Abstract
The stiffness of cancer cells is attributable to intermediate filaments such as keratin. Perinuclear reorganization via phosphorylation of specific serine residue in keratin is implicated in the deformability of metastatic cancer cells including the human pancreatic carcinoma cell line (PANC-1). 12-O-Tetradecanoylphorbol-13-acetate (TPA) is a potent tumor promoter and protein kinase C (PKC) activator. However, its effects on phosphorylation and reorganization of keratin 8 (K8) are not well known. Therefore, we examined the underlying mechanism and effect of TPA on K8 phosphorylation and reorganization. TPA induced phosphorylation and reorganization of K8 and transglutaminase-2 (Tgase-2) expression in a time- and dose-dependent manner in PANC-1 cells. These effects peaked after 45 min and 100 nM of TPA treatment. We next investigated, using cystamine (CTM), Tgase inhibitor, and Tgase-2 gene silencing, Tgase-2’s possible involvement in TPA-induced K8 phosphorylation and reorganization. We found that Tgase-2 gene silencing inhibited K8 phosphorylation and reorganization in PANC-1 cells. Tgase-2 gene silencing, we additionally discovered, suppressed TPA-induced migration of PANC-1 cells and Tgase-2 overexpression induced migration of PANC-1 cells. Overall, these results suggested that TPA induced K8 phosphorylation and reorganization via Tgase-2 expression in PANC-1 cells.
Keywords: 12-O-Tetradecanoylphorbol-13-acetate, Keratin 8 phosphorylation, Perinuclear reorganization, Transglutaminase-2, PANC-1 cells
INTRODUCTION
Cancer is a hyper-proliferative affliction that involves cellular transformation, dysregulation of apoptosis, increased proliferation as well as invasion, angiogenesis and metastasis (Ichikawa et al., 2007). Metastasis, not primary tumors, is accountable for more than 90% of all cancer deaths but understanding of the processes of invasion and metastasis, however, remains deficient (Steeg, 2006).
Invasive tumor cells mechanically soften and modify their adhesion to the extracellular matrix, which enhances their capacity to escape the primary tumor. Measurements of cancer cell softness, in fact, have uncovered a strong correlation between cell deformability and cell malignancy (Cross et al., 2007). The importance of cell elasticity or viscoelasticity in several metastatic cancer cell lines and other diseases has been reported (Beil et al., 2003). Sphingosylphosphorylcholine (SPC)-induced perinuclear reorganization of keratin 8 (K8) in human epithelial pancreatic cancer (PANC-1) cells and the resulting changes in their mechanical deformability, for example, have been examined as possible pathways facilitating pancreatic tumor cells’ migration and enhancing their metastatic competence (Park et al., 2011; Busch et al., 2012). K8 is the major component of the intermediate filaments of simple epithelia found in the intestine, liver and exocrine pancreas. A major in vivo phosphorylation site in human K8 is Serine (Ser) 431. K8 phosphorylation at Ser431 leads to keratin reorganization and improved tumor cell migration.
Transglutaminase-2 (Tgase-2) is a multifunctional protein with both intracellular and extracellular functions. In addition to catalyzing Ca2+-dependent transamidation reactions, it can bind and hydrolyze GTP/GDP with a similar affinity and catalytic rate as the α subunit of large heterotrimeric G proteins and small Ras-type G proteins (Park et al., 2011). The selective expression of Tgase-2 in chemoresistant and metastatic cancer cells, such as pancreatic, lung and ovarian tumors, as well as its ability to promote cell survival and invasion make it a promising therapeutic target (Park et al., 2012). In previous report, we demonstrated that Tgase-2 is involved in SPC-induced K8 phosphorylation and reorganization (Park et al., 2011).
12-O-Tetradecanoylphorbol-13-acetate (TPA), also commonly known as phorbol-12-myristate-13-acetate (PMA), is a diester of phorbol and a potent tumor promoter often employed in skin cancer models (Nakajima et al., 2013). Via the PKC/AP-1 pathway, TPA induces cancer cell invasion (Xu et al., 2013). TPA-induced PKC uses K8 as its substrate (Blumberg, 1988; Cadrin et al., 1992). However, the underlying mechanism and detailed effects of TPA on K8 phosphorylation and reorganization are unclear.
In this study, we found that TPA induces Ser431 phosphorylation and K8 reorganization as well as transglutaminase-2 (Tgase-2) expression. We also demonstrated that Tgase-2 is involved in TPA-induced K8 phosphorylation and reorganization as well as PANC-1 cell migration.
