Abstract
Previously, we reported that lysophosphatidylethanolamine (LPE), a lyso-type metabolite of phosphatidylethanolamine, can increase intracellular Ca2+ ([Ca2+]i) via type 1 lysophosphatidic acid (LPA) receptor (LPA1) and CD97, an adhesion G-protein-coupled receptor (GPCR), in MDA-MB-231 breast cancer cells. Furthermore, LPE signaling was suggested as like LPA1/CD97-Gi/o proteins-phospholipase C-IP3-Ca2+ increase in these cells. In the present study, we further investigated actions of LPE not only in the [Ca2+]i increasing effect but also in cell proliferation and migration in MDA-MB-231 breast cancer cells. We utilized chemically different LPEs and a specific inhibitor of LPA1, AM-095 in comparison with responses in SK-OV3 ovarian cancer cells. It was found that LPE-induced Ca2+ response in MDA-MB-231 cells was evoked in a different manner to that in SK-OV3 cells in terms of structural requirements. AM-095 inhibited LPE-induced Ca2+ response and cell proliferation in MDA-MB-231 cells, but not in SK-OV3 cells, supporting LPA1 involvement only in MDA-MB-231 cells. LPA had significant effects on cell proliferation and migration in MDA-MB-231 cells, whereas LPE had less or no significant effect. However, LPE modulations of MAPKs (ERK1/2, JNK and p38 MAPK) was not different to those by LPA in the cells. These data support the involvement of LPA1 in LPE-induced Ca2+ response and cell proliferation in breast MDA-MB-231 cells but unknown GPCRs (not LPA1) in LPE-induced responses in SK-OV3 cells. Furthermore, although LPE and LPA utilized LPA1, LPA utilized more signaling cascades than LPE, resulting in stronger responses by LPA in proliferation and migration than LPE in MDA-MB-231 cells.
Keywords: Lysophosphatidylethanolamine, LPA1, Lysophosphatidic acid, GPCR, Breast, Receptor
INTRODUCTION
Lysophosphatidic acid (LPA), a bioactive lyso-type lipid mediator, acts through G protein-coupled receptors (GPCRs) LPA1–6 (Choi and Chun, 2013). Three Edg subfamily GPCR members (LPA1–3), and three non-Edg (purinergic) GPCRs (LPA4–6) have been reported (Choi and Chun, 2013; Yanagida et al., 2013). Similar lyso-type phospholipids, such as, lysophosphatidylserine (LPS), lysophosphatidylethanolamine (LPE), lysophosphatidylglycerol (LPG), and lysophosphatidylcholine (LPC) have also been reported to act through putative GPCRs. However, few studies have been conducted on putative GPCRs (Park et al., 2005; Park et al., 2006; Park et al., 2007; Jo et al., 2008; Lee et al., 2008; Makide et al., 2009). Recently, GPR34, GPR174, and P2Y10 were reported to be GPCRs for 2-acyl LPS (Inoue et al., 2012; Kitamura et al., 2012; Makide and Aoki, 2013). However, it is controversial whether GPR34 acts as an LPS receptor in mice (Sugo et al., 2006; Iwashita et al., 2009; Liebscher et al., 2011; Kitamura et al., 2012; Ritscher et al., 2012).
LPE, a lyso-type phospholipid, has been detected in human serum at concentrations of about several hundreds of ng/ml (Misra, 1965; Makide et al., 2009). In 2007, the intracellular Ca2+ ([Ca2+]i) enhancing actions of LPE was supposed to be mediated through GPCRs, but not through GPCRs for LPA, in SK-OV3 and OVCAR-3 ovarian cancer cells (Park et al., 2007). In a previous study, we found that LPE-induced [Ca2+]i increases might be mediated via the GPCRs of LPA1 and CD97 in MDA-MB-231 cells (a breast cancer cell line) (Park et al., 2013). Furthermore, LPE signaling was proposed as like LPA1/CD 97-Gi/o proteins-phospholipase C-IP3-Ca2+ increase in the cells (Park et al., 2013). In the present study, we compared LPE-induced [Ca2+]i increases and cell proliferation in MDA-MB-231 and SK-OV3 cells induced by different LPEs and in the presence of an LPA1 antagonist. In addition, we tested the effect of LPE and LPA on cell migration and MAPK activities.
MATERIALS AND METHODS
Materials
1-Oleoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (18:1 LPE), 1-stearoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (18:0 LPE), 1-octadecyl-2-hydroxy-sn-glycero-3-pho sphoethanolamine (ether-linked 18:0 LPE), 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (16:0 LPE), 1- my ristoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (14:0 L PE), and 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate (sodium salt) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Fura 2-AM was purchased from Sigma-Aldrich (St. Louis, MO). AM-095 was obtained from Chemscene (New Jersey, USA), and WST from Daeil lab service (Seoul, Korea).
