Abstract
The state between aging with no cognitive impairment and dementia has become a major focus for intervention. The neuropathological and neurobiological correlates of this intermediate state are therefore of considerable interest, particularly from population representative samples. Here we investigate the neuropathological profile associated with different cognitive ability levels measured using strata defined by Mini Mental State Examination (MMSE) scores. One hundred and fifty one individuals were stratified into three cognitive groups including: non-, mildly, and moderately impaired at death. Alzheimer’s disease, atrophy, and vascular pathologies were investigated. Mild impairment was associated with an increased risk of vascular pathologies including small vessel disease and lacunes. In contrast, the moderately impaired group showed a more extensive pattern of pathology, including tangles and neuritic plaques (entorhinal/hippocampus), atrophy (cortical and hippocampal), and vascular disease (small vessel disease, lacunes, and infarcts). In a population-based sample of older people, MMSE score defined strata are associated with multiple pathologies. The profile of AD and vascular changes becomes more complex with increased cognitive impairment and these changes are likely to constitute a major substrate for age associated cognitive impairment. The results highlight the need for rigorous investigation of both neurodegenerative and vascular risks factors in old age.
Keywords: Aging, Alzheimer’s disease, cognitive impairment, neuropathology, vascular pathology
INTRODUCTION
Variability exists in the level of cognition in non-demented individuals before death. Knowledge of the underlying mechanisms associated with successful, unsuccessful, and pathological cognitive aging in the non-demented population is limited. Most research has been focused at particular ages or clinical states rather than investigating cognitive levels within populations without manifest dementia that include individuals of high functioning, age related cognitive decline (ARCD) and moderate impairment. A better understanding of the pathological profile associated with different cognitive levels will be important to identify those factors that pose the greatest risk for decline.
Heterogeneity in cognitive ability in the non-demented population has been supposed to reflect, at least partly, the common underlying conditions that also lead to frank decline including Alzheimer’s disease (AD) and vascular dementia [1, 2]. However, controversy exists in the severity of pathological changes needed to induce cognitive change. In some controls, the extent of AD and vascular related pathology can be equivalent to or even greater than that of a person with clinical dementia while in others, pathology is virtually absent [1, 3–5]. Clinico-pathological thresholds can be modified by age [6, 7], genetic factors [8], tolerance of pathology due to reserve (e.g., education or social networks) [9, 10], and the presence of coexisting AD and vascular pathologies [6, 11]. In control groups, grades of cognitive impairment including none, age-related, mild, and moderate decline may have different pathological profiles of increased neuropathological severity that when considered as a single group makes it difficult to distinguish from dementia. Indeed, individuals with amnestic mild cognitive impairment (aMCI) usually have a neuropathological profile similar to that of AD and dementia cases (e.g., increased neurodegenerative pathologies and atrophy) [12, 13]. Further, cognitive aging without dementia may reflect a brain free of pathology, or alternatively a brain in which the presence of pathology is masked by protective factors such as higher education.
Mental status in older aged populations is commonly assessed using the Mini Mental State Examination (MMSE) [14]. While in some studies, the MMSE is found to be insensitive to mild impairments, in others, the MMSE is found to have better predictive accuracy for incident dementia compared to more complex criteria for intermediate cognitive states, including criteria for aMCI or ARCD [15, 16]. Classification of dementia risk based on MMSE categories is easier to obtain compared to using criteria for aMCI/ARCD, particularly in population-based samples. Given this, we therefore tested whether grades of impairment in a non-demented population defined using MMSE strata can be linked to a specific neuropathological profile from subtle changes to more complex disease.
METHODS
Participants were from the Medical Research Council Cognitive Function and Ageing Study (CFAS) neuropathological resource across each of the study centers including Cambridgeshire, Gwynedd, Liverpool, Newcastle, Nottingham, and Oxford. Full details of the study design have been described previously [17]. CFAS is a large population representative study of people aged 65 years and older that began in 1991. In total, 13,004 participants completed a standardized questionnaire at their place of residence with a trained interviewer. Information on sociodemographics, health states, and cognitive ability were collected. A stratified sample of 20% (n = 2,640) was selected for a more detailed evaluation that included full mood and organicity sections of the Geriatric Mental State (GMS) Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT) [18]. Individuals selected for an assessment interview were approached for brain donation. The percentage willing to take part in donation varied among centers (20–55%) [4]. Those in the brain donor sample have been reinterviewed biennially with some annual follow-ups. The analysis here is based on the brains donated up to 1st August 2004 (n = 456) representing 3.8% of the 11,921 participants who entered the study and died before this time across each of the study centers (Data Version 3.1).