MATERIALS AND METHODS
Material
TPA was purchased from Cell Signaling Technology (Beverly, MA, USA). The phosphospecific antibody for detection of K8 Ser431, K8 Ser23, and K8 Ser73 was purchased from Abcam (Cambridge, UK). The anti-Tgase-2 and anti-β-actin antibodies were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA), as were peroxidase-labeled secondary antibodies. Alexa Fluor 488 goat anti-rabbit antibody and Alexa Fluor 594 goat anti-mouse antibody were obtained from Molecular Probes, Inc. (Eugene, OR, USA). All of these chemicals were freshly prepared for each experiment.
Cell culture
The human pancreatic carcinoma cell line PANC-1 was acquired from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with penicillin-streptomycin (10,000 IU/ml and 10,000 μg/ml, respectively) and maintained in medium containing 10% (v/v) fetal bovine serum (FBS). They were then incubated at 37°C in a humidified 10%-CO2 atmosphere. Finally, 18 hours before the respective experiments, the cells were washed twice in serum-free DMEM and incubated in serum-free DMEM.
Western blot
The cells were harvested and lysed in 50 mM Tris-Cl (pH7.5), 150 mM NaCl, 1% triton X-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, sterile solution, and protease inhibitors (Gendepot, Barker, TX, USA). The protein concentrations of the supernatants were determined using Coomassie Plus (Pierce Biotechnology Inc., Rockford, IL, USA), as recommended by the manufacturer. The protein lysates were loaded onto a 10% SDS-polyacrylamide gel, and the proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Pall, Pensacola, FL, USA). The membranes were blocked in 3% non-fat milk and probed with the appropriate primary antibodies including anti-K8 Ser431 (1:1000, Abcam), anti-K8 Ser23 (1:1000, Abcam), anti-K8 Ser73 (1:1000, Abcam), anti-Tgase-2 (1:1000, Santa Cruz Biotechnology, Inc.), and anti-β-actin (1:1000, Santa Cruz Biotechnology, Inc.). The membranes were then incubated for 60 min with HRP-labeled anti-rabbit or anti-mouse IgG secondary antibody (1:5000, Santa Cruz Biotechnology, Inc.) at room temperature. The protein-antibody complexes were detected by HRP-conjugated secondary antibodies followed by enhanced chemiluminescence Western blotting detection reagents (Amersham, Arlington Heights, IL, USA).
Confocal microscopy
PANC-1 cells were grown on coverslips and fixed 24 hours later with cold methanol for 30 min at room temperature. These fixed cells were permeabilized with a 10 min wash in 0.1% Triton X-100 at room temperature followed by several washes in PBS with 3% bovine serum albumin (PBS/BSA). The phosphospecific antibody-detecting K8 Ser431 (Abcam) primary antibody and anti-Tgase-2 were incubated with coverslips overnight at 4°C. Excess antibody was removed by four washes in PBS/BSA. Species-specific second antibodies conjugated to goat anti-rabbit IgG antibody (Alexa Fluor 488, 1:500 Molecular Probes) or goat anti-mouse IgG antibodies (Alexa Fluor 594, 1:500, Molecular Probes) were reacted with the coverslips for 1 hour at room temperature and then washed four times in PBS/BSA. The final samples were mounted onto slides and visualized under Zeiss Axiophot confocal microscopy.
Transfection with small-interference RNA (siRNA) or plasmid-containing Tgase-2
Tgase-2 siRNA was purchased from Invitrogen. Preparatory to siRNA or Tgase-2 transfection experiments, the cells were plated in six wells until attaining 70% confluence, and were then transfected with siRNA (100 pmol) using the LipofectamineTM2000 reagent according to the manufacturer's instructions. Forty-eight (48) hours after transfection, the PANC-1 cells were grown in a complete culture medium. Subsequently, they were starved for 15 hours and treated with specific chemicals. Finally, proteins were extracted from the cells and analyzed by Western blot.
Cell migration assays
Migration assays were performed using a multi-well chamber (Neuroprobe Inc., Gaithersburg, MD, USA) coated with 10 μg/ml fibronectin as a chemoattractant (Park et al., 2011). Briefly, PANC-1 cells were suspended in DMEM at a 1×105 cells/ml concentration, and a 25 μl aliquot of this suspension was treated with the indicated concentrations of TPA for 45 mins. The PANC-1 cells plated in the upper chamber were allowed to migrate for 5 hours to establish the temporal kinetics of migration. The transwell membranes were then fixed and stained using the Diff-Quik® staining kit (Kobe, Japan). Membranes were removed from the transwells, and cells on the undersurface of the millipore membrane were counted under light microscopy (average of 5 semi-random non-overlapping fields under 200× magnification). All of the treatments were performed in triplicate wells.
