Abstract
The main purpose of this study was to develop a novel, in situ gel system for sustained delivery of ranitidine hydrochloride. Ranitidine in situ gels at 0.2%, 0.5%, and 1.0% gellan gum concentration (w/v) were prepared, respectively, and characterized in terms of preparation, viscosity and in vitro release. The viscosity of the gellan gum formulations in solution increased with increasing concentrations of gellan gum. In vitro study showed that the release of ranitidine from these gels was characterized by an initial phase of high release (burst effect) and translated to the second phase of moderate release. Single photon emission computing tomography technique was used to evaluate the stomach residence time of gel containing 99mTc tracer. The animal experiment suggested in situ gel had feasibility of forming gels in stomach and sustained the ranitidine release from the gels over the period of at least 8 h. In conclusion, the in situ gel system is a promising approach for the oral delivery of ranitidine for the therapeutic effects improvement.
Keywords: Oral sustained delivery, In situ gel, Ranitidine hydrochloride, Gellan gum, Scintigraphic studies, In vivo
INTRODUCTION
H2-antagonists or proton pump inhibitors were clinically used in treating chronic conditions like peptic ulcer and reflux oesophagitis. H2-antagonists competitively inhibit histamine actioned at all H2-receptors, but were mainly used clinically as inhibitors of gastric acid secretion (Rang et al., 2003). Local availability of H2-antagonists in stomach had a greater clinical significance in treatment of peptic ulcer (Pellinger et al., 2010).
Ranitidine hydrochloride is a histamine H2-receptor antagonist. It was widely prescribed in active duodenal ulcers, gastric ulcers, Zollinger-Ellison syndrome, gastroesophageal reflux disease, and erosive esophagitis. The recommended adult oral dosage of ranitidine was 150 mg twice daily or 300 mg once daily. The effective treatment of erosive esophagitis required administration of 150 mg of ranitidine 4 times a day. A conventional dose of 150 mg can inhibit gastric acid secretion up to 5 hours but not up to 10 hours. An alternative dose of 300 mg lead to plasma fluctuations; thus a sustained release dosage form of ranitidine was desirable (Betlach et al., 1991). Moreover, due to the short biological half-life of drug (∼2.5–3 hours), and consequently, a frequent dosing regimen was needed.
Several approaches have been used in designing oral ranitidine sustained forms with high absorption and lasting drug effects. For example, floating drug delivery made of hydroxypropyl methylcellulose (Dave et al., 2004), carbopol (Adhikary and Vavia, 2008) ethyl cellulose (Mastiholimath et al., 2008), sodium alginate (Rohith et al., 2009) and osmotic technology (Kumar et al., 2008) can increase the drug retain in the stomach and resulting in increased absorption. However, due to high viscosity floating drug delivery have the disadvantage of being difficult to develop.
Oral in situ gel, or environment sensitive gel, is a new dosage form which has been applied in drug delivery recently. Compared with traditional formulations, in situ gels were administered as low viscosity solutions, and under the sensitive environment, the polymer changed conformation producing a gel, so it cannot only prolong the contact time between the drug and the absorptive sites in the stomach, but also release drug slowly and continuously, hence, it was especially useful for those drugs used chronically. Among oral in situ gel, the phase transition can be induced by a shift in temperature as for the thermo gelling xyloglucan (Miyazaki et al., 2001) or by the presence of cations as for gellan gum (Miyazaki et al., 2001), sodium alginate, pectin (Kubo et al., 2004).