Cell culture
The human MDA-MB-231 breast cancer cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured at 37°C in a 5% CO2 humidified incubator, and maintained in high glucose DMEM, containing 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin, 50 μg/ml streptomycin, 2 mM glutamine, and 1 mM sodium pyruvate. The SK-OV3 human ovarian cancer cell line was maintained as recommended by the supplier (Park et al., 2013).
Measurement of [Ca2+]i concentrations
Cells were trypsin-digested, allowed to sediment, resuspended in Hepes-buffered medium (HBM), consisting of 20 mM Hepes (pH 7.4), 103 mM NaCl, 4.8 mM KCl, 1.2 mM KH2 PO4, 1.2 mM MgSO4, 0.5 mM CaCl2, 25 mM NaHCO3, 15 mM glucose and 0.1% bovine serum albumin (fatty acid free), and then incubated for 40 min with 5 μM of fura 2-AM. [Ca2+]i levels were estimated by measuring changes in fura 2 fluorescence using an emission wavelength of 510 nm and excitation wavelengths of 340 nm and 380 nm every 0.1 sec using a F4500 fluorescence spectrophotometer (Hitachi, Japan). The ratios of fluorescence intensities (λ340/λ380) at these two wavelengths were used as a surrogate of [Ca2+]i, as previously described (Chang et al., 2006; Ahn et al., 2012).
Cell proliferation
MDA-MB-231 cells (5×103 per well) were seeded into 96-well plates and incubated in serum-deprived medium overnight. LPEs and LPA were dissolved in 0.1% BSA at appropriate concentrations and administered to medium for 24 h. Cell proliferation assays were carried out using the Enhanced Cell Viability Assay Kit EZ-CyTox WST protocol (Daeil Lab Service, Seoul, Korea), and fluorescences were measured using a microplate reader at 450 nm.
Cell migration
Cell migration was monitored using the in vitro wound-healing assay. Briefly, MDA-MB-231 cells (2×105 per well) were seeded into 6-well plates with DMEM media containing 0.5% FBS and allowed to adhere overnight. A linear scratch was made across the cell monolayer using the sharp end of a 1000-μl sterile pipette tip. Medium and non-adherent cells were removed, and cells were washed twice with PBS, and new medium containing LPE or LPA was added. Cells were permitted to migrate into wound area for 24 h. Wound closure was observed under a microscope.
Reverse transcriptase-PCR
After treatment with LPE or LPA for 5 h, first strand cDNA was synthesized using total RNA isolated using Trizol reagent (Invitrogen, USA). Synthesized cDNA products and specific primers were used for PCR with Promega Go-Taq DNA polymerase (Madison, WI, USA). The primers used to amplify 400, 294, 181, 173, and 396 bps fragments of MMPs and β-actin were as follows: MMP-2 (sense 5′-CAG GCT CTT CTC CTT TCA CAA C-3′, antisense 5′-AAG CCA CGG CTT GGT TTT CCT C-3′), MMP-3 (sense 5′-CTC ACA GAC CTG ACT CGG TT-3′, antisense 5′-CAC GCC TGA AGG AAG AGA TG-3′), MMP-7 (sense 5′-TAC AGT GGG AAC AGG CTC AGG-3′, antisense 5′-GGC ACT CCA CAT CTG GGC T-3′), MMP-9 (sense 5′-TGG GCT ACG TGA CCT ATG ACA T-3′, antise-nse 5′-GCC CAG CCC ACC TCC ACT CCT C-3′), and β-actin (sense 5′-CAC CAC ACC TTC TAC AAT GAG CTG-3′, antisense 5′-GAG GAG CAA TGA TCT TGA TCT TCA TT-3′). PCR was performed over 30 amplification cycles (denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and elongation at 72°C for 30 s) in an Eppendorf Mastcycler gradient unit (Hamburg, Germany). Aliquots of the PCR products (7 μl) so obtained were electrophoresed in 1.2% agarose gels and stained with ethidium bromide.
Western blot
MDA-MB-231 cells (5×105 per well) were seeded in 60-mm dishes, and incubated in DMEM medium containing 0.5% FBS overnight. After treatment with LPE, cells were trypsinized and collected by centrifugation at 1500 rpm for 3 min. After washing twice with PBS, cell pellets were dissolved and boiled in 200 μl of sample buffer containing 62.5 mM Tris-HCl (pH 6.8), 10% glycerol, 2% SDS, 5% 2-mercaptoethanol, and 0.05% bromophenol blue. Proteins (40 μg) were resolved by 8% SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose. Blots were incubated with specific primary antibodies recognizing the phosphorylated forms of p44/42 MAP kinase (ERK), p38 MAP kinase, or SAPK/JNK, and then with HRP-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). Signals were developed using an enhanced chemiluminescence system (Pierce Biotechnology Inc., Rockford, IL, USA).
