Abstract
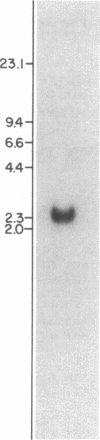
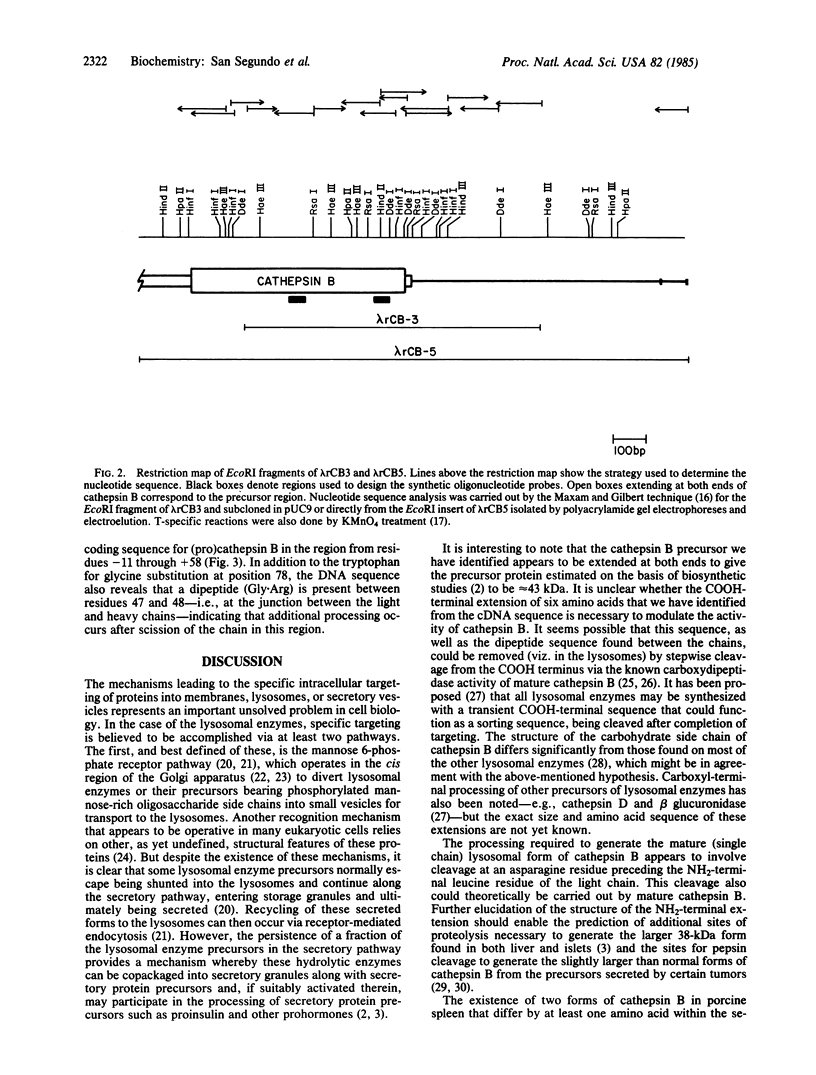
Recent studies have suggested that many lysosomal enzymes, including cathepsin B (EC 3.4.22.1), may be synthesized as larger precursors and proteolytically processed to their mature forms. To determine the structure of the primary translation product of cathepsin B, we have screened a phage cDNA library for clones encoding rat liver cathepsin B. We synthesized two extended DNA oligonucleotides to use as hybridization probes: a 50-mer corresponding to the coding segment for residues 215-231 of mature cathepsin B and a 54-mer corresponding to residues 117-134. After screening 600,000 plaques, five clones were obtained that hybridized to the 32P-labeled 50-mer; of these, two (lambda rCB3 and lambda rCB5) also reacted with the 54-mer. DNA sequence analysis confirmed that lambda rCB3 and lambda rCB5 both encoded rat liver cathepsin B, and the translated sequence is in agreement with the sequence determined [Takio, K., Towatari, T., Katunuma, N., Teller, D. C. & Titani, K. (1983) Proc. Natl. Acad. Sci. USA 80, 3666-3670], except for a tryptophan for glycine substitution at residue 78 and the presence of two amino acids at the junction site of the light and heavy chains. Moreover, the DNA sequence reveals an open reading frame extending beyond the 5' (NH2 terminus), and the predicted COOH terminus of the coding sequence for the mature protein is extended by six amino acids. These results confirm that the biosynthesis of cathepsin B involves a larger precursor form and demonstrate the effectiveness of long oligonucleotide probes for screening to detect rare cloned mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Barrett A. J. The specificity of cathepsin B. Hydrolysis of glucagon at the C-terminus by a peptidyldipeptidase mechanism. Biochem J. 1978 Jun 1;171(3):759–765. doi: 10.1042/bj1710759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszuk A. M., Deugau K. V., Sherwood J., Michalak M., Glick B. R. An efficient method for the sequence analysis of oligodeoxyribonucleotides. Anal Biochem. 1983 Feb 1;128(2):281–286. doi: 10.1016/0003-2697(83)90376-7. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Barrett A. J. Degradation of fructose-1,6-bisphosphate aldolase by cathepsin B. Biochem J. 1980 Jul 1;189(1):17–25. doi: 10.1042/bj1890017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. J., Farquhar M. G. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984 Feb;36(2):295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R., Steiner D. F. Identification of a 31,500 molecular weight islet cell protease as cathepsin B. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3245–3249. doi: 10.1073/pnas.80.11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C., Steiner D. F. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem. 1984 May 25;259(10):6041–6044. [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Carboxyl-terminal proteolytic processing during biosynthesis of the lysosomal enzymes beta-glucuronidase and cathepsin D. Biochemistry. 1983 Oct 25;22(22):5201–5205. doi: 10.1021/bi00291a021. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Leduc M. S., Recklies A. D. Characterization of a latent cysteine proteinase from ascitic fluid as a high molecular weight form of cathepsin B. Biochim Biophys Acta. 1983 Feb 22;755(3):369–375. doi: 10.1016/0304-4165(83)90240-4. [DOI] [PubMed] [Google Scholar]
- Mullins D. E., Rohrlich S. T. The role of proteinases in cellular invasiveness. Biochim Biophys Acta. 1983 Dec 29;695(3-4):177–214. doi: 10.1016/0304-419x(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Pohlmann R., Waheed A., Hasilik A., von Figura K. Synthesis of phosphorylated recognition marker in lysosomal enzymes is located in the cis part of Golgi apparatus. J Biol Chem. 1982 May 25;257(10):5323–5325. [PubMed] [Google Scholar]
- Recklies A. D., Mort J. S., Poole A. R. Secretion of a thiol proteinase from mouse mammary carcinomas and its characterization. Cancer Res. 1982 Mar;42(3):1026–1032. [PubMed] [Google Scholar]
- Rubin C. M., Schmid C. W. Pyrimidine-specific chemical reactions useful for DNA sequencing. Nucleic Acids Res. 1980 Oct 24;8(20):4613–4619. doi: 10.1093/nar/8.20.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Docherty K., Carroll R. Golgi/granule processing of peptide hormone and neuropeptide precursors: a minireview. J Cell Biochem. 1984;24(2):121–130. doi: 10.1002/jcb.240240204. [DOI] [PubMed] [Google Scholar]
- Stevens T., Esmon B., Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982 Sep;30(2):439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Schmidt P. G., Tang J. Novel carbohydrate structures of cathepsin B from porcine spleen. J Biol Chem. 1984 May 25;259(10):6059–6062. [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Teller D. C., Titani K. Homology of amino acid sequences of rat liver cathepsins B and H with that of papain. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3666–3670. doi: 10.1073/pnas.80.12.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Berman C. H., Dull T. J., Gray A., Lee J. M. Isolation of the human insulin-like growth factor I gene using a single synthetic DNA probe. EMBO J. 1984 Feb;3(2):361–364. doi: 10.1002/j.1460-2075.1984.tb01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]