Gellan gum is an anionic deacetylated, exocellular polysaccharide secreted by Pseudomonas elodea with a tetrasaccharide repeating unit of 1β-L-rhamnose, 1β-D-glucuronic, acid and 2β-D-glucose. The mechanism of gelation involves the formation of double-helical junction zones followed by aggregation of the double-helical segments to form a 3-D network by complexation with cations and hydrogen bonding with water (Grasdalen and Smidsroed, 1987; Chanrasekaran et al., 1988; Chanrasekaran and Thailambal, 1990). Many paper previously examined the feasibility of using gellan formulations for the oral sustained delivery of drug (Miyazaki et al., 2001). The proposed formulation was a gellan solution containing calcium carbonate (as a source of Ca++ ions) and sodium citrate, which complexed the free Ca++ ions and released them only in the highly acidic environment of the stomach. In this way the formulation remained in liquid form until it reached the stomach, when gelation was instantaneous.
In the present study, a oral sustained delivery system of ion-activated in situ gel for ranitidine with gellan gum was developed; and its viscosity, release, hydrogel formation in vitro and in vivo animal study were investigated.
MATERIALS AND METHODS
Materials
Ranitidine was gifted by the Department of Pharmaceutics histonepharm Pharmaceuticals Ltd. (Jiangsu, China). Gellan gum was obtained from ZhongWei Biochemical Ltd. (Shanghai, China). DTPA (Diethylene triamine pentacetate acid) was gifted by the department of radiotherapy of our hospital. All other reagents were of commercially analytical-grade.
Preparation of in situ gel
Gellan gum solutions of concentrations 0.25, 0.5 and 1.0% w/v were prepared by adding the gum to ultrapure water containing 0.17% w/v sodium citrate and heating to 90°C while stirring. After cooling to below 40°C appropriate amounts of calcium carbonate (0.75% w/v) and ranitidine (1% w/v) were then dissolved in the resulting solution.
Measurement of viscosity of in situ gel
The viscosity of gells prepared in water were determined with a rotational viscometer (NDJ-5S, Shanghai, China) using a 20 mL aliquot of the sample. Measurements were performed using suitable spindle number at 6, 12, 30, 60 r/min, and the temperature was maintained at 37°C. The viscosity was read directly from the viscometer display. All measurements were made in triplicate.
Measurement of drug release rate from gels
The in vitro release of ranitidine from the gels was measured as described by (Miyazaki et al., 1984) with slight modification using USP dissolution test apparatus (USP 36, 2013) with a paddle stirrer at 50 rpm. The dissolution medium used was 500 ml of 0.01N HCl (pH 2.0), and temperature was maintained at 37 ± 0.2°C. Ten milliliter of formulation was drawn up using disposable syringe, the needle was wiped clean and excess formulation was removed from the needle end. Ten milliliter of in situ gel solution was placed into Petri dish and Petri dish containing formulation was kept in the dissolution vessel containing dissolution medium. At every time interval, a precisely measured sample of the dissolution medium was removed and replenished with pre-warmed (37°C) fresh medium. The amount of ranitidine in each sample was determined by HPLC (LC-10A, Shimadzu Co Ltd, Kyoto, Japan).
Scintigraphic studies
In vivo residence time of the developed formulation was assessed by gamma scintigraphy. Twelve white male rabbits weighing 2.5 ± 0.2 kg were divided into 2 groups at random. Single photon emission computing tomography (ZLC 3700, Münich, Germany) auto was tuned to detect the 140 KeV radioactivity of 99mTc-DTPA. In situ gel incorporating 99mTc-DTPA (74 MBq/ml) at the gellan gum concentration of 1% was prepared as described earlier (without drug). The rabbit was positioned 10 cm in front of the probe and 2 ml of the radio labeled gel, which was stored in 20°C for 30 min before use, were administered orally. Recording started 5 s after administration and continued using a 128×128 pixel matrix at predetermined time intervals. Each animal was used only once throughout these trials.