Statistics
Results are expressed as means ± SEs for the indicated number of determinations. The significances of differences were determined by ANOVA, and statistical significance was accepted for p values of <0.05.
RESULTS
Effects of different LPEs on [Ca2+]i concentration in MDA-MB-231 and SK-OV3 cells
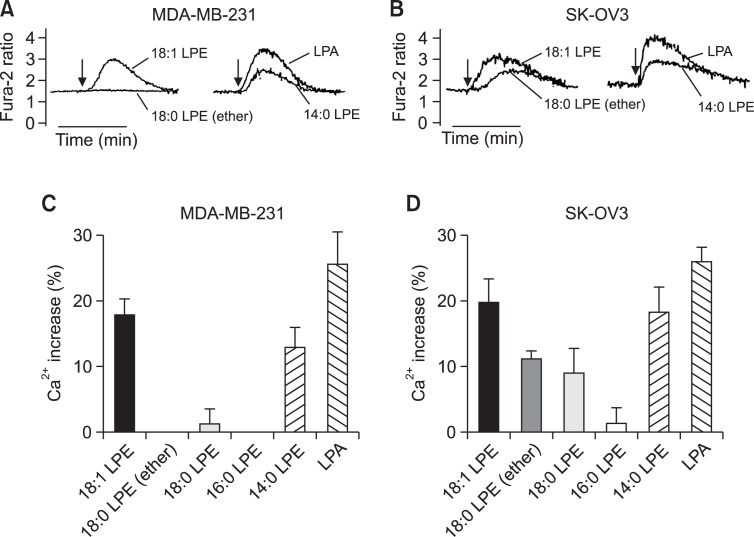
Previously, we observed LPE-induced increases of [Ca2+]i in MDA-MB-231 breast cancer cells and SK-OV3 ovarian cancer cells (Park et al., 2007; Park et al., 2013) (Fig. 1A, B). In the present study, we applied structurally different LPEs, that is oleoyl LPE (18:1 LPE), stearoyl LPE (18:0 LPE), octadecanyl LPE (ether-linked 18:0 LPE), palmitoyl LPE (16:0 LPE), and myristoyl LPE (14:0 LPE). As shown in Fig. 1A, C, 18:0 LPE, ether-linked 18:0 LPE, and 16:0 LPE did not evoke a [Ca2+]i increase in MDA-MB-231 cells, whereas 14:0 and 18:1 LPE did (Fig. 1A, C). In SK-OV3 cells, 18:0 LPE and ether-linked 18:0 LPE evoked a [Ca2+]i increase, though less than that caused by 18:1 LPE (Fig. 1B, D). Although 16:0 LPE did not increase [Ca2+]i in SK-OV3 cells, 14:0 LPE did induce (Fig. 1D). 18:1 LPE was the most potent of the LPEs tested in both cell types (Fig. 1). 18:0 LPE and ether-linked 18:0 LPE induced a [Ca2+]i increase in SK-OV3 cells only (Fig. 1). Because 18:1 LPE was the most potent LPE among tested LPEs, we used 18:1 LPE in the further study and it was stated as LPE without specification of 18:1 hereafter.
Fig. 1.
Structure-activity relationships of LPE in [Ca2+]i increases in MDA-MB-231 and SK-OV3 cells. Representative [Ca2+]i traces of MDA-MB-231 cells (a breast cancer cell line) (A) and of SK-OV3 cells (an ovarian cancer cell line) (B) treated with 10 μM of LPE or LPA. Arrows indicate when LPE or LPA was added. The data shown are representative of more than four independent experiments. Efficacies of different chain-length LPE- and LPA-induced Ca2+ responses as compared to digitonin in MDA-MB-231 cells (C) and SK-OV3 cells (D) are shown as histograms. Results are the means ± SEs of three independent experiments.