Dementia diagnosis
Full details of the approach to the diagnosis of dementia in CFAS have been published previously [7]. A study diagnosis of dementia was made using multiple information including death certificate notification of dementia or diagnosis at any interview according to the AGECAT algorithm (defined as an organicity rating of 3 or above) [18], that is equivalent to diagnosis based on the DSM-III-R. If the interview time was more than 6 months from death, additional information was gathered using a structured retrospective interview with knowledgeable informants (RINI) that covers the diagnostic domains required for clinical diagnosis including separation from terminal decline, and a final decision was made by clinicians working with CFAS. Dementia was diagnosed in 243 of the 456 brain donors. In 30 individuals, dementia status at death could not be established. These individuals, in addition to those with a diagnosis of dementia, were excluded. The remaining 183 were deemed to be non-demented; all had information sufficient to confirm absence of dementia (e.g., study diagnosis or RINI).
Cognitive assessment
The MMSE was chosen to define the cognitive groups as it has previously been found to be a good indicator for dementia risk in CFAS [15]. Currently, there is no agreement on the cut-off scores that should be used to define different cognitive categories. Here the cognitive categories were defined using cut-off scores that have previously been found to have high sensitivity and specificity for two-year risk of incident dementia [15] including scores in the range of 23–26 (e.g., mildly impaired). Individuals with scores in the range of 18–22 were classified as moderately impaired and individuals with scores in the range of 27–30 were classified as non-impaired. Individuals were categorized into each group based on their MMSE score at their last interview before death. Individuals with a missing MMSE score were excluded (n = 13). Individuals where the interval between death and last known MMSE score was greater than 2.5 years were also excluded (n = 19) to reduce the likelihood that they would be classified into the incorrect cognitive group (i.e., they may have progressed to a more severe impairment). A sensitivity analysis restricting the interval to 1.5 years was also run and while the associations were attenuated they remained in the same direction (See Supplementary Appendix 1).
Health assessment
Information about health status was included as part of the questionnaires and from the informant questionnaires, the RINIs, and death certificates. Five conditions previously associated with increased risk of dementia were selected including: angina, hypertension, diabetes mellitus, stroke, or heart attack. Individuals were dichotomized into whether they had the condition or not. Individuals were considered to have the condition based on a positive response from any information source. Only positive evidence from the death certificates was considered using ICD9 codes 430–438, 410, 250, and 413 for stroke, heart attack, diabetes mellitus, and angina.
Neuropathology protocol
At autopsy, frozen samples of brain tissue were removed for storage. The remainder of the brain was fixed for standardized assessment on paraffin-embedded tissues, following the protocol of the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) with minor modification [4, 19]. All procedures were standardized across centers with the exception of staining (See Supplementary Appendix 2 for stain details). While we cannot exclude the possibility that staining differences between centers may have affected the scoring of pathologies, the CERAD protocol is validated for cross-center comparisons including the use of different stains. Neuropathological examination was completed blind to knowledge of clinical, interview or RINI data. Information on AD, atrophy, and vascular pathologies were selected for this analysis.
Amyloid-β protein pathology and neurofibrillary tangles (NFTs) were assessed in the hippocampus (CA1), entorhinal cortex and in the frontal (Brodman Area 8/9), temporal (BA21), occipital (BA17/18) and parietal (BA7) lobes. Severity of pathology was scored as none, mild, moderate, or severe. Plaque pathology was assessed with Congo red, silver stains (including Bielschowsky, Palmgren and Gallyas), or immunohistochemistry. NFTs were assessed with immunohistochemistry (AT8 or 11/57). All slides were counterstained with Ehrlich’s hematoxylin and visualized with diaminobenzidine. For this analysis, burden of classical AD features was taken from the CERAD ratings in the entorhinal and hippocampal regions combined and in the neocortex. Each variable was defined as the maximum score in each region.