In vivo experiments
Twelve white male rabbits weighing 2.5 ± 0.2 kg were fasted for 24 h prior to the experiments but allowed free access to water. Rabbits were divided into 2 groups at random. A yoke was used to avoid the possibility of coprophagy, in addition to the fasting process, which ensured that very little food was present in the stomach (from visual observation). Gels containing ranitidine were produced in situ by oral administration of 10 ml of the appropriate solution containing 100 mg of drug using a stomach sonde needle for rabbits. A stomach sonde needle was also used for oral administration of ranitidine suspension (100 mg in 10 ml). At given intervals, 0.5 ml blood samples were taken from the ear vein and analyzed as described below. The animal experiment was carried out in compliance with the protocol of Animal Use and Care by Medical Center of Jiaotong University (China).
Determination of ranitidine
The analysis of ranitidine levels in vitro and in vivo were carried out using an RP-HPLC method in a system equipped with a LC-10ATVP pump, a SPD-10AVP UV-Vis detector (Shimadzu, Kyoto, Japan), and a HS2000 interface (Empire Science & Tech, Hangzhou, China) operated at 230 nm. A reversed-phase column (Gemini 5 μm C18, 150×4.6 mm, Phenomenex, California, USA) was used at 40°C. The mobile phase consisted of 0.01 M phosphate buffer at pH 6.2 containing 2.5 g/l heptanesulfonic acid:acetonitrile (75:25) at a rate of 1.0 ml/ min. Samples of 20 μl were injected into the HPLC column for all the analysis.
Tissue samples, 100 μl of plasma was added 100 μl of cimetidine solution (10 μg /ml) as internal standard, 100 μl of 1 M sodium hydroxide, 100 μl of saturated solution of potassium carbonate, and 1ml of ethyl acetate-isoamyl alcohol (96:4) and the sample was vortex-mixed and centrifuged. To 100 μl supernatant was added 100 μl of 0.01 M hydrochloric acid. After shaking and centrifugation, the aqueous phase was passed through a Millipore filter (0.45 μm) and injected into the HPLC column for all the analysis.
RESULTS
Characteristic of in situ gel
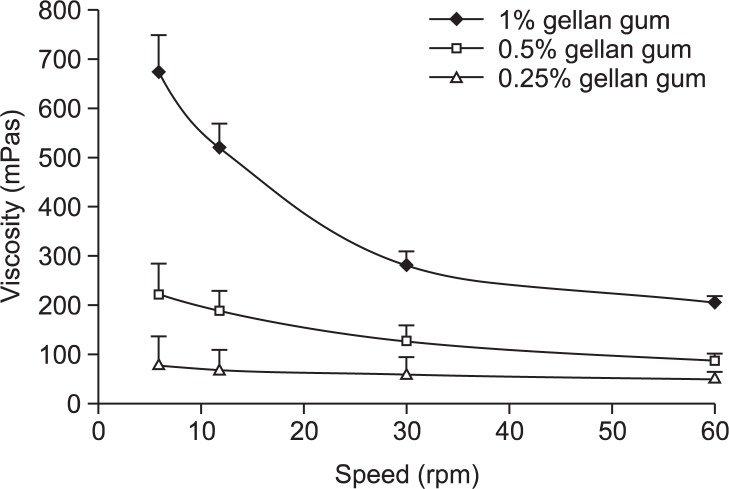
The developed formulations met all the pre-requisites to perform an in situ gelling system, behave like a fluid, but form a rigid gel when at the pH conditions of the stomach (Fig. 1). The calcium carbonate present in the formulation as insoluble dispersion was dissolved and releases carbon dioxide on reaction with acid of the stomach and the in situ released calcium ions result in formation of gel with floating characteristics. The solutions were typically of pseudo plastic systems and showed a marked increase in viscosity with increasing concentration of gellan as shown in Fig. 2.
Fig. 1.
Photograph showing the appearance of gellan gel formed in simulated gastric fluid pH 2.0.
Fig. 2.
Viscosity for the various gellan gum solution.
