactions of 32P-end-labeled oligonucleotide containing the κB sequence. The activation of NF-κB was investigated using EMSA as described in Materials and Methods (A). The NF-κB binding activity was confirmed with supershift assay with anti p50 antibody (0.5 μg) (B).
DISCUSSION
Lung cancer remains the most common cancer diagnosed worldwide and has one of the lowest survival rates of all cancers. Surgery remains the only curative treatment option but because most patients are either diagnosed at advanced stages or are unfit for surgery, less than a third of all lung cancer patients will undergo a surgical resection (Jahangeer et al., 2013). Natural killer (NK) cells play critical roles in host immunity against cancer. In response, cancers develop mechanisms to escape NK cell attack or induce defective NK cells. Currently NK cell-based cancer immunotherapy can aim to overcome NK cell paralysis by the use of chemotherapeutic to activate NK cells. However a multimodal therapy approach, such as combining chemotherapy agents with cellular immunotherapy, suffers from potential drug-mediated toxicity to immune-effector cells. Overcoming such toxic effects of anticancer cellular products is a potential critical barrier to the development of combined therapeutic approaches. In our present study we evaluated the effect of chemotherapeutic drug snake venom on immunocompetent cells’ (NK-92MI) activation. These immune effector cells efficiently reduced the viability of non-small cell lung cancer cell lines (A549 and NCI-H460), which are also sensitive to snake venom. These data show there is a benefit to using drug-resistant cellular therapy when combined with cytotoxic chemotherapy approaches. NK cells are known to release several cytokines such as granulocyte-macrophage colony-stimulating factor and interferon-γ, which can induce a tumor-killing effect (Yasumura et al., 1994; Glas et al., 2000; Brady et al., 2010). NK cells also kill cancer cells by the release of cytoplasmic granules that contain a number of proteins, such as perforin and granzymes, which lyses target cells.
Besides these cytokines, there are several tumor necrosis factor (TNF) family ligands such as FAS ligand (FASL), TNF and TNF-related apoptosis-inducing ligand (TRAIL), which are also released by NK cells and have been shown to induce cancer cell apoptosis (Cerwenka and Lanier, 2001; Cooper et al., 2001; Smyth et al., 2001; Smyth et al., 2002; Screpanti et al., 2005). Previously our studies reported that anti-cancer effect of snake venom in colon (Park et al., 2012a) and ovarian cancer (Song et al., 2012). Several studies also reported the anti-cancer effect of snake venom in multiple myeloma (Al-Sadoon et al., 2013), breast cancer and prostate cancer (Badr et al., 2013). Snake venom induces apoptosis of colon cancer cells through DR4 and DR5 (Park et al., 2012a) and inhibits cell growth through inactivation of NF-κB and STAT3 (Song et al., 2012). This ability of both NK-92MI cells and snake venom to activate death receptor ligands and death receptors respectively is critical when co-administered to lung cancer cells. In present study the snake venom activated NK-92MI cells lead to activation of ligands like Apo3L and FasL on NK-92MI cells. Concomitantly the receptors like DR3, and Fas are activated on lung cancer cells. DRs contain a cysteine-rech cytoplasmic domain, called “death domain”. DRs are activated by interaction of DRs with their ligands (interaction of DR1 with TNF; DR2 with FasL; DR3 with Apo3L; DR4 and DR5 with TRAIL) (Yoshida et al., 2010; Inoue et al., 2011). Activated DRs induce apoptosis through caspase activation (Ashkenazi, 2008). When DRs bind to their ligands, the death domains recruit the intracellular adaptor protein FADD (Fas-associated death domain protein) which results in the activation of caspase-8 that leads to the activation of downstream caspases, including caspases-3, as well as Bax (Elrod and Sun, 2008; Sun, 2011). Similarly, the snake venom activated NK-92MI cells on NSCLC cells lead to the activation of Bax, cleaved caspase-3 and cleaved caspase-8.
The members of the nuclear factor kappa B (NF-κB) family also play an important role in the development and progression of several human malignancies (Nakshatri et al., 1997; Baud and Karin, 2009). Previously our studies showed anti-cancer activity snake venom in ovarian cancer cells through activation of caspase-3 pathway via inactivation of NF-κB (Song et al., 2012). Snake venom also showed anti-arthritic effect by inhibition of inflammation mediator generation by suppression of NF-κB. In the present study, we found an enhanced inactivation ability of snake venom activated NK-92MI cells. Thus in the present study, the administration of snake venom in NK-92MI cells and consecutive co-culture of these NK-92MI cells with NSCLC cells (A549 and NCI-H460) lead to decrease in cell viability of lung cancer cells. This decreased cell viability is found to be due to activation of TNF-α, Apo3L and FasL in NK-92MI cells and TNFR2, DR3, and Fas in both A549 and NCI-H460. Furthermore, the result causes by decreasing the activation of NF-κB, and increasing apoptotic proteins in non-small cell lung cancer cells.
Acknowledgments
This work was supported by the National Research Foundation of Korea [NRF] grant funded by the Korea government (MSIP) (No. MRC,2008-0062275).