Vascular pathologies were assessed for each area examined using haematoxylin-eosine slides. Cerebrovascular pathology measures included the presence or absence of hemorrhages, infarcts (parenchymal ischaemic lesions >10 mm), lacunes (parenchymal ischaemic lesions <10 mm) and small vessel disease (diffuse pallor of myelin staining in white matter associated with hyaline degeneration of subcortical arteries and arterioles, micro-infarcts or a combination of these features). Lewy bodies (LB) were identified using hematoxylin-eosin and ubiquitin immunohistochemistry in the cortices, locus coeruleus, substantia nigra, nucleus basalis of Meynert, raphe nuclei, and dorsal efferent nucleus of vagus nerve. However, as LB disease was rare in the non-demented sample it was excluded from analysis due to a lack of statistical power.
Atrophy was assessed macroscopically, without knowledge of microscopic findings. Two regions were selected including the cortex (present versus absent) and hippocampus (classified as absent, mild, moderate, or severe). Braak staging was optional and due to the large number of missing data is not analyzed.
MRI data of formalin-fixed, post-mortem brain slices (~1 cm thick), was used to assess white matter lesions as detailed previously [20, 21]. Two rounds of MRI scanning were undertaken (1999/2000 and 2004/2005) with fixation time varying from a few months to six years. MRI was performed at 1.0 T (Siemens) using pulse sequences optimized for postmortem WMLs: T2-weighted spin echo (2500/98 ms; TR/TE excitations, where TR = repetition time and TE = echo time); proton density (2500/25 ms); and T1-weighted inversion recovery image (TR 6838 ms, inversion time of 600 ms, with a TE of 60 ms). MRI scans were scored by 3 observers using a validated semi-quantitative rating scale [22]. Scores for periventricular lesions (PVLs) and deep subcortical white matter lesions (DWML) were reported separately.
The CERAD assessment was used to determine the neuropathological spectrum of AD and other dementias. Based on the neuritic plaque scores, a CERAD neuropathological diagnosis was made of ‘normal brain’ (absent plaques), ‘plaques and tangles insufficient for Alzheimer’s disease’ (low plaques) or ‘Alzheimer’s disease’ (intermediate or high plaques). Other changes noted included Parkinson’s disease (PD) and cortical Lewy body disease (including Lewy bodies insufficient for PD) and vascular disease (combining the presence of infarcts, multiple lacunes, SVD, Binswanger’s disease, hemorrhage, or other vascular changes). An ‘other’ category was created to include changes consistent with mixed AD/PD, mixed VaD/PD, the presence of metastic tumor or white matter disease.
To ensure consistency between centers inter-rater reliability was assessed by circulation of macroscopic brain photographs and microscopic slides. This was found to be acceptable [23]. APOE genotypes were determined on frozen brain tissue samples using a similar method to that described previously [24]. Individuals were dichotomized based on their profile of ε4 alleles: none versus 1 or more.
Statistics
Summary statistics (mean and standard deviation) were used to compare baseline demographic characteristics across cognitive groups. ANOVA was used to test for group differences in age, MMSE, education, and the interval between last assessment and death. Group differences in APOE ε4 allele frequency, gender, and health status were tested using the Chi-squared statistic. AD and atrophy variables were dichotomized so that the absent ratings (“no evidence of pathology”) were compared to a combined score including the mild, moderate, and severe ratings (“evidence of pathology”). Logistic regression was used to test the relationship between neuropathology and cognitive group, with the non-impaired group as the referent. As age at death, the interval between death and last MMSE assessment and education may confound the association between neuropathology and cognitive function these were controlled in all models. The odds ratio (OR) and 95% confidence interval (95%CI) for each fully adjusted model are reported. All analyses were undertaken using Stata Version 11.
RESULTS
Participant characteristics
There were 151 individuals who died without dementia and had a MMSE score at their final interview before death. In total, 53 individuals died in a non-impaired state, 62 had mild impairment, and 36 had moderate impairment. The demographic profile of each group is shown in Table 1. There were no group differences in years of education, gender, APOE ε4 allele frequency, or disease status. Age at death and the interval between death and last MMSE assessment varied, with the moderately impaired group being oldest and having the smallest interval.