In vitro drug release
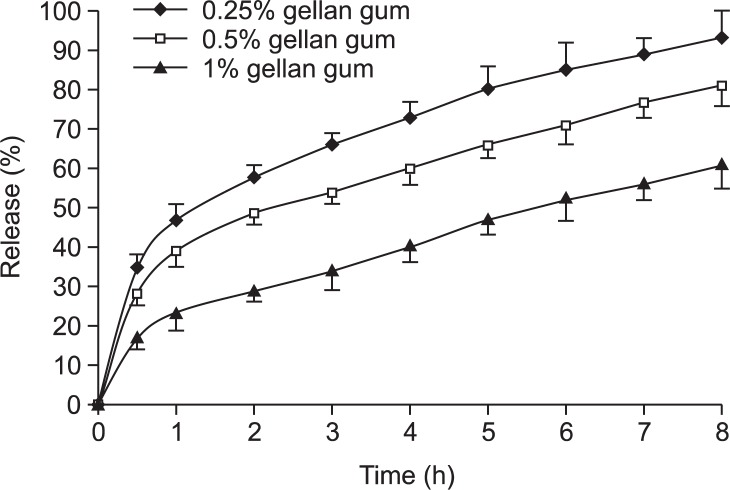
The effect of polymer concentration on in vitro drug release from in situ gels was shown in Fig. 3. The results showed that the release of ranitidine from these gels was characterized by an initial phase of high release (burst effect). However, during the hydrogel formation, a portion of ranitidine might be loaded into the hydrogel phase, and the remaining drug was released at a slower rate followed by a second phase of moderate release. This bi-phasic pattern of release is a characteristic feature of matrix diffusion kinetics. In addition, the release rate also depended on the gellan gum concentration. The release rate from various gellan gum formulations could be ranked as follows: 0.25%>0.5%>1%.
Fig. 3.
Release profiles of drug from various gellan gum formulations.
Scintigraphic studies
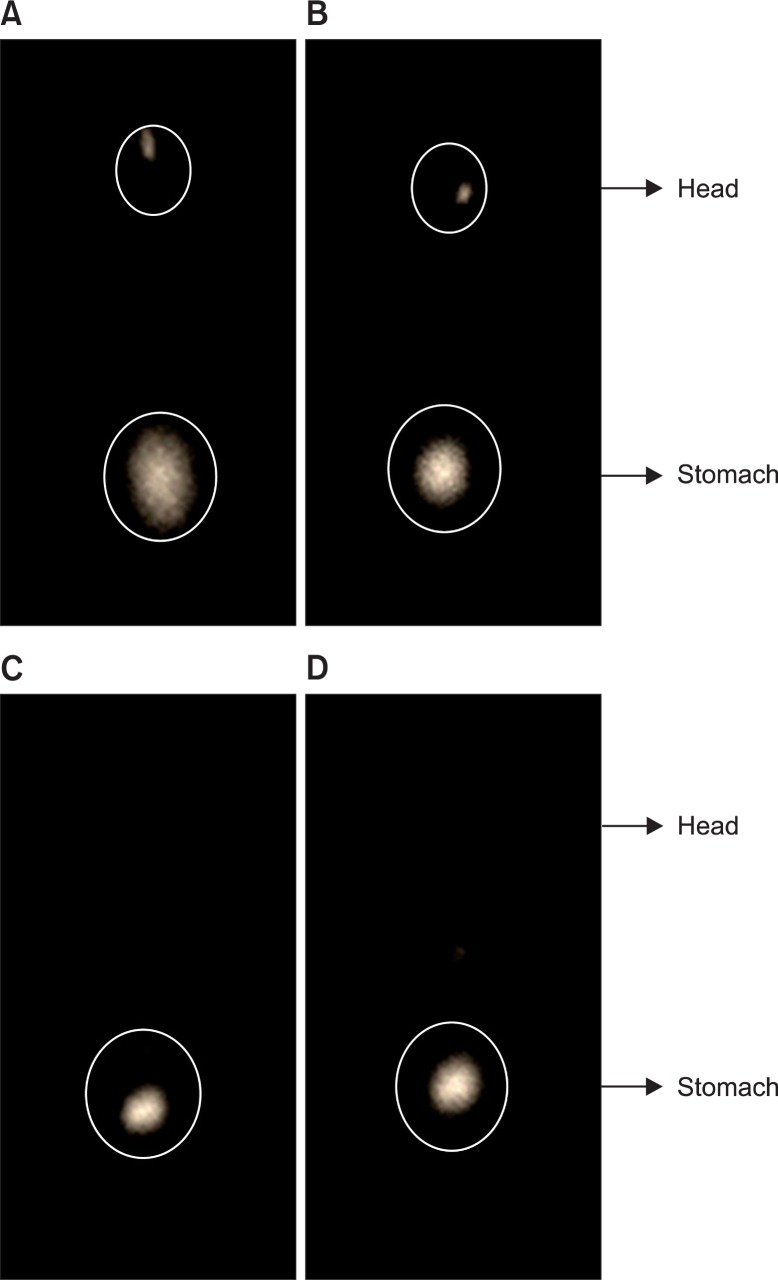
The in vivo bio-adhesion of the 99mTc-labeled gels is shown in Fig. 4. As expected, the rabbits taken after 8-h post-administration of in situ gels showed the presence of major portion of gels in the stomach indicating increase the residence time of the formulation. The more quantitative data were further demonstrated by our following reports. Form the point of imaging data, during 1 h the radiation intensity of gel suspension and in-situ gelling were almost the same, but over