REFERENCES
- Al-Sadoon MK, Rabah DM, Badr G. Enhanced anti-cancer efficacy of snake venom combined with silica nanoparticles in a murine model of human multiple myeloma: molecular targets for cell cycle arrest and apoptosis induction. Cell Immunol. 2013;284:129–138. doi: 10.1016/j.cellimm.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19:325–331. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Badr G, Al-Sadoon MK, Rabah DM. Therapeutic efficacy and molecular mechanisms of snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles in the treatment of breast cancer- and prostate cancer-bearing experimental mouse models. Free Radic Biol Med. 2013;65:175–189. doi: 10.1016/j.freeradbiomed.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Bae JH, Kim JY, Kim MJ, Chang SH, Park YS, Son CH, Park SJ, Chung JS, Lee EY, Kim SH, Kang CD. Quercetin enhances susceptibility to NK cell-mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70. J Immunother. 2010;33:391–401. doi: 10.1097/CJI.0b013e3181d32f22. [DOI] [PubMed] [Google Scholar]
- Bae JH, Kim SJ, Kim MJ, Oh SO, Chung JS, Kim SH, Kang CD. Susceptibility to natural killer cell-mediated lysis of colon cancer cells is enhanced by treatment with epidermal growth factor receptor inhibitors through UL16-binding protein-1 induction. Cancer Sci. 2012;103:7–16. doi: 10.1111/j.1349-7006.2011.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J, Carotta S, Thong RP, Chan CJ, Hayakawa Y, Smyth MJ, Nutt SL. The interactions of multiple cytokines control NK cell maturation. J Immunol. 2010;185:6679–6688. doi: 10.4049/jimmunol.0903354. [DOI] [PubMed] [Google Scholar]
- Burke S, Lakshmikanth T, Colucci F, Carbone E. New views on natural killer cell-based immunotherapy for melanoma treatment. Trends Immunol. 2010;31:339–345. doi: 10.1016/j.it.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Du G, Ye L, Zhang G, Dong Q, Liu K, Tian J. Human IL18-IL2 fusion protein as a potential antitumor reagent by enhancing NK cell cytotoxicity and IFN-gamma production. J Cancer Res Clin Oncol. 2012;138:1727–1736. doi: 10.1007/s00432-012-1248-5. [DOI] [PubMed] [Google Scholar]
- Elrod HA, Sun SY. Modulation of death receptors by cancer therapeutic agents. Cancer Biol Ther. 2008;7:163–173. doi: 10.4161/cbt.7.2.5335. [DOI] [PubMed] [Google Scholar]
- Glas R, Franksson L, Une C, Eloranta ML, Ohlen C, Orn A, Karre K. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype. An adaptive component of NK cell-mediated responses. J Exp Med. 2000;191:129–138. doi: 10.1084/jem.191.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercend T, Farace F, Baume D, Charpentier F, Droz JP, Triebel F, Escudier B. Immunotherapy with lymphokine-activated natural killer cells and recombinant interleukin-2: a feasibility trial in metastatic renal cell carcinoma. J Biol Response Mod. 1990;9:546–555. [PubMed] [Google Scholar]
- Inoue N, Matsuda F, Goto Y, Manabe N. Role of cell-death ligand-receptor system of granulosa cells in selective follicular atresia in porcine ovary. J Reprod Dev. 2011;57:169–175. doi: 10.1262/jrd.10-198e. [DOI] [PubMed] [Google Scholar]
- Jahangeer S, Forde P, Soden D, Hinchion J. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat Rev. 2013;39:862–871. doi: 10.1016/j.ctrv.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Jo M, Park MH, Kollipara PS, An BJ, Song HS, Han SB, Kim JH, Song MJ, Hong JT. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258:72–81. doi: 10.1016/j.taap.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Kim IY, Kim EH, Yoon MJ, Kim SU, Kwon TK, Choi KS. Paxilline enhances TRAIL-mediated apoptosis of glioma cells via modulation of c-FLIP, survivin and DR5. Exp Mol Med. 2011;43:24–34. doi: 10.3858/emm.2011.43.1.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Latz C, Huang Q, Kapadia MK, Freitag SK. Metastasis to eyelid as initial presentation of non-small cell carcinoma. Ophthal Plast Reconstr Surg. 2009;25:406–408. doi: 10.1097/IOP.0b013e3181b3b3df. [DOI] [PubMed] [Google Scholar]
- Magne N, Bay JO, Blay JY, Goncalves A, Massard C, Thariat J, Wislez M, Andre T, Vignot S. [American Society of Clinical Oncology (ASCO) 2012 essential data: the editorial board of the Bulletin du Cancer point of view] Bull Cancer. 2012;99:1209–1217. doi: 10.1684/bdc.2012.1670. [DOI] [PubMed] [Google Scholar]
- Markovic SN, Murasko DM. Role of natural killer and T-cells in interferon induced inhibition of spontaneous metastases of the B16F10L murine melanoma. Cancer Res. 1991;51:1124–1128. [PubMed] [Google Scholar]