Statistical analyses
The student’s t-test was used to determine the statistical significance of the value differences between experimental and control groups. The data expressed as the means ± standard deviation (SD) of at least three independent experiments each performed in triplicate. p-values of 0.05 or less were considered statistically significant.
RESULTS
Effect of TPA on Tgase-2 expression and K8 phosphorylation in PANC-1 cells
The effect of TPA on K8 phosphorylation was examined using specific monoclonal antibodies for K8 Ser23, Ser73, and Ser431 in PANC-1, a pancreatic cancer cell line well-suited for use in keratin phosphorylation and reorganization studies (Park et al., 2012; Byun et al., 2013). TPA dose-dependently induced K8 phosphorylation and Tgase-2 expression in the PANC-1 cells (Fig. 1A). We found that K8 Ser431 phosphorylation was especially remarkable in TPA-treated PANC-1 cells (Fig. 1A). Thus, subsequent studies on PANC-1 cells with the specific antibody to K8 Ser431 were conducted. Maximum K8 Ser431 phosphorylation and Tgase-2 expression were achieved at 100 nM TPA. Increased induction of K8 phosphorylation and Tgase-2 expression were first detected after 15 min, attaining the maximum after 45 min incubation with TPA (Fig. 1B).
Fig. 1.
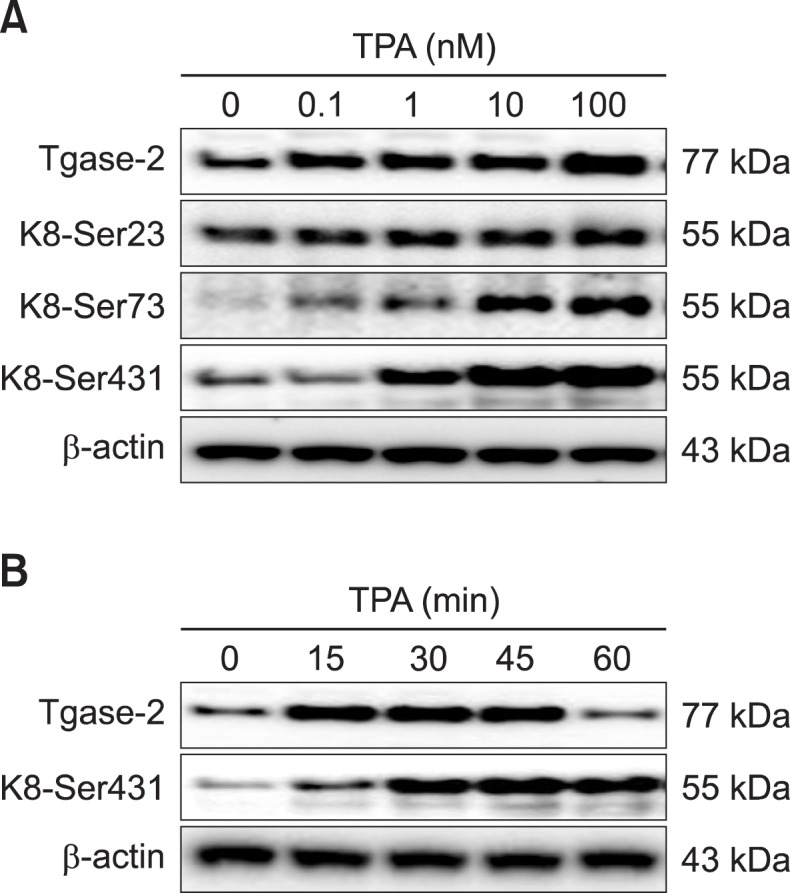
TPA induces phosphorylation of K8 and expression of Tgase-2 in PANC-1 cells. (A) Expression levels of Tgase-2 in PANC-1 cells stimulated with indicated concentrations of TPA for 45 min. (B) Time-dependent expression of Tgase-2. PANC-1 cells were treated with 100 nM TPA for the durations indicated.