Effect of AM-095 on LPE-induced [Ca2+]i responses
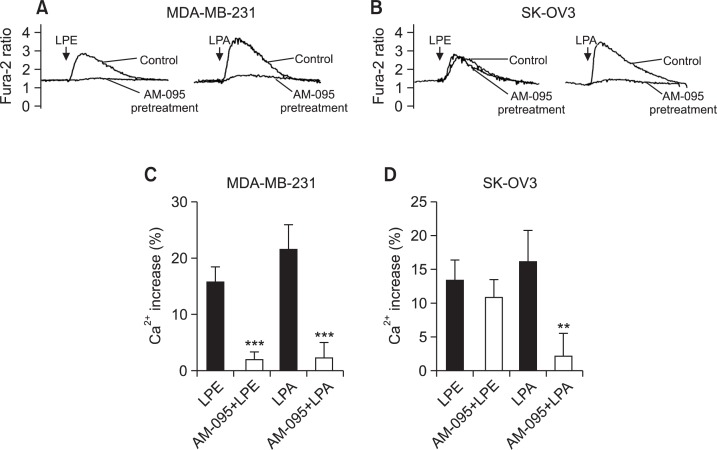
We tested the effect of AM-095 (a specific LPA1 antagonist) to verify the involvement of endogenously expressed LPA1 receptors in LPE-induced [Ca2+]i response in MDA-MB-231 cells and SK-OV3 cells (Castelino et al., 2011; Swaney et al., 2011). As shown in Fig. 2, AM-095 (500 nM) completely inhibited LPA-induced [Ca2+]i responses in both cell lines and LPE-induced [Ca2+]i responses in MDA-MB-231 cells (Fig. 2). On the other hand, AM-095 (500 nM) did not affect LPE-induced [Ca2+]i responses in SK-OV3 cells (Fig. 2B, D). Therefore, LPE appeared to increase [Ca2+]i mainly via LPA1 receptors in MDA-MB-231 cells. On the other hand, LPE appeared to increase [Ca2+]i in another manner in SK-OV3 cells.
Fig. 2.
Effect of AM-095 on LPE-induced [Ca2+]i increases in MDA-MB-231 and SK-OV3 cells. Representative [Ca2+]i traces of MDA-MB-231 (A) and SK-OV3 cells (B) pretreated with AM-095 or vehicle for 5 min and then treated with 10 μM of LPE or LPA. Arrows indicate when LPE or LPA was added. The data shown are representative of more than four independent experiments. Efficacies of LPE- and LPA-induced Ca2+ responses as compared to digitonin in MDA-MB-231 (C) and SK-OV3 cells (D) are shown as histograms. Results are the means ± SEs of three independent experiments. Statistical significance: **p<0.01, ***p<0.001 vs. AM-095 non-treated cells.
Effects of LPE and LPA on the proliferation of MDA-MB-231 cells
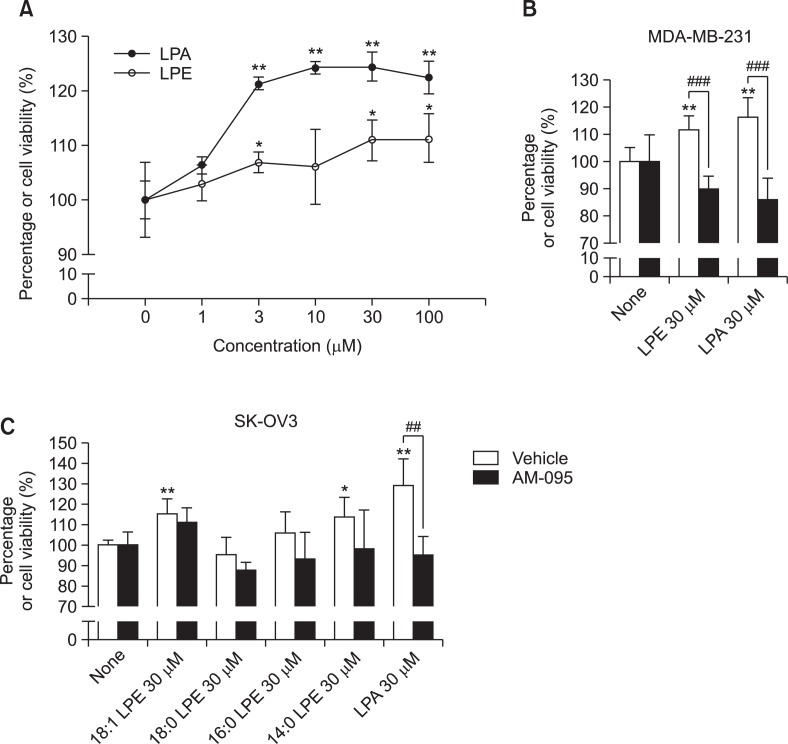
In PC12 neuronal cells, LPEs from Grifola frondosa induced neuronal differentiation and suppressed serum-deprivation induced apoptosis via MAPK activation (Nishina et al., 2006). Therefore, in order to determine the significance of LPE-induced [Ca2+]i response, we investigated the effects of LPE and LPA on cell proliferation in MDA-MB-231 breast cancer cells. LPA induced a significant and concentration-dependently increase in cell proliferation (Fig. 3A). However, LPE did induce cell proliferation significantly but less than LPA (Fig. 3A). Structually different LPEs and AM-095 were also applied to cell proliferation responses in both cell lines. Proliferation stimulatory effects of LPA and LPE were inhibited by treatment of AM-095, suggesting involvement of LPA1 in the proliferation stimulatory response of LPE in MDA-MB-231 cells (Fig. 3B). In SK-OV3 cells, 18:1 LPE and 14:0 LPE along with LPA significantly increased cell viability (Fig. 3C). In the presence of AM-095, LPA-induced proliferation stimulatory effect was inhibited, but not LPE-induced ones (Fig. 3C), suggesting no involvement of LPA1 in the response of LPE in SK-OV3 cells.
