Abstract
Uterine leiomyosarcoma (ULMS) usually follows an aggressive clinical course, although a small proportion of ULMS exhibit a more indolent course, which in turn reflects heterogeneity within this disease and the need to identify and characterise this distinct subgroup. The role of hormone therapy in ULMS is yet to be elucidated. We report a patient with well-differentiated metastatic ULMS on hormone replacement therapy (HRT) at the time of the diagnosis. The withdrawal of the HRT led to a significant decrease in the tumour burden and symptomatic improvement. The patient further benefited from aromatase inhibitor treatment once the benefit from the HRT withdrawal reached a plateau. The present case report describes for the first time hormone-dependency for tumour growth in a ULMS. We propose that a subset of ULMS that follow a protracted/indolent course might depend on hormone stimulation for tumour proliferation, and antihormone treatment can therefore be useful in these patients.
Background
Uterine leiomyosarcoma (LMS) represents the most frequent uterine sarcoma and may follow an aggressive clinical course, with a high rate of local and distant relapse (45–80%) and an approximate median overall survival of 2 years once the disease is disseminated.1
Surgery is the only curative approach for localised disease, and chemotherapy is regarded as a palliative treatment for advanced/recurrent disease. Interestingly, a small proportion of uterine LMS exhibit a more indolent course,1 2 reflecting heterogeneity within this disease and the need to identify and characterise this distinct subgroup, particularly as treatment is being planned.
Case presentation
A 42-year-old woman with a previous history of uterine fibroids underwent total abdominal hysterectomy and bilateral salpingo-oophorectomy for progressively increasing pelvic discomfort due to an enlarging uterine mass. Surgical specimen revealed a 6.8 cm mass in the uterine fundus. Ovaries, fallopian tubes and peritoneal washings were unremarkable. Histological evaluation demonstrated a cellular smooth muscle neoplasm showing multifocal significant nuclear atypia, mitotic count of 1/10 high-power fields (HPF) and a lack of geographic tumour cell necrosis (figure 1). Of note, the tumour infiltrated normal myometrium and involved vascular spaces. Giving these findings, the neoplasm was classified as a smooth muscle tumour of uncertain malignant potential (STUMP). Approximately 40% of tumour cells had diffuse and heterogeneous expression of oestrogen receptor (ER), and 90% of tumour cells had diffuse and strong expression of progesterone receptor (PR) (figure 2). CT scan examination after surgery did not demonstrate any evidence of distant disease.
Figure 1.
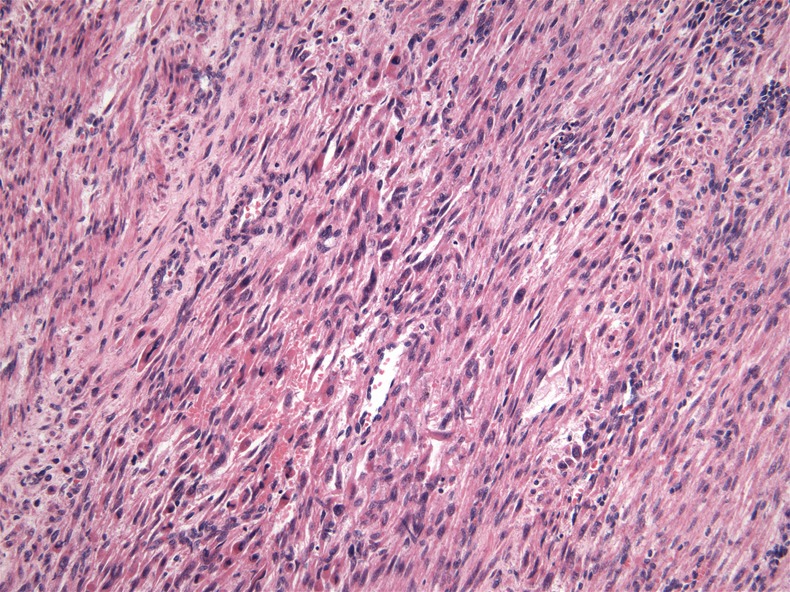
Primary uterine tumour demonstrates histological atypia.
Figure 2.
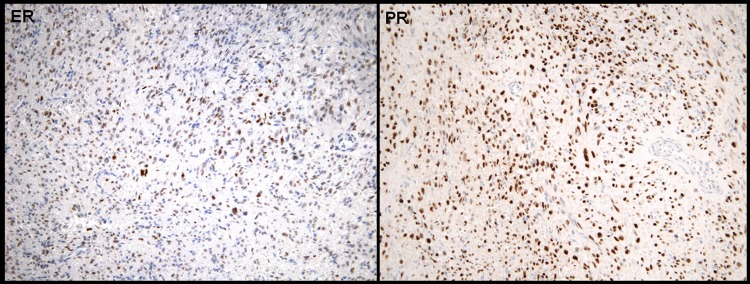
Primary uterine tumour. (A) Oestrogen receptor expression of primary tumour and (B) progresterone receptor expression of primary tumour.