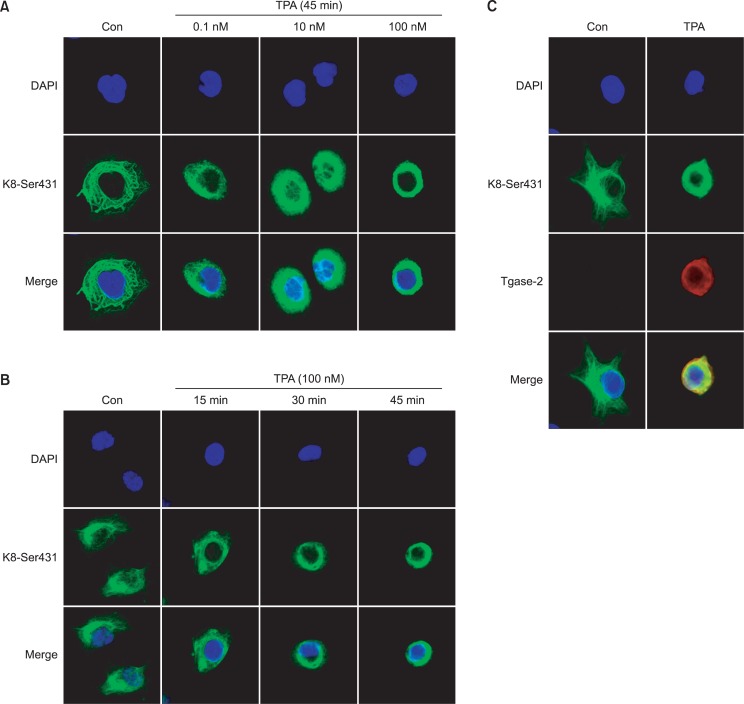
Effect of TPA on perinuclear reorganization of K8 in PANC-1 cells
PANC-1 cells exhibited the typical pan-cytoplasmic pattern of keratin filaments (Fig. 2A). Specifically, several doses of TPA induced perinuclear reorganization of keratin filaments (Fig. 2A). This effect was rapid, attaining the maximum after 45 min (Fig. 2B). TPA induced Tgase-2 expression patterns that, under confocal microscopy, were consistent with the ring formation of K8 perinuclear reorganization (Fig. 2C). These results were all strongly indicative of Tgase-2’s possible involvement in TPA-induced K8 reorganization.
Fig. 2.
TPA induces perinuclear reorganization of K8 and increases expression of Tgase-2 in PANC-1 cells. (A) Confocal microscopic examination of phosphorylation of K8 Ser431. PANC-1 cells were treated with the indicated concentrations of TPA for 45 min. (B) Confocal microscopic examination of time-dependent phosphorylation of K8 Ser431. PANC-1 cells were treated with 100 nM TPA for the durations indicated. (C) Confocal microscopic examination of Tgase-2 and K8 Ser431 phosphorylation. PANC-1 cells were treated with TPA (100 nM) for 45 min. Immunostaining was performed using K8 Ser431 (green), Tgase-2 (red), and DAPI (blue).
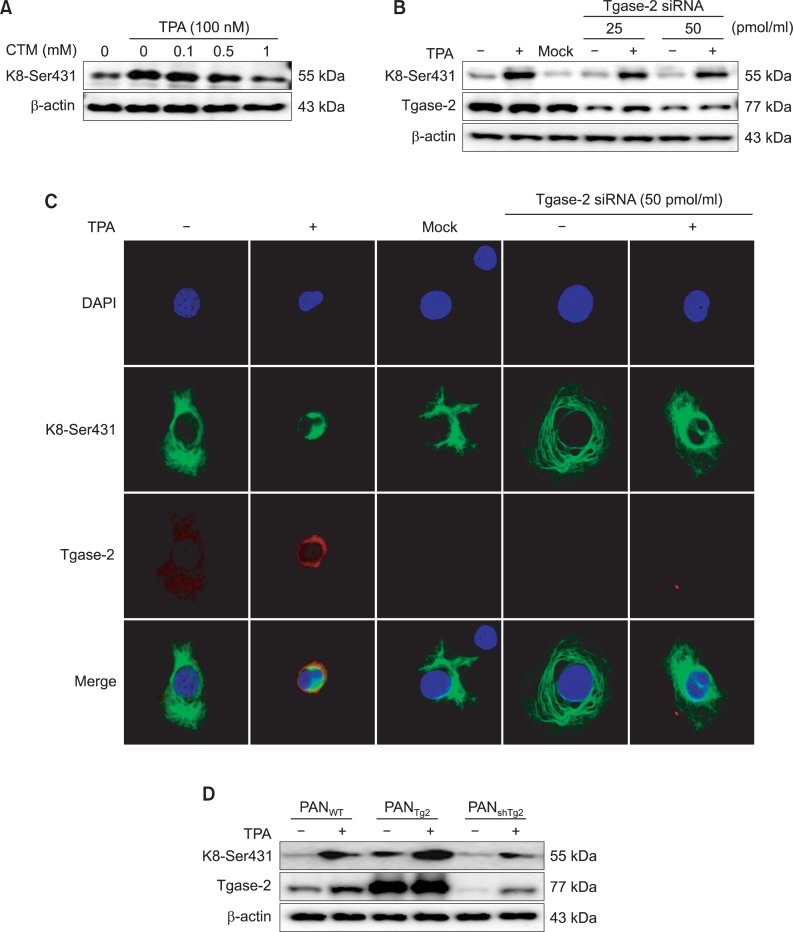
Involvement of Tgase-2 expression in TPA-induced K8 phosphorylation and reorganization
The effect of Tgase-2 on TPA-induced K8 phosphorylation and reorganization was examined both using a well-known Tgase inhibitor, cystamine (CTM), and by Tgase-2 gene silencing. In the results, CTM inhibited TPA-induced K8 phosphorylation, as confirmed by Western blotting (Fig. 3A). Gene silencing of Tgase-2 also suppressed K8 phosphorylation