time, the suspension had been gradually eliminated, basically no radiological marker inside stomach. However, in the group of in-situ gelling, with the passage of time due to the formation of a gel in the stomach, it maintained a certain intensity of radiation during 3 h and 8 h.
Fig. 4.
Scintigraphic image of rabbits after gel and suspension administration. A: suspension (1 h); B: in situ gel (1 h); C: in situ gel (3 h); D: in situ gel (8 h).
In vivo release
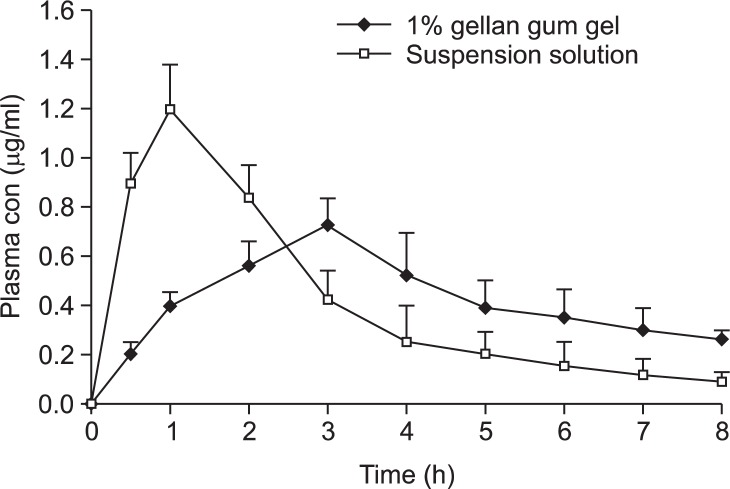
Plasma drug levels following oral administration to rabbits of ranitidine from 1.0% (w/v) gellan gum gel and from the suspension of ranitidine, are compared in Fig. 5. The area under the plasma concentration-time curve (AUC) and the mean residence time (MRT) obtained from the plasma concentration-time data of each animal using a personal computer program for model-independent analysis are summarized in Table 1. For the pharmacokinetic analysis of plasma, the mean (SD) values obtained for the in situ gel and suspension formulations were as follows: Tmax, 2.8 (0.45) and 1.3 (0.67) h; Cmax, 0.72 (0.12) and 1.21 (0.15) μg/ml; AUC0–8h, 3.37 (0.27) and 3.51 (0.36) μg·h/mL; MRT, 3.65 (0.22) and 2.27 (0.31) h, respectively. The mean residence times of ranitidine when released from the gels were significantly longer than that following the oral administration of this drug in solution.