Table 1. Donor sample by cognitive group, all non-demented.
| Non impaired (MMSE 27–30) |
Mildly impaired (MMSE 23–26) |
Moderately impaired (MMSE 18–22) |
|
|---|---|---|---|
| Total Number (n) | 53 | 62 | 36 |
| % Female | 39.6 | 50 | 61.1 |
| Mean MMSE (SD)* | 28.7 (1.0) | 24.7 (1.2) | 20.1 (1.6) |
| Mean Age at Death (SD)* | 81.5 (7.4) | 83.1 (7.9) | 85.7 (7.6) |
| Mean Education (SD) | 9.8 (1.7) | 9.5 (1.4) | 9.8 (1.9) |
| Mean Interval (yrs)* | 1.3 (0.6) | 1.1 (0.7) | 0.9 (0.6) |
| APOE (% ε4+) | 29.7 (11) | 23.9 (11) | 16.7 (5) |
| Median Braak (q1, q3) | 2 (1, 3) | 2 (2, 3) | 2 (2, 3) |
| % Stroke (n) | 18.9 (10) | 22.6 (14) | 36.1 (13) |
| % Angina (n) | 22.6 (12) | 27.4 (17) | 36.1 (13) |
| % Diabetes (n) | 13.5 (7) | 12.9 (8) | 11.1 (4) |
| % Heart Attack (n) | 22.6 (12) | 32.3 (20) | 36.1 (13) |
| % Hypertension (n) | 41.5 (22) | 45.2 (28) | 50 (18) |
MMSE=Mini mental state examination; q1 = 25th Percentile; q3 = 75th Percentile; SD = standard deviation;
Significant group differences.
CERAD diagnosis
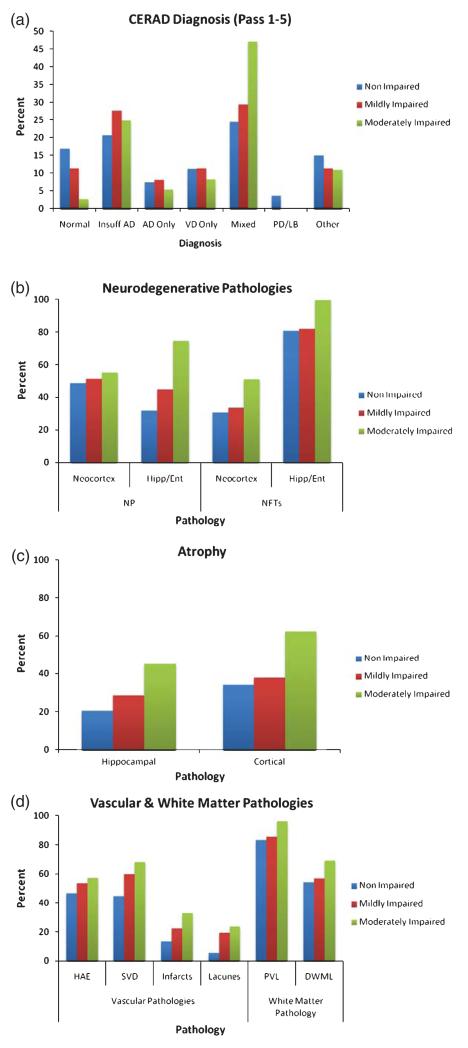
Figure 1a shows the distribution of CERAD neuropathological diagnosis across the groups. It was rare for brains to be classified as normal, particularly in the moderately impaired group. A diagnosis of pure AD or pure VaD was also rare (<15%). In contrast, with increased severity of decline there was an increase in the number of individuals with plaques and tangles of insufficient severity for AD or a mixed AD/VaD diagnosis.
Fig. 1. Pathological profile across cognitive groups:
(a) CERAD neuropathological diagnosis (collapsed across 5 assessments); (b) AD pathologies; (c) atrophy; and, (d) vascular and white matter pathologies.
AD pathology
Plaques and tangles were present in all groups and increased with cognitive impairment as shown in Fig. 1b. There were no significant differences between the non-impaired and mildly impaired groups on any AD variable as shown in Table 2. However, there was a significant increase in the risk of neuritic plaques in the hippocampus/entorhinal region in the moderately impaired group (OR = 3.6, 95%CI 1.2–10.6). Further, all individuals with moderate impairment showed NFTs in the hippocampus/entorhinal region.