induced by TPA (Fig. 3B). Under the TPA-treatment condition, gene silencing of Tgase-2 in PANC-1 cells restored the keratin filaments’ typical pan-cytoplasmic pattern (Fig. 3C). Control si-RNA-treated PANC-1 cells did not affect TPA-induced keratin-reorganization (Fig. 3C). The effect of Tgase-2 also was examined in Tgase-2 knockdown and Tgase-2 stably expressed PANC-1 cells (PANshTg2 or PANTg2) that had been established by transfection of lentiviral Tgase-2 shRNA or lentiviral vector containing a Tgase-2 gene, respectively (Park et al., 2011). The PANshTg2 cells showed decreased K8 phosphorylation with TPA treatment; the PANTg2 cells, by contrast, showed increased K8 phosphorylation (Fig. 3D).
Fig. 3.
Tgase-2 is involved in TPA-induced K8 phosphorylation and reorganization of PANC-1 cells. (A) Effect of CTM on TPA-induced K8 phosphorylation. PANC-1 cells were treated with various amounts of CTM and TPA (100 nM). (B) Effect of Tgase-2 siRNA on TPA-induced K8 phosphorylation. PANC-1 cells were transfected with the indicated amounts of Tgase-2 siRNA or control siRNA (mock) and stimulated with or without TPA (100 nM). (C) Effect of Tgase-2 on perinuclear keratin organization in TPA-treated PANC-1 cells. In (B) and (C), PANC-1 cells were treated with TPA (100 nM) or vehicle for 45 min. For Tgase-2 gene silencing, the PANC-1 cells were treated with Tgase-2 siRNA and then with TPA or vehicle for 45 min. Immunostaining was performed using K8 Ser431 (green), Tgase-2 (red), and DAPI (blue). (D) Effect of TPA on keratin-8phosphorylation in PANC-1 cells (PANWT), Tgase-2 overexpressing cells (PANTg2), and Tgase-2 silenced cells (PANshTg2). PANC-1 cells (PANWT, PANTg2, and PANshTg2 cells) were treated with TPA (100 nM) for 45 min.