Fig. 5.
Plasma concentrations of ranitidine in rabbits after oral administration of 1% gellan gum gel and an aqueous solution. All formulations contained 100 mg ranitidine. Each value represents mean ± S.E. of five determinations.
Table 1.
Comparison of bioavailability parameters of ranitidine administered from gels of gellan formed in situ in rabbit stomach and from suspension solution
| Parameter | In situ gel | Suspension |
|---|---|---|
| Tmax (h) | 2.8±0.45 | 1.3±0.67 |
| Cmax (μg/ml) | 0.72±0.12 | 1.21±0.15 |
| AUC0–8h (μg·h/ml) | 3.37±0.27 | 3.51±0.36 |
| MRT (h) | 3.65±0.22* | 2.27±0.31 |
p<0.05 compared with suspension solution.
DISCUSSION
In this study, in situ gels at three different gellan gum concentrations were prepared. The two main pre-requisites of in situ gelling systems are optimum viscosity and gelling capacity (speed and extent of gelation). The formulation should have an optimum viscosity that will allow easy swallowing as a liquid, which then undergoes a rapid sol-gel transition due to ionic interaction. The rheological properties of the solutions are of importance in view of their proposed oral administration. The observed increase in viscosity with increase in concentration has been proposed that as the concentration of gellan gum increased, the polymer chains approached closer, and the number of interactions between the polymer chains increased which lead to a denser 3-D network structure (Nickerson and Paulson, 2004).
Because the release rate of a drug directly affected its absorption process in vivo, ranitidine release through the different gellan gum formulations was examined using the dissolution method. Release results indicated that the structure of the gel became more closely packed and functioned as an increasingly resistant barrier to drug release as the concentration of polymer increased.
Several methods, both in vitro and in vivo, have been used to evaluate transport rates (Zou et al., 2007). Advantages of the gamma scintigraphic technique lie in the ability to non-invasively monitor the deposition and clearance of drug formulations, allowing both quantitative and photographic illustrations of distribution and clearance of the radio labeled formulation. Employing this technique to evaluate the clearance of in situ gels requires a radiotracer which is stable and non-diffusible to prevent absorption into the vascular compartment. 99mTc tracer is reported as technically easy to perform and met all the requisites. Therefore, 99mTc-DTPA was used in this study.
The in situ gel contained the optimum levels of sodium citrate and calcium carbonate and formed gels in the stomach at 37°C. Rapid absorption from the suspension produced a peak plasma drug concentration of 1.2 μg/ml at 1 h. A sustained release of drug from the gels was evident from the concentration-time profiles. For example, release of ranitidine from the in situ gel decreased gradually from about 0.7–0.2 μg/ml over the 2 h period following administration. All of the formulations are homogeneous liquids and do not have the problems associated with the administration of suspensions. In addition, it may be possible to achieve a more sustained release by manipulation of the concentrations of the components of the in situ gelling formulations.
In amount, ranitidine in situ gel can be prepared by mixing the ranitidine, gellan gum. The gel was typically of pseudo plastic systems and presented undergoes a sol-gel transition at the pH conditions of the stomach in vitro study. The animal experiment suggested in situ gel has feasibility of forming gels in stomach and sustaining the ranitidine release from the gels over the period of at least 8 h. In conclusion, the in situ gel system is a promising approach for the oral delivery of ranitidine for the therapeutic effects improvement.