Fig. 3.
Effects of LPE and LPA on cell proliferation in MDA-MB-231 human breast cancer cells. MDA-MB-231 human breast cancer cells were treated with 0-100 μM 18:1 LPE or LPA for 24 h (A). Cell viabilities were measured using an MTT assay, as described in Materials and Methods. MDA-MB-231 human breast cancer cells were pretreated with 500 nM of AM-095 or vehicle for 5 min and treated with 30 μM of LPE or LPA for 24 h (B). SK-OV3 human ovarian cancer cells were pretreated with 500 nM of AM-095 or vehicle for 5 min and treated with 30 μM of LPE or LPA for 24 h (C). Results are the means ± SEs of three independent experiments. Statistical significance: *p<0.05, **p<0.01 vs. none-treated cells and ##p<0.01, ###p<0.001 vs. vehicle-treated cells.
Effects of LPE and LPA on cell migration in MDA-MB-231 cells
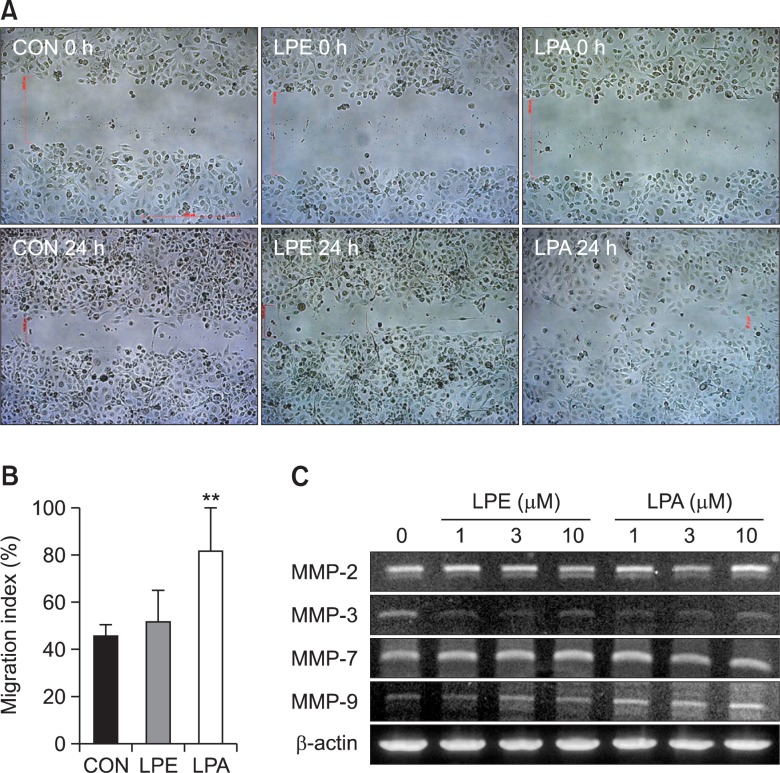
In SK-OV3 human ovarian cancer cells, LPE stimulated chemotactic migration and cellular invasion (Park et al., 2007). Therefore, we investigated effects of LPE and LPA on cell migration using a wounding assay. LPA induced a significant increase in MDA-MB-231 cell migration after 24 hr (Fig. 4A, B), but LPE did not (Fig. 4A, B). In addition, we measured the expression levels of matrix metalloproteinases (MMP) by RT-PCR. LPE did not affect the gene expressions of MMPs (MMP-2, MMP-3, MMP-7, and MMP-9) (Fig. 4C), whereas LPA induced an increase in the expression of MMP-9 only (Fig. 4C).
Fig. 4.