The patient started hormone replacement therapy (HRT) with low-dose oral oestrogens for menopausal symptoms and was followed annually with gynaecological and physical examinations. Nine years after the initial surgery, at the age of 51, the patient reported a 3-week history of chest discomfort with deep breathing. A subsequent CT-scan revealed multiple bilateral pulmonary and pleural-based nodules (figure 3A) and an 11 cm mass in the retroperitoneum, consistent with disseminated disease. Biopsy of a pulmonary nodule demonstrated metastatic leiomyosarcoma (LMS) and displayed similar features to the primary uterine tumour. The pulmonary lesion demonstrated strong and diffuse immunoreactivity for both ER and PR in greater than 95% of tumour nuclei (figure 4). Based on the clinical behaviour, the tumour was best considered as a metastatic low-grade LMS. Given the diffuse ER/PR expression, the patient was instructed to discontinue HRT.
Figure 3.
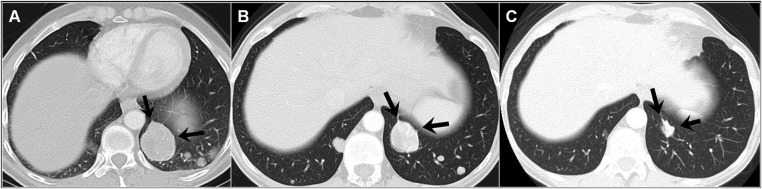
Representative pulmonary metastasis. (A) Baseline; (B) following withdrawal of hormone replacement therapy and (C) following 2.5 years of aromatase inhibition.
Figure 4.
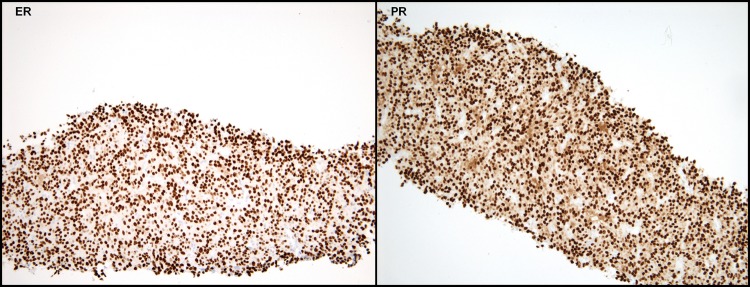
Pulmonary metastasis. (A) Oestrogen receptor expression and (B) progesterone receptor expression.
After 6 weeks of HRT withdrawal, all symptoms resolved and there was an overall decrease in size of the pulmonary metastases (figure 3B) and no change in size of the retroperitoneal mass. Six months later, the pulmonary lesions remained stable. The retroperitoneal mass increased slightly in size and was resected. The surgical specimen showed similar histological features to the primary tumour and the lung biopsy, although the mitotic rate was higher (5:10 HPF) and PR were negative. At this time, the patient started treatment with an aromatase inhibitor (letrozol) to further suppress the systemic oestrogen levels. After 2.5 years of letrozol, the patient continues to demonstrate further reduction in size of the pulmonary metastasis (figure 3C) and remains asymptomatic.
The largest pulmonary lesions have reduced by over 50% in maximum unidimensionalmeasurement, and some of the smaller lesions have become imperceptible by imaging.
Discussion
In the present case, the primary uterine tumour did not demonstrate features consistent with a conventional LMS; it showed diffuse nuclear atypia but lacked sufficient mitotic rate and coagulative necrosis in order to meet at least two of the three Stanford criteria for the diagnosis of uterine LMS.3 However, the tumour infiltrated both blood vessels and normal myometrium, and neither of these two findings is observed in strictly benign uterine lesions, hence the designation as STUMP. In addition, the tumour eventually metastasised, but remarkably, it was detected unusually late compared with the early recurrences (often within 2–3 years) commonly observed in conventional LMS.1 Disease-free interval between primary diagnosis of STUMP and recurrence of distant disease may be many years.4 Together, these features are consistent with the diagnosis of a so-called ‘low-grade’ or ‘well-differentiated’ uterine LMS,2 although this entity is yet to be fully understood or characterised.
There may be subtle histological gradation from benign leiomyoma to STUMP to LMS, which makes diagnosis of low-grade uterine LMS challenging. Moreover, most gynecological pathologists consider all uterine LMS as high-grade tumours, and typically do not provide a grade, despite some studies having concluded that grade might be a prognosis factor.5
We show an unequivocal dependency on oestrogen and progesterone hormones for tumour growth in a case of metastatic leiomyosarcoma following a STUMP.
The withdrawal of the HRT led to a significant decrease in the tumour burden and symptomatic improvement of the patient. Aromatase inhibitor treatment further significantly reduced the size of the metastases once the benefit from the HRT withdrawal reached a plateau. The role of hormone-dependency for uterine LMS growth and progression remains controversial. Some studies have suggested that the use of HRTs6 and tamoxifen7 may be related to an increased risk of developing uterine sarcoma. Up to 40% of uterine LMS are ER and/or PR positive.8 In addition, studies have suggested that the presence of ER/PR receptors by imunohistochemistry (IHC) identifies a subset of uterine LMS with a more favourable course.8 Anecdotal case reports and one retrospective series have reported some benefit of antihormone therapies in patients with metastatic uterine LMS9 and STUMP. However, these reports may be biased towards patients with a more favourable outcome due to inherent tumour biology. Current clinicopathological criteria are extremely limited in order to identify a priori a subgroup of patients with ULMS who have a greater chance to have a hormonally driven tumour.