Table 2. Distribution of pathology (mild/moderate/severe) across cognitive groups and associations (OR and 95%CI) between pathology and cognitive state at death.
| Pathological Profile (%)+ | Logistic Regression* | ||||||
|---|---|---|---|---|---|---|---|
| Non | Mild | Moderate | Non vs. Mild | Non vs. Mod | |||
| OR | 95%CI | OR | 95%CI | ||||
| Maximum Score Neuritic Plaques | |||||||
| Neocortex | 49.1 | 51.6 | 55.6 | 0.7 | (0.3–1.7) | 0.7 | (0.3–1.9) |
| Entorhinal cortex and hippocampus | 32.1 | 45.2 | 75.0 | 1.1 | (0.5–2.7) | 3.6 | (1.2–10.6) |
| Maximum Score Neurofibrillary Tangles | |||||||
| Neocortex | 30.8 | 33.9 | 51.4 | 1.1 | (0.5–2.8) | 1.6 | (0.6–4.4) |
| Entorhinal cortex and hippocampus | 81.1 | 82.3 | 100.0 | 0.2 | (0.0–1.0) | Omitted | |
| Atrophy | |||||||
| Hippocampus** | 20.8 | 29.1 | 45.7 | 2.2 | (0.7–6.2) | 4.8 | (1.5–15.6) |
| Cortical | 34.6 | 38.3 | 62.9 | 1.5 | (0.6–3.9) | 4.4 | (1.5–12.6) |
| White Matter Changes | |||||||
| Periventricular lesions | 83.7 | 86.2 | 96.6 | 1.6 | (0.5–5.5) | 6.8 | (0.7–68.4) |
| Deep Subcortical Lesions | 54.9 | 57.4 | 69.7 | 1.1 | (0.5–2.7) | 1.8 | (0.6–5.2) |
| Vascular | |||||||
| Small vessel disease | 45.1 | 60.3 | 68.6 | 2.5 | (1.1–6.1) | 3.6 | (1.3–10.4) |
| Infarcts | 13.7 | 23.0 | 33.3 | 2.6 | (0.8–9.0) | 4.8 | (1.2–18.9) |
| Lacunes | 6.0 | 19.7 | 24.2 | 6.4 | (1.3–31.2) | 8.8 | (1.6–49.4) |
| Haemorrhage | 10.0 | 1.6 | 3.1 | 0.2 | (0.0–1.9) | 0.3 | (0.0–3.8) |
Mild = mild impairment; Mod = moderate impairment; Non = non-impaired; OR = odds ratio; 95%CI, 95% confidence interval;
Percent of individuals with mild, moderate or serve pathology ratings;
Adjusted for age, education and time between last MMSE score and death;
Significant age by cognition interaction.
Atrophy
Cortical and hippocampal atrophy increased with cognitive impairment as shown in Fig. 1c. Risk of atrophy did not differ between the non-impaired and mildly impaired groups. In contrast, individuals with moderate impairment were at increased risk of atrophy in the cortex (OR = 4.4, 95%CI 1.5–12.6) and hippocampus (OR = 4.8, 95% CI 1.5–15.6).
Vascular pathology
Vascular features also increased with cognitive impairment as shown in Fig. 1d. Mild impairment was associated with an increased risk of SVD (OR = 2.5, 95%CI 1.1–6.1) and lacunes (OR = 6.4, 95%CI 1.3–31.2). The moderately impaired group were at increased risk of SVD (OR = 3.6, 95%CI 1.3–10.4), infarcts (OR = 4.8, 95%CI 1.2–18.9) and lacunes (OR = 8.8, 95%CI 1.6–49.4).
White matter pathology
Evidence of white matter pathology including periventricular lesions and deep subcortical lesions were observed in all groups and generally increased with cognitive impairment. However, associations were not significant.
DISCUSSION
Here we characterized the neuropathological profile of non-demented older adults with different levels of cognitive function at death from a population-based autopsy sample. Most individuals across all groups showed evidence of pathological changes despite not expressing the clinical symptoms of dementia. AD pathologies were not found to be associated with mild impairment, but were associated with moderate impairment. Moderate impairment was also associated with an increased risk of atrophy and vascular pathologies. These results highlight the importance of mixed pathologies in increasing risk of cognitive impairment in non-demented individuals. Overall, the results suggest that AD pathology and atrophy, particularly when vascular pathologies are evident may be the key contributors to impaired cognitive function.