Effects of LPE and LPA on the migration of MDA-MB-231 cells. Relative cell migration rates were measured using a wound-healing assay. Cells were treated with 10 μM 18:1 LPE or LPA for the indicated times (A). Results are the means ± SEs of three independent experiments (B). Statistical significance: **p<0.01 vs. control cells. To examine whether 18:1 LPE affects mRNA expression level of MMPs in MDA-MB231 human breast cancer cells, we used RT-PCR analysis of MMPs. Cells were treated with indicated concentrations of 18:1 LPE or LPA for 5 h. The data shown are representative of more than three independent experiments (C).
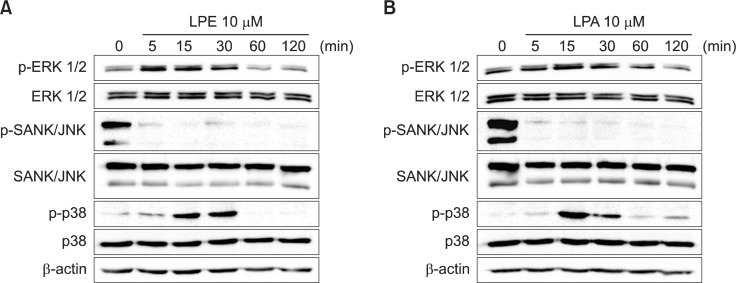
Effects of LPE and LPA on MAPKs in MDA-MB-231 cells
We measured the activities of three modules of MAPKs, namely, ERK1/2, SAPK/JNK, and p38 MAPK, which are down-stream signaling molecules of a [Ca2+]i increase. Neither LPE nor LPA changed their protein levels (Fig. 5). However, LPE and LPA induced a transient activation of ERK1/2 after 5 and 30 min of treatment (Fig. 5). Furthermore, LPE and LPA significantly inhibited SAPK/JNK activities after 5 min of treatment (Fig. 5). On the other hand, p38 MAPK was activated by LPE and LPA after 15 to 30 min of treatment (Fig. 5). Further activation of p38 MAPK was observed after 2 h in LPA-treated cells (Fig. 5B).
Fig. 5.
Effects of LPE and LPA on the activities of MAPKs in MDA-MB-231 cells. Effects of 18:1 LPE and LPA on the phosphorylations of MAPKs were estimated by Western blotting. MDA-MB-231 cells were treated with 10 μM 18:1 LPE (A) or LPA (B) for the indicated times. Western blotting was conducted using cell lysates. Results are representative of three independent experiments.
DISCUSSION
In the previous study, LPE-induced [Ca2+]i increase was found to be mediated via LPA1 and CD97 in MDA-MB-231 breast cancer cells (Park et al., 2013). In the present study, by applying structurally different LPEs and AM-095 (a selective LPA1 antagonist), we found differences between MDA-MB-231 breast cancer cells and SK-OV3 ovarian cancer cells. In SK-OV3 cells, LPE-induced [Ca2+]i increases were not inhibited by AM-095, implying no involvement of LPA1, which is consistent with a previous report by Park et al. (2007), who concluded no involvement of any LPA receptor in the LPE-induced response. In MDA-MB-231 breast cancer cells, LPE-induced [Ca2+]i increases were inhibited by AM-095, implying LPA1 involvement, which is consistent with our recent finding (Park et al., 2013). Therefore, our present results strongly support an idea of two previous papers about LPE action mechanisms. That is, LPE acts through GPCRs in two different ways depending on cell types; 1) using LPA1 and CD97 in MDA-MB-231 cells (Park et al., 2013) and 2) using unknown GPCRs different from LPA receptors in SK-OV3 cells (Park et al., 2007).
It was previously suggested that LPE-induced response in SK-OV3 ovarian cancer cells differs mechanistically from that in MDA-MB-231 cells (Park et al., 2007; Park et al., 2013). This difference is demonstrated in the present study by the synthetic LPE analogues, especially 18:0 LPE and ether-linked 18:0 LPE. Ether-linked 18:0 LPE and ester-linked 18:0 LPE produced half the response elicited by ester-linked 18:1 LPE in SK-OV3 cells, but did not produce any response in MDA-MB-231 cells. In the present study, response to LPE in SK-OV3 cells was affected by structural modifications, such as, ether linkage and chain length, but response in MDA-MB-231 cells was restricted to ester-linked 18:1 LPE. Furthermore, myristoyl LPE (14:0) induced [Ca2+]i increases in MDA-MB-231 cells and SK-OV3 cells, but its efficacy was less than that of 18:1 LPE and LPA in both cell lines. Myristoyl LPE-induced [Ca2+]i and 18:1 LPE-induced [Ca2+]i increases may be driven by different mechanisms, because palmitoyl LPE (16:0), which possesses a medium-size fatty acid chain (intermediate between 18:1 and 14:0 LPE) did not induce significant a [Ca2+]i increase in either cell-line.