- Mohammed N, Kestin LL, Grills IS, Battu M, Fitch DL, Wong CY, Margolis JH, Chmielewski GW, Welsh RJ. Rapid disease progression with delay in treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:466–472. doi: 10.1016/j.ijrobp.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan TR, O’Sullivan GC, McKenna SL. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy. 2011;7:509–524. doi: 10.4161/auto.7.6.15066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Lee SH, Son DJ, Oh KW, Kim KH, Song HS, Kim GJ, Oh GT, Yoon DY, Hong JT. Antiarthritic effect of bee venom: inhibition of inflammation mediator generation by suppression of NF-kappaB through interaction with the p50 subunit. Arthritis Rheum. 2004;50:3504–3515. doi: 10.1002/art.20626. [DOI] [PubMed] [Google Scholar]
- Park MH, Choi MS, Kwak DH, Oh KW, Yoon do Y, Han SB, Song HS, Song MJ, Hong JT. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-kappaB. Prostate. 2011;71:801–812. doi: 10.1002/pros.21296. [DOI] [PubMed] [Google Scholar]
- Park MH, Jo M, Won D, Song HS, Han SB, Song MJ, Hong JT. Snake venom toxin from Vipera lebetina turanica induces apoptosis of colon cancer cells via upregulation of ROS- and JNK-mediated death receptor expression. BMC Cancer. 2012a;12:228. doi: 10.1186/1471-2407-12-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Son DJ, Kwak DH, Song HS, Oh KW, Yoo HS, Lee YM, Song MJ, Hong JT. Snake venom toxin inhibits cell growth through induction of apoptosis in neuroblastoma cells. Arch Pharm Res. 2009;32:1545–1554. doi: 10.1007/s12272-009-2106-0. [DOI] [PubMed] [Google Scholar]
- Park MH, Song MJ, Cho MC, Moon DC, Yoon do Y, Han SB, Hong JT. Interleukin-32 enhances cytotoxic effect of natural killer cells to cancer cells via activation of death receptor 3. Immunology. 2012b;135:63–72. doi: 10.1111/j.1365-2567.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Martinez I, Martin-Villalba A. Tyrosine phosphorylation and CD95: a FAScinating switch. Cell Cycle. 2009;8:838–842. doi: 10.4161/cc.8.6.7906. [DOI] [PubMed] [Google Scholar]
- Screpanti V, Wallin RP, Grandien A, Ljunggren HG. Impact of FASL-induced apoptosis in the elimination of tumor cells by NK cells. Mol Immunol. 2005;42:495–499. doi: 10.1016/j.molimm.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, Yagita H, Okumura K. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–670. doi: 10.1084/jem.193.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- Son DJ, Park MH, Chae SJ, Moon SO, Lee JW, Song HS, Moon DC, Kang SS, Kwon YE, Hong JT. Inhibitory effect of snake venom toxin from Vipera lebetina turanica on hormone-refractory human prostate cancer cell growth: induction of apoptosis through inactivation of nuclear factor kappaB. Mol Cancer Ther. 2007;6:675–683. doi: 10.1158/1535-7163.MCT-06-0328. [DOI] [PubMed] [Google Scholar]
- Song JK, Jo MR, Park MH, Song HS, An BJ, Song MJ, Han SB, Hong JT. Cell growth inhibition and induction of apoptosis by snake venom toxin in ovarian cancer cell via inactivation of nuclear factor kappaB and signal transducer and activator of transcription 3. Arch Pharm Res. 2012;35:867–876. doi: 10.1007/s12272-012-0512-1. [DOI] [PubMed] [Google Scholar]
- Sun SY. Understanding the role of the death receptor 5/FADD/caspase-8 death signaling in cancer metastasis. Mol Cell Pharmacol. 2011;3:31–34. [PMC free article] [PubMed] [Google Scholar]
- Tam YK, Miyagawa B, Ho VC, Klingemann HG. Immunotherapy of malignant melanoma in a SCID mouse model using the highly cytotoxic natural killer cell line NK-92. J Hematother. 1999;8:281–290. doi: 10.1089/106161299320316. [DOI] [PubMed] [Google Scholar]
- Triller N, Korosec P, Kern I, Kosnik M, Debeljak A. Multidrug resistance in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed disease. Lung Cancer. 2006;54:235–240. doi: 10.1016/j.lungcan.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Yasumura S, Amoscato A, Hirabayashi H, Lin WC, White-side TL. Proliferation of hematopoietic cell lines induced by a soluble factor derived from human squamous cell carcinomas of the head and neck. Cancer Immunol Immunother. 1994;39:407–415. doi: 10.1007/BF01534429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Horinaka M, Sakai T. “Combination-oriented molecular-targeting prevention” of cancer: a model involving the combination of TRAIL and a DR5 inducer. Environ Health Prev Med. 2010;15:203–210. doi: 10.1007/s12199-009-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HP, Takayama K, Su B, Jiao XD, Li R, Wang JJ. Effect of sunitinib combined with ionizing radiation on endothelial cells. J Radiat Res. 2011;52:1–8. doi: 10.1269/jrr.10013. [DOI] [PubMed] [Google Scholar]