This study is based on a large population-representative cohort of brain donors. Importantly this makes the findings generalizable to the population as a whole compared to findings from clinical, hospital, or specialist autopsy series. Further, while the brain donor sample represents only a small proportion of the total CFAS baseline, donors do not differ from baseline sample with respect to sex, social class, and length of time in education (years) [23]. Cognitive function was assessed using the MMSE at every interview wave. The advantage of the MMSE is that it is quick and easy to administer and has previously been shown to identify an at-risk state with higher progression to dementia compared to other more detailed classification criteria for intermediate cognitive states (e.g., aMCI) [15]. MMSE defined categories may therefore provide some guidance into the underlying pathologies that drive changes in global cognitive scores leading to an increased risk of dementia. The results however, are subject to the limitations of the CERAD protocol and the staining methods used (e.g., lack of methods based on α-synuclein for Lewy body pathology). Evidence of pathology is based on semi-quantitative assessment and this methodology was retained during the course of the study for all autopsy data to be consistent across CFAS study centers. Future analysis may identify further associations and potential mechanisms using a more robust approach and including newer staining methods. Despite the caveats of a semi-quantitative approach the assessment of pathologies showed acceptable inter-rater reliability and we choose to dichotomize variables for ease of interpretation into: (1) no evidence of pathological changes; and, (2) evidence of pathological changes. Further, information on other pathological features found to be related to impaired cognition, such as argyrophilic grain disease and hippocampal sclerosis in addition to other health conditions such as pulmonary and renal function were not available. Further studies are needed to determine the impact of these pathologies and their interaction with other pathological features on increasing risk of cognitive decline in non-demented population-representative samples.
AD pathology was common in all groups. Similar to previous studies we found that NFTs in the entorhinal/hippocampal region are highly prevalent irrespective of cognitive group (>80%), with few NFTs in the neocortex, except in the moderately impaired group [25, 26]. The pattern of plaques was also consistent with previous reports of a higher density in the neocortex compared to the hippocampal/entorhinal region, again with the moderately impaired group being an exception [5, 6, 25, 27–29]. In terms of risk, however, AD pathology does not appear to be associated with mild impairment, but does appear to play a role in moderate impairment. The results depended on location. Changes in the neocortex were not significant, while an increase in the presence of neuritic plaques and NFTs in the entorhinal/hippocampal region were associated with moderate impairment. This is consistent with previous studies where an increase in AD pathology has been found to be associated with MCI cases that transition to dementia at death [30] as well as reports where AD changes have been linked to subtle cognitive impairments in non-demented individuals [31, 32] as well as being associated with variability in MMSE scores [33]. Individuals in the moderately impaired group may represent “pre-clinical” AD and had they lived longer they may have progressed to dementia.
Moderate, but not mild impairment was associated with atrophy in the cortex and hippocampus. This finding supports previous neuroimaging reports of hippocampal and whole brain atrophy and neocortical loss in vivo at early dementia stages, becoming more severe with disease progression [34]. Further, individuals with MCI (defined using a Clinical Dementia Rating Score of 0.5) have been found to show substantial cell loss in the hippocampus at autopsy [31, 35–37]. Together, the data suggests that in the earliest signs of cognitive decline therapeutic targets should focus on preventing brain loss.
Group differences were also found in the presence of vascular pathological features including SVD, lacunes, and infarcts. The prevalence of vascular co-morbidity associated with angina, hypertension, diabetes mellitus, stroke, and heart attack did not differ across groups and therefore cannot explain these results. Vascular changes have been found to increase with age [38] and have previously been identified in relation to more severely impaired and dementia groups [39–42]. Further, the presence of vascular disease may lead to clinical expression of AD symptoms even when the burden of neurodegenerative pathology is relatively low [43]. However, the significance of specific vascular pathologies varies between studies. This may be due to the heterogeneity of vascular disease, differences between the cognitive status of the study samples, differences in sample selection or a combination of these factors. The findings here extend the role of vascular features in cognitive decline toward all impaired states, regardless of severity [30, 44].
The prevalence of white matter abnormalities generally increased with increasing cognitive impairment, but group differences were not significant. While results from other studies are not consistent with regard to specific white matter pathology and are variable in terms of the exact location, the presence of white matter changes has been associated with cognitive decline in both postmortem and MRI studies [39, 40, 45–47]. The pathological basis of white matter changes is thought to be complex and may be due to multiple processes, including aging, health related co-morbidity (e.g., hypertension, diabetes), reduced neuropil density, and myelin pathology. Further work is needed to determine the pattern of inter-relations between these and how they relate to cognitive symptoms. While, both Gold et al. [39] and Soderlund et al. [46] found that periventricular lesions are significantly associated with cognition, our results are not significant. The differences between these studies may be due to differences between the cognitive status in the different samples, sample selection criteria and heterogeneity in vascular diseases and associated white matter disease mechanisms.