In PC12 neuronal cells, LPEs from Grifola frondosa were reported to induce neuronal differentiation and suppressed serum-deprivation-induced apoptosis via MAPK activation (Nishina et al., 2006). In the present study, the significance of LPE-induced [Ca2+]i increase on cell proliferation was investigated in MDA-MB-231 cells. LPA induced a significant and concentration-dependent increase in cell proliferation in MDA-MB-231 cells, whereas LPE did less. Both responses were inhibited by AM-095, supporting involvement of LPA1 in the cells. However, in SK-OV3 cells LPA-induced cell proliferation was inhibited by AM-095, but LPE-induced responses were not, which again supports no involvement of LPA1 in SK-OV3 cells.
In SK-OV3 cells, LPE stimulates chemotactic migration and cellular invasion (Park et al., 2007). The significance of LPE-induced [Ca2+]i increase was investigated on cell migration using the wounding assay in MDA-MB-231 cells. LPA induced a significant increase in cell migration after 24 hr of treatment, whereas LPE did not. LPE is less potent than LPA at increasing Ca2+ increasing action (Park et al., 2013), but efficacy of 10 μM LPE was similar to that of 10 μM LPA. Therefore, in order to explain the discrepancy of 10 μM LPE- and 10 μM LPA-induced responses in cell proliferation and cell migration, there must be additional signaling(s) for LPA in addition to [Ca2+]i increase. LPE-induced [Ca2+]i increase was not enough to induce the same degree of proliferation and migration observed with LPA in MDA-MB-231 cells. However, LPA and LPE similarly modulated three modules of MAPKs in the cells. Therefore, it appears LPA triggers another signaling pathway in addition to the [Ca2+]i signaling and MAPK modulations in MDA-MB-231 cells, although further investigation of this topic is evidently required.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2011-0021158) and by the Korean National Research Foundation funded by the Korean government (MSIP) (Grant no. 2009-0083538).
REFERENCES
- Ahn BR, Moon HE, Kim HR, Jung HA, Choi JS. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid β peptide-induced toxicity in PC12 cells. Arch Pharm Res. 2012;35:1989–1998. doi: 10.1007/s12272-012-1116-5. [DOI] [PubMed] [Google Scholar]
- Castelino FV, Seiders J, Bain G, Brooks SF, King CD, Swaney JS, Lorrain DS, Chun J, Luster AD, Tager AM. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Lee YK, Lee EH, Park JJ, Chung SK, Im DS. Structure-activity relationships of dimethylsphingosine (DMS) derivatives and their effects on intracellular pH and Ca2+ in the U937 monocyte cell line. Arch Pharm Res. 2006;29:657–665. doi: 10.1007/BF02968250. [DOI] [PubMed] [Google Scholar]
- Choi JW, Chun J. Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta. 2013;1831:20–32. doi: 10.1016/j.bbalip.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Ishiguro J, Kitamura H, Arima N, Okutani M, Shuto A, Higashiyama S, Ohwada T, Arai H, Makide K, Aoki J. TGFα shedding assay: an accurate and versatile method for detecting GPCR activation. Nature methods. 2012;9:1021–1029. doi: 10.1038/nmeth.2172. [DOI] [PubMed] [Google Scholar]
- Iwashita M, Makide K, Nonomura T, Misumi Y, Otani Y, Ishida M, Taguchi R, Tsujimoto M, Aoki J, Arai H, Ohwada T. Synthesis and evaluation of lysophosphatidylserine analogues as inducers of mast cell degranulation. Potent activities of lysophosphatidylthreonine and its 2-deoxy derivative. J Med Chem. 2009;52:5837–5863. doi: 10.1021/jm900598m. [DOI] [PubMed] [Google Scholar]
- Jo SH, Kim SD, Kim JM, Lee HY, Lee SY, Shim JW, Yun J, Im DS, Bae YS. Lysophosphatidylglycerol stimulates chemotactic migration in human natural killer cells. Biochem Biophys Res Commun. 2008;372:147–151. doi: 10.1016/j.bbrc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Makide K, Shuto A, Ikubo M, Inoue A, Suzuki K, Sato Y, Nakamura S, Otani Y, Ohwada T, Aoki J. GPR34 is a receptor for lysophosphatidylserine with a fatty acid at the sn-2 position. J Biochem. 2012;151:511–518. doi: 10.1093/jb/mvs011. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee HY, Kim SD, Jo SH, Shim JW, Lee HJ, Yun J, Bae YS. Lysophosphatidylserine stimulates chemotactic migration in U87 human glioma cells. Biochem Biophys Res Commun. 2008;374:147–151. doi: 10.1016/j.bbrc.2008.06.117. [DOI] [PubMed] [Google Scholar]