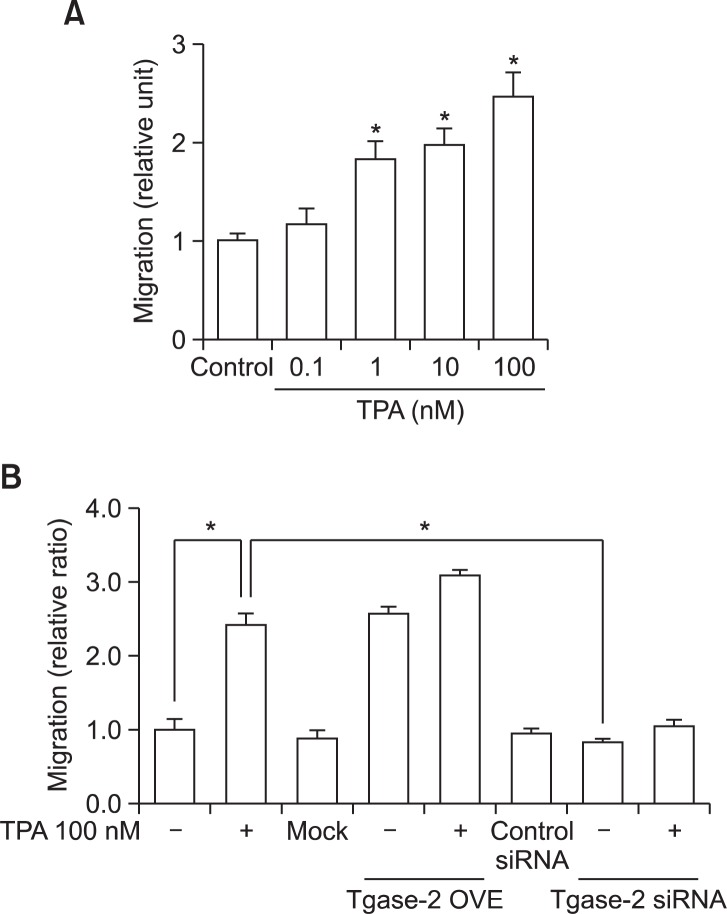
Involvement of Tgase-2 in TPA-induced migration of PANC-1 cells
The expected final result of the reorganization of the keratin network in PANC-1 cells is enhanced migratory properties. Therefore, we examined the effects of TPA on PANC-1 cell migration, indeed finding that TPA dose-dependently induced migration (Fig. 4A). We then examined the involvement of Tgase-2 in TPA-induced PANC-1 cell migration. The results showed that Tgase-2 gene silencing effectively suppressed TPA-induced PANC-1 cell migration and overexpression of Tgase-2 induced migration of PANC-1 cell without TPA treatment (Fig. 4B).
Fig. 4.
Tgase-2 is involved in TPA-induced migration of PANC-1 cells. (A) Migration of PANC-1 cells through size-limited (8 μm) pores in response to TPA. PANC-1 cells (5×104 cells per well) were treated with various concentrations of TPA, as indicated, for 45 min. (B) Effect of Tgase-2 gene silencing on TPA-induced migration of PANC-1 cells. For Tgase-2 gene silencing, the PANC-1 cells were transfected with Tgase-2 siRNA (50 nM) or control siRNA (mock) and treated with TPA (100 nM) or vehicle for 45 min. In (A), and (B), the migration assays were performed as described. A representative of three independent experiments + s.e.m., each performed in triplicate, is shown. *p<0.05 compared to control, **p<0.05 compared to TPA (100 nM).
DISCUSSION
Metastatic cancer cells have unique mechanical characteristics, for example, softness and elasticity (Cross et al., 2007). Keratins are among the main intermediate filaments that control such characteristics (Bordeleau et al., 2008). The effects of TPA on keratin reorganization in PANC-1 cells and on the underlying mechanisms of their migratory properties were the foci of the present study. Even though TPA has long been known to promote tumorigenesis and PKC activator (Blumberg, 1988; Nakajima et al., 2013), its influence on keratin phosphorylation and reorganization had not previously been investigated.
Using confocal microscopy, we demonstrated that TPA rapidly induces a redistribution of intact keratin filaments from the cell periphery to the perinuclear region (Fig. 2). K8 is an intermediate filament consisting of a conserved central coiled-coil α-helical rod domain flanked by N- (head) and C-terminal (tail) domains (Park et al., 2012). For K8, three phosphorylation sites have been reported, namely K8pSer23, K8pSer73, and K8pSer431. K8 phosphorylation is regulated by various protein kinases such as protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) (Ridge et al., 2005; Park et al., 2011; Park et al., 2012). For example, shear stress, causes disassembly of keratin IF in lung alveolar epithelial cells by protein kinase Cδ-mediated phosphorylation of K8 Ser-73 (Ridge et al., 2005). Leukotriene B4 (LTB4) induced ERK-mediated phosphorylation of K8 Ser-431 via BLT2 (Park et al., 2012).
We determined, by the site-specific K8 phosphoserine antibodies anti-Ser23, anti-Ser73, and anti-Ser431, the exact K8 phosphoserine residues affected by TPA. ERK and JNK is involved in Ser431 and p38 and PKCδ is responsible for Ser73 (Park et al., 2011; Busch et al., 2012; Park et al., 2012). We found that PP2A is involved in ERK and JNK-mediated phosphorylation of K8 Ser431 (Park et al., 2012). Phosphorylation of K8 Ser73 and Ser431 in the TPA-treated PANC-1 cells was remarkable (Fig. 1). However, we focused on phosphorylation of K8 Ser431 since previous report suggested that phosphorylation of Ser431 is important in perinuclear reorganization of K8 (Beil et al., 2003).