The CERAD results demonstrate that each cognitively defined group includes individuals with no pathology, AD related only, vascular only, and mixed AD/vascular pathology. This heterogeneity suggests that progression to cognitive impairment, even at early stages, is complex. Indeed, while in clinical or hospital based autopsy cohorts the most common neuropathogical diagnosis is AD usually followed by vascular dementia, the results here support findings from community studies where mixed pathological features tend to be most common [48]. Further, the presence of individuals that lack these pathologies in all cognitively defined groups suggests that other factors associated with cognitive impairment have not been identified. Overall, the individual pathological findings and the CERAD diagnostic results suggest that more severe impairment is associated with multiple pathologies. However, a major issue in interpretation is whether the effect is additive, interactive or due to an as yet unidentified factor.
CONCLUSION
It appears that a buildup of pathological features including neurodegenerative pathology and atrophy in combination with vascular changes may underlie the cognitive transition across non-demented states captured using MMSE scores. Further research is needed to determine the threshold of change associated with each cognitive profile and those changes responsible for progression to dementia.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported in part by: a Special Project grant and a Programme grant from the MRC and the Department of Health; the UKNIHR Biomedical Research Centre for Ageing and Age-related Disease Award to the Newcastle up Type Hospitals Foundation Trust; the Cambridge Brain Bank is supported by the NIHR Cambridge Biomedical Research Centre; The Cambridgeshire and Peter-borough NIHR CLAHRC; Nottingham University Hospitals NHS Trust; University of Sheffield and the Sheffield Teaching Hospitals NHS Foundation Trust; The Thomas Willis Oxford Brain Collection, supported by the Oxford Biomedical Research Centre; The Walton Centre NHS Foundation Trust, Liverpool. BS is funded by the Joint European Post-Doctoral Programme: The European Research Area in Ageing (ERA-AGE) Network FLARE Programme. FM and GM are funded by UK.MRC.U.1052.00.013. We would like to acknowledge the essential contribution of the liaison officers, the general practitioners, their staff, and nursing and residential home staff. We are grateful to our respondents and their families for their generous gift to medical research, which has made this study possible.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=1081).
REFERENCES
- [1].Tyas SL, Snowdon DA, Desrosiers MF, Riley KP, Markesbery WR. Healthy ageing in the Nun Study: Definition and neuropathologic correlates. Age Ageing. 2007;36:650–655. doi: 10.1093/ageing/afm120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Jr, Xiong C, Grant E, Storandt M, Morris JC. Predictors of pre-clinical Alzheimer disease and dementia: A clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- [3].Bouras C, Hof PR, Morrison JH. Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett. 1993;153:131–135. doi: 10.1016/0304-3940(93)90305-5. [DOI] [PubMed] [Google Scholar]
- [4].Neuropathology Group of the Medical Research Council Cognitive Function Ageing Study Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- [5].Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- [6].Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [7].Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C, the Medical Research Council Cognitive Function and Ageing S Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- [8].Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- [9].Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol. 2006;5:406–412. doi: 10.1016/S1474-4422(06)70417-3. [DOI] [PubMed] [Google Scholar]
- [10].Bennett DAM, Wilson RSP, Schneider JAM, Evans DAM, Mendes de Leon CFP, Arnold SEM, Barnes LLP, Bienias JLS. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- [11].Tyas SL, Salazar JC, Snowdon DA, Desrosiers MF, Riley KP, Mendiondo MS, Kryscio RJ. Transitions to mild cognitive impairments, dementia, and death: Findings from the Nun Study. Am J Epidemiol. 2007;165:1231–1238. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Haroutunian V, Hoffman LB, Beeri MS. Is there a neuropathology difference between mild cognitive impairment and dementia? Dialogues Clin Neurosci. 2009;11:171–179. doi: 10.31887/DCNS.2009.11.2/vharoutunian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- [14].Folstein M, Folstein S, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [15].Stephan BCM, Savva GM, Brayne C, Bond J, McKeith IG, Matthews FE, Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Optimizing mild cognitive impairment for discriminating dementia risk in the general older population. Am J Geriatr Psychiatry. 2010;18:662–673. doi: 10.1097/jgp.0b013e3181e0450d. [DOI] [PubMed] [Google Scholar]