REFERENCES
- Adhikary A, Vavia PR. Bioadhesive ranitidine hydrochloride for gastroretention with controlled microenvironmental pH. Drug Dev Ind Pharm. 2008;34:860–869. doi: 10.1080/03639040801928812. [DOI] [PubMed] [Google Scholar]
- Betlach CJ, Straughn AB, Meyer MC, Bialer M, Vashi VI, Liebermann P, González MA. The effect of raising gastric pH with ranitidine on the absorption and elimination of theophylline from a sustained-release theophylline tablet. Pharm Res. 1991;8:1516–1519. doi: 10.1023/a:1015846417085. [DOI] [PubMed] [Google Scholar]
- Chanrasekaran R, Puigjaner LC, Joyce KL, Arnott S. Cation interaction in gellan: an X-ray study of the potassium salt. Carbohydr Res. 1988;181:23–40. [Google Scholar]
- Chanrasekaran R, Thailambal VG. The influence of calcium ions, acetate and L-glycerate groups on the gellan double helix. Carbohydr Polym. 1990;12:431–442. [Google Scholar]
- Dave BS, Amin AF, Patel MM. Gastroretentive drug delivery system of ranitidine hydrochloride: formulation and in vitro evaluation. AAPS PharmSciTech. 2004;5:e34. doi: 10.1208/pt050234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasdalen H, Smidsroed O. Gelation of gellan gum. Carbohydr Polym. 1987;7:371–393. [Google Scholar]
- Kubo W, Miyazaki S, Dairaku M, Togashi M, Mikamib R, Attwood D. Oral sustained delivery of ambroxol from in situ-gelling pectin formulations. Int J Pharm. 2004;271:233–240. doi: 10.1016/j.ijpharm.2003.11.027. [DOI] [PubMed] [Google Scholar]
- Kumar P, Singh S, Mishra B. Gastroretentive drug delivery system of Ranitidine hydrochloride based on osmotic technology: development and evaluation. Curr Drug Deliv. 2008;5:332–342. doi: 10.2174/156720108785914943. [DOI] [PubMed] [Google Scholar]
- Mastiholimath VS, Dandagi PM, Gadad AP, Mathews R, Kulkarni AR. In vitro and in vivo evaluation of ranitidine hydrochloride ethyl cellulose floating microparticles. J Microencapsul. 2008;25:307–314. doi: 10.1080/02652040801973101. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Takeuchi S, Yokouchi C, Takada M. Pluronic F 127 gels as a vehicle for topical administration of anticancer agents. Chem Pharm Bull. 1984;32:4205–4208. doi: 10.1248/cpb.32.4205. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Kawasaki N, Kubo W, Endo K, Attwood D. Comparison of in situ gelling formulations for the oral delivery of cimetidine. Int J Pharm. 2001;220:161–168. doi: 10.1016/s0378-5173(01)00669-x. [DOI] [PubMed] [Google Scholar]
- Nickerson MT, Paulson AT. Rheological properties of gellan, k-carrageenan and alginate polysaccharides: effect of potassium and calcium ions on macrostructure assemblages. Carbohydr Polym. 2004;58:15–24. [Google Scholar]
- Pellinger TK, Simmons GH, Maclean DA, Halliwill JR. Local histamine H(1-) and H(2)-receptor blockade reduces postexercise skeletal muscle interstitial glucose concentrations in humans. Appl Physiol Nutr Metab. 2010;35:617–626. doi: 10.1139/H10-055. [DOI] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th ed. Churchill Livingstone; Edinburgh: 2003. pp. 370–371. [Google Scholar]
- Rohith G, Sridhar BK, Srinatha A. Floating drug delivery of a locally acting H2-antagonist: an approach using an in situ gelling liquid formulation. Acta Pharm. 2009;59:345–354. doi: 10.2478/v10007-009-0021-z. [DOI] [PubMed] [Google Scholar]
- Zou H, Jiang XT, Kong LS, Gao S. Design and gamma-scintigraphic evaluation of a floating and pulsatile drug delivery system based on an impermeable cylinder. Chem Pharm Bull. 2007;55:580–585. doi: 10.1248/cpb.55.580. [DOI] [PubMed] [Google Scholar]