Several inducers, such as EGF, phosphatase inhibitor, sheared stress, and LTB4, can induce phosphorylation and reorganization of keratin in many types of cells (Kasahara et al., 1993; Ku and Omary, 1997; Sivaramakrishnan et al., 2009). SPC-induced keratin reorganization via phosphorylation, meanwhile, is related to tumor cells’ viscoelasticity and migration (Beil et al., 2003). And TPA, additionally, is among the compounds that induce keratin phosphorylation and reorganization (Fig. 1, 2).
We observed that TPA induced a Tgase-2 expression similar to phosphorylation of K8. This prompted us to consider a potential role for Tgase-2 in TPA-induced keratin phosphorylation and reorganization (Fig. 3). Even though Tgase-2 mediates the metastasis and chemoresistance of several cancer cells, to date there are no reported links between it and TPA-induced keratin reorganization.
Transglutamination reactions are also needed to maintain keratin structures (Omary et al., 1998). However, the role of Tgase-2 in the keratin structures of normal, simple epithelia or epithelial cancer, such as pancreatic cancer, is unclear. In a previous report, we demonstrated that SPC-induced Tgase-2 activated c-Jun N-terminal kinase (JNK) (Park et al., 2011). And in the present study, as shown in Fig. 3, CTM and gene silencing of Tgase-2 suppressed K8 phosphorylation and perinuclear keratin reorganization. These observations suggest that Tgase-2 is involved in TPA-induced K8 phosphorylation and perinuclear reorganization. An additional observation suggestive of Tgase-2’s involvement in TPA-induced keratin reorganization was its colocalization to the region of the perinuclear K8 ring structure in TPA-treated PANC-1 cells (Fig. 2C). Further, the effects of gene silencing on the TPA-induced migration of PANC-1 cells suggest that Tgase-2 also is involved in TPA-induced migration (Fig. 4). Indeed, an earlier finding, that TPA-induced Tgase-2 expression is similar to SPC-induced Tgase-2 expression, indicates that Tgase-2 might have a general role in keratin phosphorylation and reorganization of PANC-1 cells (Park et al., 2011). In addition, ethacrynic acid, also suppressed the SPC-induced K8 phosphorylation via Tgase-2 inhibition (Byun et al., 2013).
TPA is an activator of the Ca2+/phospholipid-dependent protein kinase and K8 possibly is a substrate of PKC (Blumberg, 1988; Cadrin et al., 1992). The specific K8 serine residues affected by TPA are not known. TPA-induced phosphorylation and reorganization of keratin might occur by way of PKC, since TPA is the PKC activator (Blumberg, 1988). However, TPA also induces the MAP kinases p38 and ERK, and so their involvement must be considered. Currently, studies seeking to determine the exact kinases involved in TPA-induced K8 phosphorylation are ongoing.
In the present study, migration of PANC-1 cells through size-limited (8 μm) pores possibly was increased by TPA via keratin reorganization (Fig. 4A), and Tgase-2 gene silencing suppressed TPA-induced migration and Tgase-2 overexpression induced migration of PNAC-1 cells (Fig. 4B). This latter observation, that Tgase-2 gene silencing or Tgase-2 overexpression itself reduced or induced PANC-1 cell migration, without TPA treatment, suggests that Tgase-2 is an important factor such migration. And it is consistent with one other report (Park et al., 2011).
In conclusion, TPA induces K8 phosphorylation and reorganization, and Tgase-2 is involved in TPA-induced phosphorylation and reorganization of keratin. This suggests that Tgase-2 can be a new target for modulation of TPA-induced K8 phosphorylation and reorganization.
Acknowledgments
This study was supported by grants from the Korea Healthcare Technology R&D project (no. A101836), the Ministry of Health Welfare and Family Affairs, the Research Program for New Drug Target Discovery (2011-0030173), and the GRRC program of Gyeonggi Province (GRRC Dongguk2013-B01).