- [16].Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment1: Prevalence and predictive validity according to current approaches. Acta Neurol Scand. 2003;108:71–81. doi: 10.1034/j.1600-0404.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- [17].MRC CFAS. MRC CFAS Medical Research Council Cognitive Function and Ageing Study Group Cognitive function and dementia in six areas of England and Wales: The distribution of MMSE. Psychol Med. 1998;28:319–335. doi: 10.1017/s0033291797006272. [DOI] [PubMed] [Google Scholar]
- [18].Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16:89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- [19].Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, Belle Gv, Berg L, participating Cn The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD): Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- [20].Fernando MS, O’Brien JT, Perry RH, English P, Forster G, McMeekin W, Slade JY, Golkhar A, Matthews FE, Barber R, Kalaria RN, Ince PG. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol. 2004;30:385–395. doi: 10.1111/j.1365-2990.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- [21].Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O’Brien JT, Ince PG, on behalf of the MRC Cognitive Function, Ageing Neuropathology Study Group White matter lesions in an unselected cohort of the elderly: Molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- [22].Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, Steinling M, Valk J. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- [23].Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: Attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6:e1000180. doi: 10.1371/journal.pmed.1000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nicoll JA, Burnett C, Love S, Graham DI, Dewar D, Ironside JW, Stewart J, Vinters HV. High frequency of apolipoprotein E epsilon 2 allele in hemorrhage due to cerebral amyloid angiopathy. Ann Neurol. 1997;41:716–721. doi: 10.1002/ana.410410607. [DOI] [PubMed] [Google Scholar]
- [25].Price JL. The relationship between tangle and plaque formation during healthy aging and mild dementia. Neurobiol Aging. 1993;14:661–663. doi: 10.1016/0197-4580(93)90062-g. [DOI] [PubMed] [Google Scholar]
- [26].Haroutunian V, Perl DP, Purohit DP, Marin D, Khan K, Lantz M, Davis KL, Mohs RC. Regional Distribution of Neuritic Plaques in the Nondemented Elderly and Subjects With Very Mild Alzheimer Disease. Arch Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- [27].Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, Renbing X, Peck A. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- [28].Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: Neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- [29].Kazee AM, Eskin TA, Lapham LW, Gabriel KR, McDaniel KD, Hamill RW. Clinicopathologic correlates in Alzheimer disease: Assessment of clinical and pathologic diagnostic criteria. Alzheimer Dis Assoc Disord. 1993;7:152–164. doi: 10.1097/00002093-199307030-00004. [DOI] [PubMed] [Google Scholar]
- [30].Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, Tangalos EG, Boeve BF, Knopman DS, Braak H, Petersen RC. Neuropathologic outcome of mild cognitive impair ment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- [31].Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- [33].Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, Vainio P, Vanninen R, Partanen K, Soininen H. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- [35].Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound Loss of Layer II Entorhinal Cortex Neurons Occurs in Very Mild Alzheimer”s Disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- [37].West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [38].Longstreth WT, Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009;23:291–294. doi: 10.1097/WAD.0b013e318199fc7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gold G, Kovari E, Herrmann FR, Canuto A, Hof PR, Michel J-P, Bouras C, Giannakopoulos P. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- [40].Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel J-P, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- [41].Sonnen JA, Larson EB, Haneuse S, Woltjer R, Li G, Crane PK, Craft S, Montine TJ. Neuropathology in the adult changes in thought study: A review. J Alzheimers Dis. 2009;18:703–711. doi: 10.3233/JAD-2009-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann N Y Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- [43].Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- [44].Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- [45].Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Soderlund H, Nilsson LG, Berger K, Breteler MM, Dufouil C, Fuhrer R, Giampaoli S, Hofman A, Pajak A, de Ridder M, Sans S, Schmidt R, Launer LJ. Cerebral changes on MRI and cognitive function: The CASCADE study. Neurobiol Aging. 2006;27:16–23. doi: 10.1016/j.neurobiolaging.2004.12.008. [DOI] [PubMed] [Google Scholar]
- [47].Murray ME, Senjem ML, Petersen RC, Hollman JH, Preboske GM, Weigand SD, Knopman DS, Ferman TJ, Dickson DW, Jack CR., Jr Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol. 2010;67:1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.