The predictive capability of ER and/or PR expression to predict response to hormonal therapies remains unknown. O'Cearbhaill et al10 reported partial response in 3 of 34 patients, all of whom were ER positive (PR status was unknown). Additionally, it has been shown that PR-positive uterine LMS have lower recurrence rates and better overall survival.8 Interestingly, in the present case report, the responsive lung metastases coexpressed both ER and PR, while the progressing retroperitoneal mass was ER positive and PR negative. Based on this single experience and previous evidence,8 10 we suggest that high ER/PR coexpression in uterine LMS might help to define a subgroup of patients whose tumours may be more likely to be hormonally dependent. Previous diagnosis of STUMP may further support the role of hormone dependency.
Long-term follow-up is advised following the diagnosis of STUMP since recurrence of disease may develop after many years in some cases.2 4 Moreover, patients with recurrent disease might potentially benefit from surgical resection, if amenable, due to the slow-growth of the disease. Additionally, the hormone-dependency for tumour growth observed in this subgroup of tumours suggests that HRT should be administered with caution after the diagnosis of STUMP.
In summary, we have shown a patient with a metastatic low-grade LMS following a STUMP who had a significant bidimensional response to HRT withdrawal and subsequent aromatase inhibition representing a strikingly distinct behaviour compared with conventional uterine LMS. We have reinforced the proof-of-concept for hormone dependency in low-grade uterine LMS following a STUMP and hypothesise that uterine LMS comprise a heterogeneous diagnosis that may include a small proportion of hormonally driven tumours. This is important as these patients may benefit from effective and less aggressive treatments, such as aromatase inhibitors.
Learning points.
Uterine leiomyosarcoma (LMS) comprise a heterogeneous population that include a small proportion of hormonally driven tumours.
Oestrogen receptor/progesterone receptor coexpression in uterine LMS might help to define a subgroup of patients whose tumours may be more likely to be hormonally dependent.
Hormone-dependent uterine LMS will benefit from antihormone therapeutic approaches.
Footnotes
Contributors: CS, MN, ST, CR and SG have contributed equally in the conception, design, acquisition and interpretation of data provided in the case report. They have also contributed equally in drafting the article, revising it critically for important intellectual content and approved the final version of the manuscript for publication.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Raut CP, Nucci MR, Wang Q, et al. Predictive value of FIGO and AJCC staging systems in patients with uterine leiomyosarcoma. Eur J Cancer 2009;45:2818–24 [DOI] [PubMed] [Google Scholar]
- 2.Veras E, Zivanovic O, Jacks L, et al. “Low-grade leiomyosarcoma” and late-recurring smooth muscle tumors of the uterus: a heterogenous collection of frequently misdiagnosed tumors associated with an overall favorable prognosis relative to conventional uterine leiomyosarcomas. Am J Surg Pathol 2011;35:1626–37 [DOI] [PubMed] [Google Scholar]
- 3.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol 1994;18:535–58 [PubMed] [Google Scholar]
- 4.Guntapalli SR, Ramirez PT, Anderson ML, et al. Uterine smooth muscle tumor of uncertain potential: a retrospective analysis. Gyn Oncol 2009;113:324–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapp DS, Shin JY, Chan JK. Prognostic factors and survival in 1396 patients with uterine leiomyosarcomas. Cancer 2008;15:820–30 [DOI] [PubMed] [Google Scholar]
- 6.Jaakkola S, Lyytinen HK, Pukkala E, et al. Use of estradiol-progestin therapy associates with increased risk for uterine sarcomas. Gynecol Oncol 2011;122:260–3 [DOI] [PubMed] [Google Scholar]
- 7.Silva EG, Tornos CS, Follen-Mitchell M. Malignant neoplasms of the uterine corpus in patients treated for breast carcinoma: the effects of tamoxifen. Int J Gynecol Pathol 1994;13:248–58 [DOI] [PubMed] [Google Scholar]
- 8.Leitao MM, Jr, Hensley ML, Barakat RR, et al. Immunohistochemical expression of estrogen and progesterone receptors and outcomes in patients with newly diagnosed uterine leiomyosarcoma. Gynecol Oncol 2012;124:558–62 [DOI] [PubMed] [Google Scholar]
- 9.Amant F, Coosemans A, Debiec-Rychter M, et al. Clinical management of uterine sarcomas. Lancet Oncol 2009;10:1188–98 [DOI] [PubMed] [Google Scholar]
- 10.O'Cearbhaill R, Zhou Q, Iasonos A, et al. Treatment of advanced uterine leiomyosarcoma with aromatase inhibitors. Gynecol Oncol 2010;116:424–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