- Liebscher I, Muller U, Teupser D, Engemaier E, Engel KM, Ritscher L, Thor D, Sangkuhl K, Ricken A, Wurm A, Piehler D, Schmutzler S, Fuhrmann H, Albert FW, Reichenbach A, Thiery J, Schoneberg T, Schulz A. Altered immune response in mice deficient for the G protein-coupled receptor GPR34. J Biol Chem. 2011;286:2101–2110. doi: 10.1074/jbc.M110.196659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makide K, Aoki J. GPR34 as a lysophosphatidylserine receptor. J Biochem. 2013;153:327–329. doi: 10.1093/jb/mvt010. [DOI] [PubMed] [Google Scholar]
- Makide K, Kitamura H, Sato Y, Okutani M, Aoki J. Em erging lysophospholipid mediators, lysophosphatidylserine, lysophosphatidylthreonine, lysophosphatidylethanolamine and lysophosphatidylglycerol. Prostaglandins Other Lipid Mediat. 2009;89:135–139. doi: 10.1016/j.prostaglandins.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Misra UK. Isolation of lysophosphatidylethanolamine from human serum. Biochim Biophys Acta. 1965;106:371–378. doi: 10.1016/0005-2760(65)90045-7. [DOI] [PubMed] [Google Scholar]
- Nishina A, Kimura H, Sekiguchi A, Fukumoto RH, Nakajima S, Furukawa S. Lysophosphatidylethanolamine in Grifola frondosa as a neurotrophic activator via activation of MAPK. J Lipid Res. 2006;47:1434–1443. doi: 10.1194/jlr.M600045-JLR200. [DOI] [PubMed] [Google Scholar]
- Park KS, Lee HY, Kim MK, Shin EH, Bae YS. Lysophosphatidylserine stimulates leukemic cells but not normal leukocytes. Biochem Biophys Res Commun. 2005;333:353–358. doi: 10.1016/j.bbrc.2005.05.109. [DOI] [PubMed] [Google Scholar]
- Park KS, Lee HY, Kim MK, Shin EH, Jo SH, Kim SD, Im DS, Bae YS. Lysophosphatidylserine stimulates L2071 mouse fibroblast chemotactic migration via a process involving pertussis toxin-sensitive trimeric G proteins. Mol Pharmacol. 2006;69:1066–1073. doi: 10.1124/mol.105.018960. [DOI] [PubMed] [Google Scholar]
- Park KS, Lee HY, Lee SY, Kim MK, Kim SD, Kim JM, Yun J, Im DS, Bae YS. Lysophosphatidylethanolamine stimulates chemotactic migration and cellular invasion in SK-OV3 human ovarian cancer cells: involvement of pertussis toxin-sensitive G-protein coupled receptor. FEBS Lett. 2007;581:4411–4416. doi: 10.1016/j.febslet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Park SJ, Lee KP, Kang S, Chung HY, Bae YS, Okajima F, Im DS. Lysophosphatidylethanolamine utilizes LPA1 and CD97 in MDA-MB-231 breast cancer cells. Cell Signal. 2013;25:2147–2154. doi: 10.1016/j.cellsig.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Ritscher L, Engemaier E, Staubert C, Liebscher I, Schmidt P, Hermsdorf T, Rompler H, Schulz A, Schoneberg T. The ligand specificity of the G-protein-coupled receptor GPR34. Biochem J. 2012;443:841–850. doi: 10.1042/BJ20112090. [DOI] [PubMed] [Google Scholar]
- Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, Kikuchi K, Nagi T, Harada M, Ogi K, Ebisawa M, Mori M. Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun. 2006;341:1078–1087. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- Swaney JS, Chapman C, Correa LD, Stebbins KJ, Broadhead AR, Bain G, Santini AM, Darlington J, King CD, Baccei CS, Lee C, Parr TA, Roppe JR, Seiders TJ, Ziff J, Prasit P, Hutchinson JH, Evans JF, Lorrain DS. Pharmacokinetic and pharmacodynamic characterization of an oral lysophosphatidic acid type 1 receptor-selective antagonist. J Pharmacol Exp Ther. 2011;336:693–700. doi: 10.1124/jpet.110.175901. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Kurikawa Y, Shimizu T, Ishii S. Current progress in non-Edg family LPA receptor research. Biochim Biophys Acta. 2013;1831:33–41. doi: 10.1016/j.bbalip.2012.08.003. [DOI] [PubMed] [Google Scholar]