REFERENCES
- Beil M, Micoulet A, von Wichert G, Paschke S, Walther P, Omary MB, Van Veldhoven PP, Gern U, Wolff-Hieber E, Eggermann J, Waltenberger J, Adler G, Spatz J, Seufferlein T. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–811. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- Blumberg PM. Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. Cancer Res. 1988;48:1–8. [PubMed] [Google Scholar]
- Bordeleau F, Bessard J, Sheng Y, Marceau N. Keratin contribution to cellular mechanical stress response at focal adhesions as assayed by laser tweezers. Biochem Cell Biol. 2008;86:352–359. doi: 10.1139/o08-076. [DOI] [PubMed] [Google Scholar]
- Busch T, Armacki M, Eiseler T, Joodi G, Temme C, Jansen J, von Wichert G, Omary MB, Spatz J, Seufferlein T. Keratin 8 phosphorylation regulates keratin reorganization and migration of epithelial tumor cells. J Cell Sci. 2012;125:2148–2159. doi: 10.1242/jcs.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun HJ, Kang KJ, Park MK, Lee HJ, Kang JH, Lee EJ, Kim YR, Kim HJ, Kim YW, Jung KC, Kim SY, Lee CH. Ethacrynic acid inhibits sphingosylphosphorylcholine-induced keratin 8 phosphorylation and reorganization via transglutaminase-2 inhibition. Biomol Ther. 2013;21:338–342. doi: 10.4062/biomolther.2013.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadrin M, McFarlane-Anderson N, Aasheim LH, Kawahara H, Franks DJ, Marceau N, French SW. Differential phosphorylation of CK8 and CK18 by 12-O-tetradecanoylphorbol-13-acetate in primary cultures of mouse hepatocytes. Cell Signal. 1992;4:715–722. doi: 10.1016/0898-6568(92)90052-a. [DOI] [PubMed] [Google Scholar]
- Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Nakamura Y, Kashiwada Y, Aggarwal BB. Anticancer drugs designed by mother nature: ancient drugs but modern targets. Curr Pharm Des. 2007;13:3400–3416. [PubMed] [Google Scholar]
- Kasahara K, Kartasova T, Ren XQ, Ikuta T, Chida K, Kuroki T. Hyperphosphorylation of keratins by treatment with okadaic acid of BALB/MK-2 mouse keratinocytes. J Biol Chem. 1993;268:23531–23537. [PubMed] [Google Scholar]
- Ku NO, Omary MB. Phosphorylation of human keratin 8 in vivo at conserved head domain serine 23 and at epidermal growth factor-stimulated tail domain serine 431. J Biol Chem. 1997;272:7556–7564. doi: 10.1074/jbc.272.11.7556. [DOI] [PubMed] [Google Scholar]
- Nakajima J, Nakae D, Yasukawa K. Structure-dependent inhibitory effects of synthetic cannabinoids against 12-O-tetradecanoylphorbol-13-acetate-induced inflammation and skin tumour promotion in mice. J Pharm Pharmacol. 2013;65:1223–1230. doi: 10.1111/jphp.12082. [DOI] [PubMed] [Google Scholar]
- Omary MB, Ku NO, Liao J, Price D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem. 1998;31:105–140. [PubMed] [Google Scholar]
- Park MK, Lee HJ, Shin J, Noh M, Kim SY, Lee CH. Novel participation of transglutaminase-2 through c-Jun N-terminal kinase activation in sphingosylphosphorylcholine-induced keratin reorganization of PANC-1 cells. Biochim Biophys Acta. 2011;1811:1021–1029. doi: 10.1016/j.bbalip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Park MK, Park Y, Shim J, Lee HJ, Kim S, Lee CH. Novel involvement of leukotriene B(4) receptor 2 through ERK activation by PP2A down-regulation in leukotriene B(4)-induced keratin phosphorylation and reorganization of pancreatic cancer cells. Biochim Biophys Acta. 2012;1823:2120–2129. doi: 10.1016/j.bbamcr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ridge KM, Linz L, Flitney FW, Kuczmarski ER, Chou YH, Omary MB, Sznajder JI, Goldman RD. Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J Biol Chem. 2005;280:30400–30405. doi: 10.1074/jbc.M504239200. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Schneider JL, Sitikov A, Goldman RD, Ridge KM. Shear stress induced reorganization of the keratin intermediate filament network requires phosphorylation by protein kinase C zeta. Mol Biol Cell. 2009;20:2755–2765. doi: 10.1091/mbc.E08-10-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- Xu X, Prough RA, Samuelson DJ. Differential 12-OTetradecanoylphorbol-13-acetate-induced activation of rat mammary carcinoma susceptibility Fbxo10 variant promoters via a PKC-AP1 pathway. Mol Carcinog. 2013. Sep 5, [Epub ahead of print] [DOI] [PMC free article] [PubMed]